
Preparation and properties of flame-sprayed Mo-FeB-Fe cermet coatings
MA Zhuang1, WANG Wei1, 2, ZOU Ji-feng1, DONG Shi-zhi1, ZHANG Lian-yong1, LI Zhi-chao1
1. College of Materials Science and Engineering, Liaoning Technical University, Fuxin123000, China;
2. State Key Laboratory of Solidification Processing, Northwestern Polytechnical University, Xi’an 710072, China
Received 22 June 2010; accepted 24 March 2011
Abstract:
The powders of Mo2FeB2 cermet were prepared with Mo powders, Fe-B alloy powders and Fe powders as raw materials. Mo2FeB2 cermet coatings were prepared on Q235 steel by reactive thermal spraying (RTS) method and heated at 1 000 °C in vacuum oven of 1 kPa for 5 h. The properties of coatings were investigated. The results indicate that Fe2B appears after milling for 15 h in the powder at room temperature, a part of ternary borides (Mo2FeB2) are generated in powder sintered at 900 °C. The coatings are composed of the major phases Mo2FeB2 and α-Fe, a little of Fe2O3, FeO and some pores. The bonding strength between the substrate and the ceramic coating is 32.73 MPa, the thermal-shock times is about 43 and the wear resistance is enhanced by approximately 5.28 times compared with that of the substrate, respectively. The comprehensive properties of Mo2FeB2 cermet coatings can be improved further after vacuum heat-treatment at 1 000 °C for 5 h.
Key words:
Mo2FeB2; cermet coating; reactive thermal spraying; hardness; thermal shock resistance; wear resistance;
1 Introduction
Boride-based ceramic-metal composite coatings have good abrasion wear and corrosion resistance as well as high hardness, and therefore in many applications they can be regarded as superior to oxide and carbide coatings [1]. Mo2FeB2 based cermet coating as a kind of new material is widely used in wear resistant materials, anticorrosion components, cutting tools and mould due to their good mechanical proporties, high hardness, good wear resistance and corrosion resistance, especially the near coefficient of the thermal expansion of the coating and Fe-matrix [2].
At present, reaction boronizing sintering is a novel strategy to form ternary boride based cermet during liquid phase sintering. This technique has successfully developed ternary boride based cermets with excellent mechanical properties, such as Mo2FeB2, Mo2NiB2 and WCoB based cermets, and has been applied in wear resistant applications such as injection molding machine parts, can make tools and dies for the extrusion of copper [3-4]. The Mo2FeB2 based cermet coating is also prepared by means of reaction boronizing sintering technique [5]. The coatings were prepared by liquid phase sintering at 1 250-1 300 °C using Mo, Fe-B alloy and Fe powders as raw materials. But this technology has an unavoidable disadvantage. For example, the microstructure and mechanical properties of steel substrate become worse at high temperatures and the preparation of coating is limited to the size of vacuum furnace. So it is difficult to obtain the coating with uniform thickness in a curved surface and vertical surfaces.
Reactive thermal spraying (RTS) represents an important and rapidly growing field of surface modification technologies. The main feature of this technique is that the ceramic phases, formed by an in-situ reaction directly, are fine, spherical, uniformly dispersed and have a clean interfacial structure with the metal matrix [6]. In RTS, the reactions for synthesizing the ceramic phases are exothermic, which provide a supplemental heat for spraying process, and enable Mo2FeB2 based cermet coatings to be prepared by a simple flame spraying device [7]. The technology maintains the original properties of steel substrate and obtains uniform thickness.
Ternary boride systems have been studied extensively as bulk materials and particles, but information is scarce on ternary boride as coating materials on the surface of Q235 steel by reactive thermal spraying. Therefore, the present work aims to investigate the properties and wear behavior of Mo2FeB2 based cermet coating using Mo, Fe-B alloy and Fe powders as raw materials on Q235 steel by reactive thermal spraying.
