- Abstract:
- 1 Introduction▲
- 2 Experimental▲
- 3 Results and discussion▲
- 4 Conclusions▲
- References
- Figure
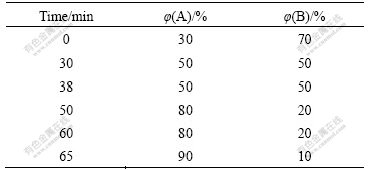
- Fig.1 TICs from essential oils in EUO barks (a), (c) and leaves (b), (d) when retention time is from 4.0 to 26.5 min ((a) and (b)) and from 7.1 to 7.3 min ((c) and (d))
- Fig.2 Results obtained through treating peak clusters A and B by combining HELP with AMWFA (EV denotes eigen value): (a) Rank profile of peak cluster A; (b) Resolved chromatograms of three components in peak cluster A; (c) Rank profile of peak cluster B; (d) MSCC; (e) IP-MSCC; (f) Result of matrixes X and Y common rank analysis
- Fig.3 Comparison of resolved mass spectra ((a) and (b)) with standard mass spectra of benzocyclobutane (c) and heptenal (d) from NIST library
- Fig.4 Chromatograms of calibration solution (a), EUO bark extraction (b) and EUO leave extraction (c): 1—Rutin; 2—Quercetin; 3—Kaempferol
- Fig.5 Ultraviolet absorbance spectra of rutin((a), (d) and (g)), quercetin ((b), (e) and (h)), and kaempferol((c), (f) and (i)) in calibration solution, barks, and leaves, respectively
J. Cent. South Univ. Technol. (2009) 16: 0371-0379
DOI: 10.1007/s11771-009-0063-x
![]()
Comparative analysis of chemical components between barks and leaves of Eucommia ulmoides Oliver
ZHOU Ju-feng(周菊峰)1, 2, ZHANG Tai-ming(张泰铭)1, CHEN Wang-ai(陈望爱)1, LIANG Yi-zeng(梁逸曾)1
(1. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. Department of Chemistry and Life Science, Xiangnan University, Chenzhou 423000, China)
Abstract:
The chemical components of the essential oils in the barks and leaves of Eucommia ulmoides Oliver were analyzed and compared by chromatograms and mass spectra technique, heuristic evolving latent projections (HELP), alternative moving window factor analysis (AMWFA) algorithms and normalization method based on the peak areas; the flavones in the barks and leaves of Eucommia ulmoides Oliver were separated on an ODS column by gradient elution carried out with the flow phase consisting of water, methanol and phosphoric acid (0.1%), and their contents were quantitatively determined by standard curve method and diode array detection (DAD) at 362 nm. The results show that 68 and 73 compounds respectively from essential oils of the barks and leaves of Eucommia ulmoides Oliver are identified, and there are 33 mutual compounds among 108 compounds determined. The total contents of these volatile components of the two samples possess 92.9% and 97.75% of the gross of the relevant essential oils, respectively; the contents of the rutin, quercetin and kaempferol in the barks and leaves of Eucommia ulmoides Oliver are 0.016 9, 0.003 6, 0.002 1 and 0.064 4, 0.030 2, 0.010 0 mg/g, respectively, and the determination recoveries are 95.2%-106.2%. The comparative analysis shows that for the barks and leaves of Eucommia ulmoides Oliver, there are significant differences in their components of the relevant essential oils and flavones.