2 Experimental
Commercial Mo powders (3-5 μm), Fe powders (4.5 μm) and FeB powders (<75 μm) were used as raw materials. Characteristics of those powders are listed in Table 1. The nominal compositions of 32FeB-48Mo- 20Fe (mass fraction, %) were used for the present work. These powders were mixed in QM-3SP2 planetary ball-mill (Nanjing University Instrument Plant of China) with zirconia balls for 5 h and 15 h at a speed of 520 r/mim. After milling, the powders were sieved less than 75 μm, pelletized with 7.5% (mass fraction) polyvinyl butyral in alcohol, sintered in ZGL-60-16 vacuum furnace (Jinguan Science and Technology Co., Ltd, China), crushed and sieved less than 106 μm. Q235 steel substrates with dimensions of 25 mm×25 mm and a thickness of 6 mm were used. Before spraying, samples were grit blasted with F24 grit at a pressure of 600 kPa and degreased with acetone.
Table 1 Characteristics of raw powders
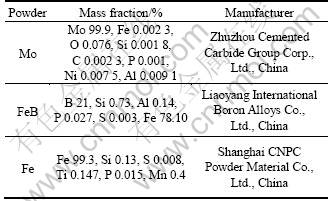
The Mo2FeB2 cermet coating was sprayed by QT-E2000-7/H flame spray gun (Shanghai Dahao Nanomaterials & Thermal Spray Co., Ltd., China). Spraying parameters of those coatings are listed in Table 2. Because of short spraying time, low flow rate and flame temperature, these factors show an important effect on the quality of thermal spray coating [8]. The Mo2FeB2 cermet coatings were remelted using the flame after flame spraying to improve the compactness and bonding strength of cermet coatings. However, because it is difficult to control the temperature during flame spraying, the final thickness of coatings is not homogeneous [9]. The flame melting specimens were performed with oxyacetylene flame to a desired temperature of 800 °C for 20 s. These specimens were heated in vacuum oven of 1 kPa at 1 000 °C for 5 h.
The phase composition of the coatings and powders were examined by X-ray diffractometer (XRD, D/MAX-RB X-ray diffractometer, Rigaku, Japan) operating with Cu Kα radiation and a step size of 0.02. The XRD patterns were collected in a 2θ range from 10° to 80° with a scanning rate of 2(°)/min. The measurement of particle size distribution of powders was carried out by laser diffraction on HYL-1076 system (Dandong Hylology Instruments Co., Ltd, China). The surface and cross-sections of feedstock powders and sprayed specimens were observed and analyzed with scanning electron microscope (SEM, SSX-550 scanning electron microscope, Shimadzu, Japan).
Table 2 Thermal spray parameters
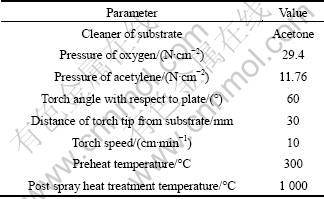
The micro-hardness of cermet coatings was tested using micro-hardness instrument (Shenzhen Shunwan Instrument Equipment Co., Ltd, China) at a load of 0.98 N with a dwell time of 10 s. The cross section of coatings was polished before indentation and the distance between two indentations was at least three times the diagonal to prevent stress-field effect from the nearby indentation [10]. The thermal shock tests were carried out according to GB5270—85. The samples were put into electric resistance furnace to heat to 400 °C, holding 10 min, and then put into water (room temperature) [11]. The tensile bond strength tests were performed according to GB8642—88 using a crosshead speed of 0.5 mm/min for all the tests. The bond strength of cermet coatings was calculated by dividing the failure load by the cross-sectional area of the specimen. Two specimens in the pair were used and the coating was deposited on only one of them. The specimens were bonded by glue and kept pressing against each other for 2 h in a furnace at 180 °C [12]. The wear test was performed using ball-on-disk equipment, M-200. The test diameter was 40 mm, the counterpart was a bulk of WC-6Co ball, the normal applied load was 30 N, and the relative velocity of sample was 120 r/min, with a total testing time of 10 min. The tests were performed at room temperature. The wear rates were calculated by means of mass loss measurements [13].
3 Results and discussion
3.1 Phase composition of powders
The X-ray diffraction patterns of the powders of ball-milling and heat-treatment are shown in Fig. 1(a). It can be seen that XRD peaks become broadening and the peak intensity decreases with the increase of milling time. The results show that the finer blending powders can be produced. The chemical composition of the powders after ball-milling for 5 h is the same as that of raw material and no new phase appears. However, a new phase Fe2B forms after ball-milling for 15 h. This is due to grain refinement, decrease of reaction activation energy and augment of surface-area reaction process of FeB+Fe=Fe2B. Based on the powders after ball-milling for 15 h which have more activation including crystal defects, dislocation, grain boundary and holes, etc, the powders ball-milled for 15 h were sintered at 900 °C, crushed, sieved and then sprayed with flame. It can be seen that a new phase Mo2FeB2 appears in the powders after heat treatment at 900 °C. DSC curve of the powders after being ball-milled for 15 h without heat treatment is shown in Fig. 1(b). The results show that an exothermic peak appears at 834 °C. Mo2FeB2 is generated at this temperature:
Mo+2FeB =Mo2FeB2+Fe (1)
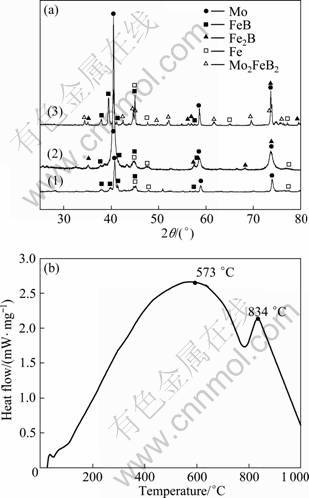
Fig. 1 XRD patterns of feedstock powders (a) and DSC pattern of mixed-powders after being ball-milled for 15 h (b) (1—Milling for 5 h; 2—Milling for 15 h; 3—Milling for 15 h and then sintering at 900 °C)
In addition, Mo, FeB and Fe remaining in the powders after heat treatment at 900 °C can have chemical reaction with each other and produce Mo2FeB2 during the spraying process.
3.2 Morphology and particle size distribution of powders
The morphology of the powders after ball-milling for 15 h and sieving less than 75 μm is illustrated in Fig. 2(a). The particle size distribution of these powders is shown in Fig. 2(b). It can be seen that the powders consist of micro-crystalline and nano-crystalline composite. The medium diameter of powders is 6.53 μm. The spraying powders at 900 °C were crushed and sieved less than 75 μm. The surface microstructures of them are shown in Figs.2(c) and (d). It is very clear that the powder prepared by sinter-crushing method is spherical with uniform particle size. The sintered powder granulation can accelerate interaction. Although the particles are irregular, they can meet the demand of reactive flame spray process well.
3.3 Phase structure of coatings
The XRD patterns of the coatings prepared by reactive flame spray method with and without heat treatment are shown in Fig. 3. It can be seen that the coating is composed of Mo2FeB2 type hard phase, Fe type binder phase and other iron oxides. The hard phase Mo2FeB2 formed in the coatings includes two parts, one is in the process of reactive flame spray and the other is in the process of vacuum heat treatment at 900 °C because there is no Mo2FeB2 in the raw materials. The FeO and Fe2O3 phases are formed during the process of flame-sprayed. The phase structure of the coating at 1 000 °C vacuum heat treatment does not change, which is shown in Fig. 3, but the diffraction intensity is increased.