Key words:
1 Introduction
Eucommia ulmoides Oliver (EUO) is actually a rare officinal plant, and its barks qua an officinal part is a valuable natural medicine (NM). In the book “Chinese Pharmacopoeia”[1], it is recorded that EUO barks have important actions to resume the functions of human livers and kidneys. The pharmacological research [2-6] has indicated that EUO barks have the actions such as anti-hypertension, anti-cancer, enhancing the strength, preventing miscarriage, diuresis, debasing fattiness, and anti-senescence. In addition, both officinal values and contents of natural active components in EUO leaves are also very high, and the components can be separated from the leaves. Therefore, EUO leaves have the potentiality to be empoldered into relevant health foods or leechdoms [7-9]. For an officinal plant, there usually are differences in chemical substance of its different parts. However, for EUO, are the differences the component ones, the content ones or the both? How big are the differences? The answers are of very important significance for the rational exploiture and sufficient utilization of the rare officinal resources as well as the research on EUO pharmacology. Therefore, it is quite necessary to analyze comparatively the chemical components in the barks and leaves. The volatile components in some traditional Chinese medicines were analyzed by combining chromatograms and mass spectra technique with one chemometric algorithm [10-11]. However, there has not been a report about the systemic comparative analysis of chemical components in different parts of EUO plants. In this work, for putting forward the method for systemic comparative analysis of the volatile components and flavones in EUO barks and leaves, the gas chromatography-mass spectrum (GC-MS) two- dimensional data of the essential oils in EUO barks and leaves were resolved by combining the heuristic evolving latent projections (HELP) [12-16] and the alternative moving window factor analysis (AMWFA) [17] in order to obtain the pure chromatograms and mass spectra from the components (comprising mutual ones) in the essential oils of EUO barks and leaves. The components in the oils were identified by the pure mass spectra and the NIST database search, and their relative contents were determined by the pure chromatograms and the area normalization method. The quantitative analyses of the flavones in EUO barks and leaves were carried out by high performance liquid chromatography-diode array detector (HPLC-DAD) detection and standard curve method.
2 Experimental
2.1 Reagents, standards and samples
Methanol from Hanbang Ltd. of Science and Technology (Jiangsu, China) was of HPLC grade; Hexane, petroleum ether, alcohol, ethyl acetate and phosphoric acid, which were from Huihong Ltd. of Chemical Reagents (Hunan, China), were of analytical reagent grade. Distilled water was used. Quercetin (batch number (BN): 100081-200406), kaempferol (BN: 110861-200405) and rutin (BN: 100080-200306) were purchased from National Institute for the Control of Pharmaceutical and Biological Products (NICPBP) (Beijing, China). All of EUO barks and leaves were from Sichuan (China), and provided by Tocan Ltd. of Bio-science and Bio-technology (Changsha, China).
2.2 Preparation of EUO barks and leaves for chromatographic analyses
The dried and powdered barks (100 g) and leaves (100 g) were placed into extractors respectively, and soxhlet extracted for 5 h according to “Chinese Pharmacopoeia (Vol.1)” [1], then, the essential oils acquired were diluted to 2.00 mL with hexane. These sample solutions were for GC-MS determination.
The dried and powdered barks (10 g) and leaves (10 g) were degreased by petroleum ether at 80 ℃ for 3 h. The residue was soaked with 100 mL of 80% alcohol at room temperature for 24 h, then extracted continuously three times with the same solvent for 1 h in an ultrasonic extractor. Then the solutions extracted were evaporated to almost dryness under vacuum at 50 ℃. The concentrate samples evaporated were extracted with ethyl acetate. Finally, the extracted solution containing flavones was diluted with methanol to 2 mL. After passing a 0.45 ?m filter membrane, the sample solution was for HPLC-DAD determination.
2.3 GC-MS determinations of essential oils in EUO barks and leaves
The apparatus was the Shimazu 2010 GC-MS system (Shimazu, Japan), supplied with electron impact ionization (EI) detector. The OV-1 capillary column was used in the experiment; spec: 30 m×0.25 mm×0.25 ?m; temperature for injecting samples: 280 ℃; temperature for detection: 280 ℃; carrier gas: He; velocity of flow: 1.0 mL/min; ratio of distributary: 10:1; injection volume: 1.0 ?L. The program change of the column temperature was as follows:
![]()
The MS conditions were as follows: 0.75 kV of EI detector voltage, 200 ℃ of ion source temperature, 30-450 U of m/z range and 0.2 s of scan interval.
2.4 HPLC-DAD determinations of flavones of EUO barks and leaves
Determination was performed on a HP 1100 HPLC-DAD system (Agilent, USA) with quaternary gradient pump, integrated vacuum degasser and DAD detector. Chromatographic column: Waters spherisorb RP18 (4.6 mm×250 mm, 5 μm) (Waters, USA); injection volume: 10 μL; velocity of flow: 0.8 mL/min; detection wavelength: 362 nm; Gradient elution solutions: methanol (A), water solution of phosphoric acid (0.1%) (B). The gradient elution conditions are listed in Table 1.
Table 1 Conditions of HPLC gradient elution
3 Results and discussion
3.1 Qualitative analysis of components of essential oils of EUO barks and leaves
The GC-MS total ion-current chromatograms (TICs) of the essential oils in EUO barks and leaves are shown in Fig.1(a) and (b), respectively. It is obvious from the TICs that although most peaks are well separated, there are also some inflexions in the chromatographic curves, which may be caused by such signals as impurity peaks, instrument background and component overlap peaks. In this work, the overlapped two-dimensional data were resolved by combining HELP with AMWFA in order to obtain the exacter results.
Let us take peak cluster A (A in Figs.1(a) and (c)) in the range of 7.10-7.30 min of retention time (namely, the range from the 873rd chromatographic scan point to the 928th) as an example to elaborate simply the application of HELP in the sample analyses. Although peak cluster A is actually a multi-compound overlapping peak, it seems a chromatographic peak of single compound. Therefore, the data-matrix of zero-concentration region of peak cluster A was first treated by local principal component analysis (LPCA) in order to deduct the data background and to obtain the selective information. At the same time, based on fixed size moving window evolving factor analysis (FSMWEFA), the data-matrix of the selective
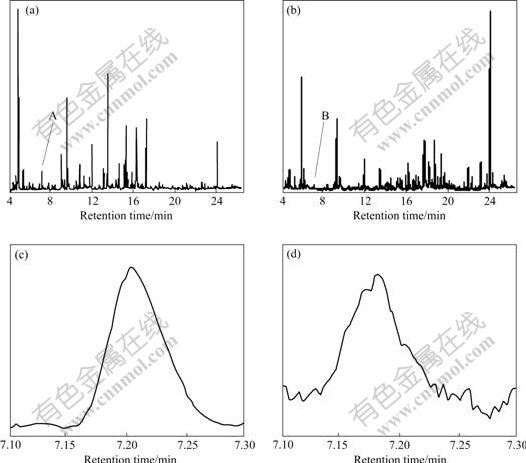
Fig.1 TICs from essential oils in EUO barks (a), (c) and leaves (b), (d) when retention time is from 4.0 to 26.5 min ((a) and (b)) and from 7.1 to 7.3 min ((c) and (d))
region was solved by local singularity value resolution to obtain the rank profile (Fig.2(a)). Furthermore, based on the selective information and the rank profile, the two-dimensional data of peak cluster A were resolved by utilizing the technique of full rank resolution so as to educe the pure chromatograms and mass spectra of the relevant components in peak cluster A. Then, matching searches were carried out by employing the pure mass spectra in the NIST standard mass spectrum library in order to confirm the compounds corresponding to pure chromatograms labeled “1”, “2” and “3”, respectively (Fig.2(b)). The results show that compounds 1, 2 and 3 are benzocyclobutane, heptenal and (E, E)-2, 4-octadienal, respectively.
HELP is a valid method for the resolution of two-dimensional overlapped data. However, it is difficult to obtain exactly qualitative results by HELP when the selective information of overlapped peaks is too weak. Nevertheless, AMWFA is a splendid supplementation for HELP. AMWFA can employ the intersectant information hiding in two data systems to confirm the number and mass spectra of the mutual components in the two systems. It can also educe the mass spectrum information of the other data system from the component information of a system. Therefore, when there is not the selective information or there is only the weak information in data-matrix I, the relevant components in this system may be confirmed by the available information extracted by AMWFA from the sub-matrix in data-matrix II.