3.4 Morphology analysis of coating
Figures 4 (a) and (b) show the surface morphologies of coatings without heat treatment and the surface morphology of coatings with heat treatment at 1 000 °C, respectively. It can be seen from Fig. 4(a) that there are massive particles which are un-melted; the surface morphology of coatings looks roughness. The molten particles are deposited on previous melting zone and lamellar structure which is formed by the half-melted
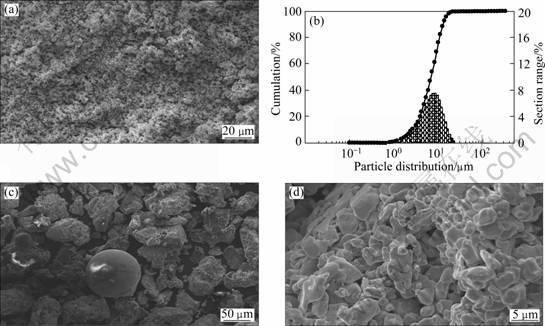
Fig. 2 SEM images (a), (c), (d) and particle size distribution (b) of powders: (a) After ball-milling for 15 h and sieving; (b) After ball-milling for 15 h and sieving less than 75 μm; (c) Heat treatment at 900 °C, crushing and sieving less than 75 μm; (d) Heat treatment at 900 °C, crushing and sieving less than 75 μm particles. The kinetic energy and heat energy of the high temperature particles have promoted temperature in a tiny zone. As a result of the low temperature and jet velocity of flame spray, the porosity of coatings formed is one of the important limiting factors for improving the quality of the coatings. In order to improve the service performance of the coatings, it is necessary to heat-treat at 1 000 °C under vacuum for 5 h. It can be seen from Fig. 4(b) that the particles which are unmelted and half melted have been melted. The surface of the coatings is smooth; the bonding strength of inter-particles increases and the porosity reduces obviously.
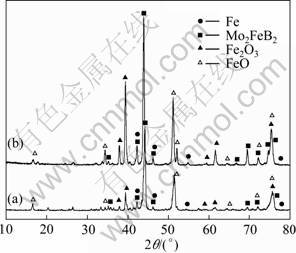
Fig. 3 XRD patterns of coating: (a) Without heat treatment; (b) Heat treatment at 1 000 °C
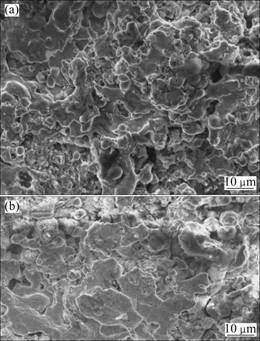
Fig. 4 SEM images of coatings: (a) Without heat treatment; (b) Heat treatment at 1 000 °C
The thickness of coating without heat treatment is about 300 μm and the other is about 400 μm (Figs.5(a) and (b)). The coating thickness depends mainly on the spraying distance, the spraying times and the moving speed along the surface. The coatings without heat treatment are loosen and have defects, while the coatings heat treated at 1 000 °C are very dense, and are composed of more melted particles than that without heat treatment. The EDS analyses indicate that there is element diffusion at the interface in Fig. 5. The diffusion of Mo atoms from coating to substrate and Fe atoms on the contrary direction do not happen in the coating without heat treatment, so the bonding mode between coating and matrix is mainly mechanical bonding; while in the coating heat treated at 1 000 °C, the Mo atoms diffuse to steel substrate and the Fe atoms in the steel substrate diffuse to the coating at the interfacial zone. Therefore, the bonding mode between coating and matrix is mainly chemical bonding.