The above-mentioned example may also be used to illustrate the application of AMWFA whose principle is elaborated in Ref.[17]. The data (namely, peak cluster B) from 7.10 to 7.30 min of retention time in the TIC of essential oils in the leaves were intercepted (see Fig.1(d) and B in Fig.1(b)). It is obvious from Fig.1 that the selective information securable from peak cluster B is very weak because the signal-to-noise is very low. Therefore, although the rank profile (Fig.2(c)) and the resolved chromatograms and mass spectra of relevant compounds may be obtained through the resolution of the two-dimensional data by HELP, only a compound, i.e. benzocyclobutane, can be confirmed, and the other can not be done veraciously because of its low similarity coefficient to the compound searched in the NIST database. However, the problem may be solved by combining AMWFA and HELP. In the process, peak
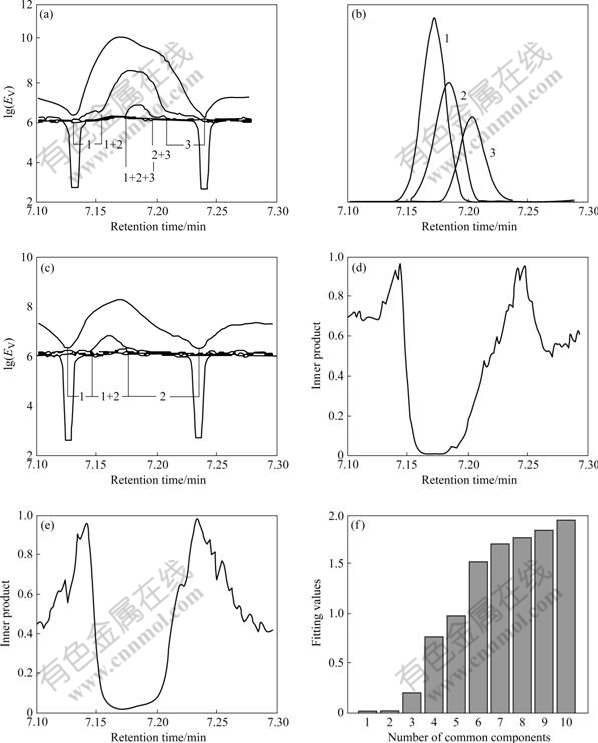
Fig.2 Results obtained through treating peak clusters A and B by combining HELP with AMWFA (EV denotes eigen value): (a) Rank profile of peak cluster A; (b) Resolved chromatograms of three components in peak cluster A; (c) Rank profile of peak cluster B; (d) MSCC; (e) IP-MSCC; (f) Result of matrixes X and Y common rank analysis
cluster A was treated as basis matrix X, and peak cluster B was taken as object matrix Y. By analyses of the multi-component spectral correlative chromatography (MSCC) (Fig.2(d)) [17] and inverse projection multi-component spectral correlative chromatography (IP-MSCC) (Fig.2(e)), the relationship between matrix X and matrix Y can be built. And then, the number of the mutual components may be confirmed by the common rank analysis (Fig.2(f)). It is obvious from Fig.2(f) that there are two mutual components in system X and system Y because the first and second fitting values are close to zero and the third one increases rapidly. The two mutual components may be identified after their pure mass spectra obtained by HELP resolution of peak cluster A (namely, basis matrix X) are searched in NIST library. The search results are benzocyclobutane and heptenal (Fig.3), namely, the two compounds existing in peak cluster B are benzocyclobutane and heptenal. The common components in data matrixes of other retention time regions in the TIC (Fig.1(b)) may be treated by the same means in order to obtain the more veracious results.
By combining GC-MS technique and chemometric algorithms, 68 compounds in the essential oil of EUO barks and 73 ones in that of EUO leaves were identified, respectively. There are 33 mutual components in the compounds, that is, the sum of the compounds identified in the two samples are 108, and all of the similarity coefficients more than 0.90 (Table 2). However, only 37 compounds in the essential oil of EUO barks and 20 ones of the leaves can be identified by the mass spectrum search without combining with chemometric resolution. By all appearances, the reliability of the qualitative results of the compounds contained in the overlap peaks is improved greatly with the help of the synthetical application of HELP and AMWFA algorithms.
3.2 Quantitative analysis and comparison of components of essential oils in EUO barks and leaves
For quantitative analyses of the overlapped peaks, there are the vertical fragmentation and the curve fitting in the traditional methods. However, the methods would usually bring more errors [18]. Therefore, the quantitative analyses of the compounds in the essential oils of EUO barks and leaves were performed by the area normalization method based on the pure chromatograms obtained by HELP resolution. The quantitative results are shown in Table 2. The total contents of the compounds identified in EUO barks and leaves account for 92.9% and 97.75% of the total contents of relevant essential oils, respectively. It is obvious from Table 2 that the compounds in the two essential oils mainly include aldehydes, alcohols, ketones, acids, alkenes, alkanes, furans, aromatic compounds and ethers. However, from the synthetical comparison of the chemical components in the essential oils of EUO barks and leaves, it is obvious that the most principal components in the essential oil of EUO barks are aldehydes whose relative total content is 44.41%; the relative total content of the
Fig.3 Comparison of resolved mass spectra ((a) and (b)) with standard mass spectra of benzocyclobutane (c) and heptenal (d) from NIST library
Table 2 Constituents and their relative contents in essential oils of EUO barks and leaves

furans is 20.18%, that of the alkenes and alkanes is 13.24%; the relative total contents of the other kinds are lower, for example, the total content of the ketones is 5.75%, the acids 4.47%, the alcohols 3.09%, the aromatic compounds 1.2%, the ethers 0.56%, and the esters less than 0.1%. Comparing with the essential oil components in EUO barks, the differences between the relative contents of the essential oil components of the leaves are less. For example, the relative total content of the ketones is 27.46%, the aldehydes 14.7%, the acids 14.0%, the total relative content of the alkenes and alkanes is 11.94%, the alcohols 11.35%, the esters 9.93%, aromatic compounds 4.93% and the furans 3.45%. The relative content (11.7%) of hexa-aldehyde and that (11.4%) of 2-methylbenzofuran are the highest in the oil of the barks. However, the relative content (13.2%) of the palm acid is the highest in the leaves. Obviously, there are biggish differences in the components and their relative contents of the essential oils of the barks and the leaves.
3.3 Quantitative analysis of flavones in EUO barks and leaves
3.3.1 Calibration curve
The relationship curves between the concentrations (Xs) of rutin, quercetin and kaempferol qua contrast substances and their chromatographic peak areas (Ys) were determined. The results show that the good linear relationships exist when the concentrations of rutin, quercetin and kaempferol are (0.021 5-0.430), (0.009 5- 0.190) and (0.008 5-0.170) mg/mL, respectively; the regression equations are Y=1.33×104X-136, Y= 1.78×104X-87.5 and Y=2.30×104X-129, respectively; the correlative coefficients are 0.999 0, 0.993 9 and 0.997 7, respectively.
3.3.2 Quantitative results and their comparison of flavones in EUO barks and leaves
Under the condition of the same gradient elution as shown in Table 1, the chromatographic elution curve was determined by the methanol calibration solution consisting of rutin, quercetin and kaempferol (Fig.4(a)). Under the same condition, the extracted solutions of EUO barks and leaves were used to determine their chromatographic curves by HPLC-DAD. The curves are shown in Figs.4(b) and (c), respectively. By the comparisons of the retention times (Fig.4) and the ultraviolet absorption spectra (Fig.5) of the compounds in the chromatographic curves of the mixed calibration solution and the sample solutions, the chromatographic peaks of rutin, quercetin and kaempferol in the samples may be confirmed easily. According to the chromatographic peak areas, the contents of the three flavones in EUO barks and leaves were quantitatively