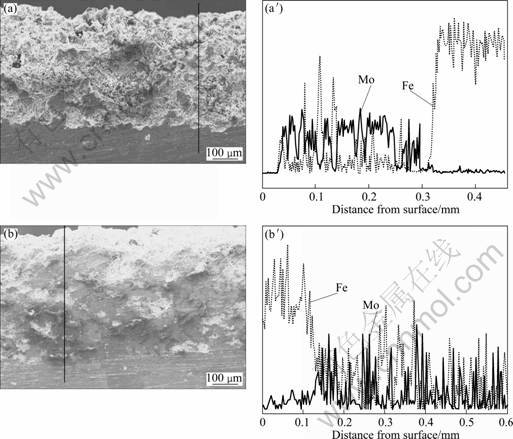
Fig. 5 SEM images (a, b) and EDS results (a′, b′) of cross section of coatings: (a), (a′) Without heat treatment; (b), (b′) Heat treatment at 1 000 °C
3.5 Microhardness of coatings
The microhardness of the coating and matrix is shown in Fig. 6. It indicates that the coating has higher microhardness HV0.11 261 as compared with the substrate. There is a long and narrow transition zone between the coating heat treated at 1 000 °C and substrate, while the microhardness of the coating without heat treatment is lower and the transition zone is not obvious in the interface zone. The microhardness of the coating is related to the quality, compactness and bonding strength of the coating. The coating without heat treatment has defects such as micro-pore, crackle and poor bonding with the substrate. So the coating with 1 000 °C heat treatment has higher microhardness as compared with the coating without heat treatment. Therefore, the microhardness of the coating is related to denser coating, and better bonding with the substrate.
3.6 Bonding strength and thermal shock resistance of coatings
From Table 3, it can be seen that the bonding strength of coating without heat treatment is 32.73 MPa, while it is 58.06 MPa after heat treatment at 1 000 °C. The times of thermal shock resistance without heat treatment is 43, while it is 65 after heat treatment at 1 000 °C. In most cases, the desquamation of coating is due to heat stress between the coating and substrate. The coating without heat treatment is mainly mechanical bonding, while the coating with 1 000 °C heat treatment is mainly chemical bonding. Therefore, the coating with 1 000 °C heat treatment has good bonding strength and good thermal shock resistance.
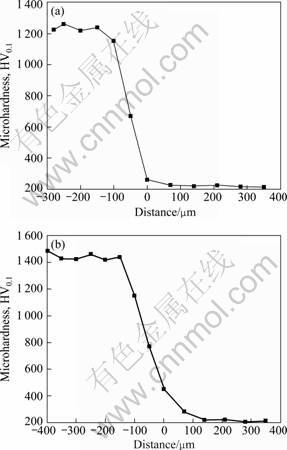
Fig. 6 Microhardness of coatings: (a) Without heat treatment; (b) Heat treated at 1 000 °C
Table 3 Bonding strength and thermal shock resistance of coatings

3.7 Abrasive resistance of coatings
The relative wear resistances of the coating and substrate are shown in Fig. 7. The average mass loss of the Q235 steel is 20.28 g/m2, the average mass loss of the coating without heat treatment is 3.23 g/m2, while the average mass loss of the coating heat treated at 1 000 °C is 2.17 g/m2. As shown in Fig. 7, the coating heat treated at 1 000 °C exhibits an excellent abrasive wear resistance which is 8.35 times higher than the substrate Q235 steel and 1.58 times that of the coating without heat treatment. Figure 8 shows the SEM images of worn surface. As shown in Fig. 8(a), there are numerous deep wear scars and severe plastic deformations on the surface of Q235 steel. For the coating without heat treatment, some unmelted and half melted particles have come out in some regions of the coating surface. The quality of the coating has an important effect on the wear resistance of coating. The wear resistance of coating without heat treatment which has some defects such as pores is not good, as shown in Fig. 8(b). It is suggested that the wear mechanism of the coating is selective removal of the binder caused probably by plastic deformation and fatigue due to the repeated action of the abrasive particles followed by the undermining of the Mo2FeB2 hard particles, resulting in their eventual pullout. The coating with 1 000 °C heat treatment has better abrasive wear resistance due to denser structure, smoother surface, higher hardness and better bonding strength (Fig. 8(c)). During the process of wearing, B2O3 and MoO2 were produced on the coating due to temperature increasing, which can form transitional films on the surface of the partner samples. And this kind of films can lubricate them well [14-16]. Therefore, the worn surface of coatings heat treated at 1 000 °C shows dips shape, which is due to the particles shedding from mating materials and particles shedding from coating in the wearing process. The worn surface of coating is a relatively flat topography, and has more shallow furrows and scallops which are produced by the deciduous hard particles. So the comparative resistance of the coating heat treated with 1000°C is better than substrate and the coating without heat treatment.