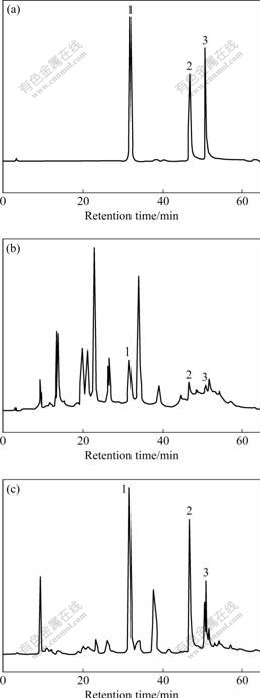
Fig.4 Chromatograms of calibration solution (a), EUO bark extraction (b) and EUO leave extraction (c): 1—Rutin; 2—Quercetin; 3—Kaempferol
determined by the standard curve method. The results are shown in Table 3 (similarly, the other compounds in the samples may also be conformed by the relevant standard substances). The relative standard deviation (RSD) and the results of standard addition recovery indicate that the method is of satisfactory precision and accuracy (see Table 3). In addition, the results (Fig.4) still show that there are significant differences in the flavone kinds of the barks and leaves, and that the contents of the same
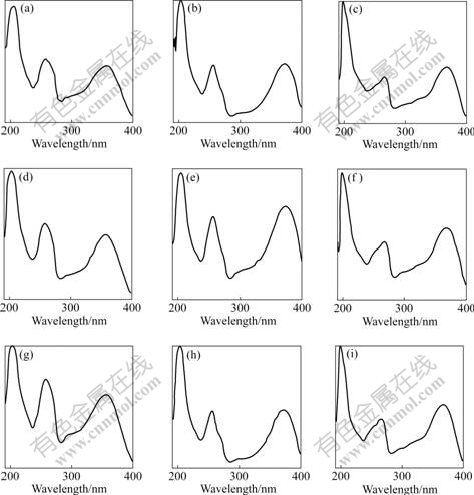
Fig.5 Ultraviolet absorbance spectra of rutin((a), (d) and (g)), quercetin ((b), (e) and (h)), and kaempferol((c), (f) and (i)) in calibration solution, barks, and leaves, respectively
Table 3 Determination results of three flavones in EUO barks and their leaves (parallel 5 times)
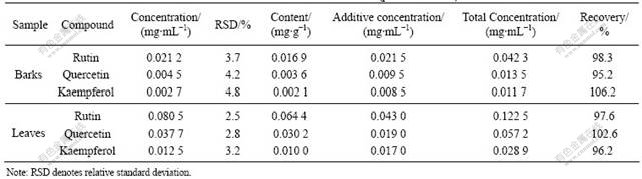
flavones in the two parts are also very different. For example, the contents of rutin, quercetin and kaempferol in the leaves are much higher than those in the barks. However, the other flavones in the barks are much more than those in the leaves. Therefore, the leaves may be used to empolder the medicines whose main effective components are rutin, quercetin and kaempferol, and the barks may be used to empolder ones whose main effective components are the other flavones.
4 Conclusions
(1) The method for systemic comparison analysis of the essential components and flavones in EUO barks and leaves is put forward. The GC-MS data from the essential oils of EUO barks and leaves are resolved by combining HELP with AMWFA algorithms in order to determine the essential components in the oils by searching their resolved mass spectra in NIST database, which may both use the good resolution power of HELP algorithm and obtain the exacter qualitative results of the mutual components whose selective signals are very weak.
(2) There are obvious differences in the compounds and their contents of the essential oils from EUO barks and leaves. The aldehydes are the main components in the essential oil of EUO barks. However, the main ones of the oil of the leaves are ketones, aldehydes and acids. The total content of the esters in the essential oil of EUO leaves is about 10% of the total content of the oil. On the contrary, there are only trace esters in the oil of EUO barks.
(3) The quantitative determination results of the flavones in EUO barks and leaves by HPLC-DAD and the standard curve method are of good precision and veracity.
(4) There are very distinct differences in the contents of the flavones in EUO barks and leaves. The contents of quercetin, kaempferol and rutin in the leaves are much higher than those in the barks. However, the other flavones in the barks are more than those in the leaves.
(5) Because of the significant differences in the components and their contents, EUO barks and leaves are suitable for empoldering the relevant-different- effective-component medicines.
References
[1] Chinese Pharmacopoeia Committee of Health Ministry of China. Chinese pharmacopoeia: Vol.1 [M]. Beijing: Publishing House of Chemical Industry, 2005. (in Chinese)
[2] CHEUNG S C M, SZETO Y T, BENZIE I F F. Antioxidant protection of edible oils [J]. Plant Foods for Human Nutrition, 2007, 62(1): 39-42.
[3] GEUN W L, HYUN C Y, SANG Y B. Inhibitory effect of Eucommia ulmoides Oliver on adipogenic differentiation through proteome analysis [J]. Enzyme and Microbial Technology,2004, 35(6/7): 632-638.
[4] OKADA N, SHIRATA K, NIWANO M. Immunosuppressive activity of a monoterpene from Eucommia Ulmoides [J]. Phytochemistry, 1994, 37(1): 281-282.