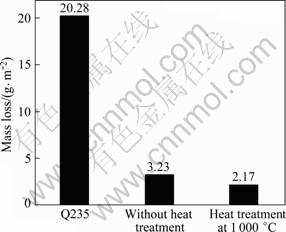
Fig. 7 Comparison of abrasive resistance of coating and substrate
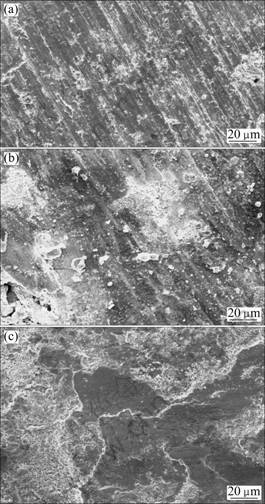
Fig. 8 SEM images of worn surface: (a) Q235 steel; (b) Without heat treatment; (c) Heat treatment at 1 000 °C
4 Conclusions
1) Fe2B phase appears after milling for 15 h in the powder, ternary boride (Mo2FeB2) is generated in sinter-crushing powders at 900 °C, and the powders by thermal spraying are composed of Mo2FeB2, Mo, FeB and Fe.
2) The coatings prepared by RTS are composed of the major phases Mo2FeB2 and α-Fe, a little of Fe2O3, FeO and some pores.
3) The microhardness of coating after flame remelt is 1 261 HV0.1; the bonding strength between the coating and substrate is 32.73 MPa; the thermal shock resistance reaches 43 times; the abrasive wear resistance is 5.28 times higher than the substrate Q235 steel.
4) The bonding strength between the coating and substrate is 58.06 MPa; the thermal shock resistance reaches 65 times; the abrasive wear resistance is 8.35 times higher than the substrate Q235 steel.
References
[1] KERANEN J, STENBERG T, MANTYLA T, LEPISTO T. Microstructural characterization of detonation gun-sprayed boride-based cermet coatings [J]. Surface and Coatings Technology, 1996, 82: 19-37.
[2] LIU Fu-tian, HUANG Wei-ling, LI Wen-hu, ZHANG Ying-cai, LI Zhao-qian. Study on bond interface between ternary boride cermet classing and steel substrate [J]. Materials Science and Technology, 2005, 13(5): 452-455. (in Chinese)
[3] YU Hai-zhou, ZHENG Yong, LIU Wen-jun, ZHENG Jian-zhi, XIONG Wei-hao. Effect of V content on the microstructure and mechanical properties of Mo2FeB2 based cermets[J]. Materials and Design, 2010, 31: 2680-2683.
[4] TAKAGI K I. Development and application of high strength ternary boride base cermets [J]. Journal of Solid State Chemistry, 2006, 179: 2809-2818.
[5] WANG Yong-guo, LI Zhao-qian. Fabrication ternary boride based wear resistance cladding material using reaction sintering technology [J]. Materials Science and Engineering, 2002, 20(2): 210-213. (in Chinese)
[6] LIU Chang-song, HUANG Ji-hua, ZHAO Yong, LIU Mu, YIN Sheng. TiC-Fe coatings prepared by flame synthesis process [J]. Transactions of Nonferrous Metals Society of China, 2000, 10(3): 405-407.
[7] LIU Hui-yuan, HUANG Ji-hua. Reactive thermal spraying of TiC-Fe composite coating by using asphalt as carbonaceous precursor [J]. Journal of Materials Science, 2005, 40: 4149-4151.
[8] MA Zhuang, LIN Peng, DONG Shi-zhi, LI Zhi-chao. Al2O3-based multiphase ceramic coating of Al-CuO system prepared by SHS reaction thermal spraying on AZ91D [J]. The Chinese Journal of Nonferrous Metals, 2009, 19(8): 1379-1384. (in Chinese)
[9] HARSHA S, DWIVEDI D K, AGARWAL A. Influence of CrC addition in Ni-Cr-Si-B flame sprayed coatings on microstructure, micro-hardness and wear behavior [J]. Int J Adv Manuf Technol, 2008, 38: 93-101.