[5] CHENG Guang-li. Analysis of effective chemical substances and development of pharmacology in Euconmria Ulmoides Oliver [J]. Chinese Traditional Patent Medicine, 2006, 28(5): 723-725. (in Chinese)
[6] HU Jia-ling. Research development on Euconmria Ulmoides Oliver [J]. Chinese Traditional and Herbal Drugs, 1999, 30(5): 394-396. (in Chinese)
[7] PARK S A, CHOI M S, KIM M J, JUNG U J, KIM H J, PARK K K, NOH H J, PARK H M, PARK Y B, LEE J S, LEE M K. Hypoglycemic and hypolipidemic action of Du-zhong (Eucommia ulmoides Oliver) leaves water extract in C57BL/KsJ-db/db mice [J]. Journal of Ethnopharmacology,2006, 107(3): 412-417.
[8] YANG J, KATO K, NOGUCHI K, DAIRAKU N, KOIKE T, IIJIMA K, IMATANI A, SEKINE H, OHARA S, SASANO H, SHIMOSEGAWA T. Tochu (Eucommia Ulmoides) leaf extract prevents ammonia and vitamin C deficiency induced gastric mucosal injury [J]. Life Sciences,2003, 73(25): 3245-3256.
[9] MATSUDA E, YOSHIZAWA Y, YOKOSAWA Y. Effects of Eucommia Ulmoides Oliver leaf extract on 3T3-L1 differentiation into adipocytes [J]. Journal of Natural Medicines, 2006, 60(1): 126-129.
[10] GONG Fan, LIANG Yi-zeng, FUNG Ying-sing. Analysis of volatile components from Cortex cinnamomi with hyphenated chromatography and chemometric resolution [J]. Journal of Pharmaceutical and Biomedical Analysis,2004, 34(5): 1029-1047.
[11] GONG F, LIANG Y Z, CUI H, CHAU F T, CHAN B T. Determination of volatile components in peptic powder by gas chromatography– mass spectrometry and chemometric resolution [J]. Journal of Chromatography A,2001, 909(2): 237-247.
[12] LIANG Yi-zeng. White, grey and black multicomponent systems and their chemometrics algorithms [M]. Changsha: Hunan Publishing House of Science and Technology, 1996: 29. (in Chinese)
[13] LIANG Y Z, KVALHEIM O M, KELLER H R. Heuristic evolving latent projections: Resolving two-way multicomponent data. Part 2: Detection and resolution of minor constituents [J]. Analytical Chemistry, 1992, 64(4): 946-953.
[14] LIXiao-ru, LANZheng-gang, LIANGYi-zeng. Analysis of volatile chemical components of Radix Paeoniae Rubra by gas chromatography-mass spectrometry and chemometric resolution [J]. Journal of Central South University of Technology, 2007,14(1): 57-61.
[15] MEHDI J H, BEHROOZ Z, HASSAN S. Characterization of essential oil components of Iranian Geranium oil using gas chromatography-mass spectrometry combined with chemometric resolution techniques [J]. Journal of Chromatography A, 2006, 1114(1): 154-163.
[16] ZHAO Chen-xi, LIANG Yi-zeng, FANG Hong-zhuang, LI Xiao-ning. Temperature-programmed retention indices for gas chromatography-mass spectroscopy analysis of plant essential oils [J]. Journal of Chromatography A, 2005, 1096(1): 76-85.
[17] ZENG Zhong-da, LIANG Yi-zeng, WANG Ya-li. Alternative moving window factor analysis for comparison analysis between complex chromatographic data [J]. Journal of Chromatography A, 2006, 1107(2): 273-285.
[18] GONG Fan, LIANG Yi-zeng, SONG You-qun. Determindation of the volatile oil of Pericarpium Citri Reticulatae with gas chromatography/mass spectrometry [J]. Chinese Journal of Analytical Chemistry, 2000, 28(7): 860-864. (in Chinese)
(Edited by YANG You-ping)
Foundation item: Project(20235020) supported by the National Natural Science Foundation of China
Received date: 2008-07-25; Accepted date: 2008-10-08
Corresponding author: ZHANG Tai-ming, Professor; Tel: +86-13873172148; E-mail: taimingzhang@163.com
- Comparative analysis of chemical components between barks and leaves of Eucommia ulmoides Oliver