[10] MAO Zheng-ping, MA Jing, WANG Jun, SUN Bao-de. Comparison of the coatings deposited using Ti and B4C powder by reactive plasma spraying in air and low pressure [J]. J Mater Sci, 2009, 44: 3265-3272.
[11] TSUNEYKI IDE, TEIICHI ANDO. Reaction sintering of a Fe-6wt pct B-48wt pct Mo alloy in the presence of liquid phases [J]. Metallurgical Transactions A, 1989, 20(1):17-24.
[12] MAGNANI M, SUEGAMA P H, ESPALLARGAS N, FUGIVARA C S, DOSTA S, GUILEMANY J M, BENEDETTI A V. Corrosion and wear studies of Cr3C2NiCr HVOF coatings sprayed on AA7050 T7 under cooling [J]. Journal of Thermal Spray Technology, 2009, 18(3): 353-363.
[13] LI Peng-liang, ZHOU Jing-en, XI Sheng-qi. Cubic AlN synthesized by high energy ball milling and its phase conversion at high temperature [J]. Journal of Inorganic Materials, 2006, 21(4): 821-826. (in Chinese)
[14] ZHAO Zheng, LIU Fu-tian, LI Wen-hu. Wear resistance property of Mo2FeB2 hard alloy cladding material [J]. Journal of the Chinese Ceramic Society, 2008, 36(S1): 95-98. (in Chinese)
[15] VENCL A, MRDAK M, BANJAC M. Correlation of microstructures and tribological properties of ferrous coatings deposited by atmospheric plasma spraying on Al-Si cast alloy substrate [J]. Metallurgical and Materials Transactions A, 2009, 40: 398-405.
[16] ZHAO Hui, LIU Zheng, CHEN Li-jia, CHEN Ji, HAN Zhong. Wear resistance of ceramic coating on AZ91 magnesium alloy by micro-arc oxidation [J]. Transactions of Nonferrous Metals Society of China, 2006, 16(s): s1814-s1818.
反应火焰喷涂Mo-FeB-Fe系金属陶瓷涂层的制备及性能
马 壮1, 王 伟1, 2, 邹积峰1, 董世知1, 张连勇1, 李智超1
1. 辽宁工程技术大学 材料科学与工程学院,阜新 123000;
2. 西北工业大学 凝固技术国家重点实验室,西安 710072
摘 要:以Mo粉、FeB合金粉、Fe粉为原料,将混合粉末在900 °C下真空热处理2 h,破碎,过75 μm筛制备喷涂喂料;采用反应火焰喷涂技术在Q235钢表面制备Mo2FeB2金属陶瓷涂层。将反应热喷涂制备的涂层在真空炉中1 000 °C下热处理5 h,测试涂层的性能。结果表明:在室温球磨15 h后粉体中有Fe2B生成,在900 °C下烧结后破碎的喷涂粉末中有部分三元硼化物(Mo2FeB2)生成;涂层由占主体的Mo2FeB2和α-Fe相和少量Fe2O3、FeO相及气孔组成。在涂层和基体的结合面处,存在由高硬度涂层到低硬度钢基体的过渡区;涂层和基体的结合强度为32.73 MPa,抗热震次数可以达到43次左右,耐磨性比钢基体提高5.28倍;涂层经过1 000 °C真空扩散热处理后,具有更加优异的力学性能。
关键词:Mo2FeB2;金属陶瓷涂层;反应火焰喷涂;硬度;抗热震性;耐磨性
(Edited by LI Xiang-qun)
Foundation item: Project (2007T069) supported by Liaoning Education Department Innovation Team, China
Corresponding author: WANG Wei; Tel: +86-418-3351727; E-mail: gackmol@hotmail.com
DOI: 10.1016/S1003-6326(11)60859-5


