DOI:10.19476/j.ysxb.1004.0609.2017.03.024
无膜微生物燃料电池处理含铜废水回收铜及其产电性能
刘维平,印霞棐,路娟娟,梁国斌,陈月美
(江苏理工学院 化学与环境工程学院,常州 213001)
摘 要:
构建双室无膜折流板式微生物燃料电池,研究双室无膜折流板微生物燃料电池的产电性能和废水处理,在不需要外部电源的情况下,直接利用微生物燃料电池产生的电能处理阴极模拟含铜废水并回收铜。结果表明:以乙酸钠为阳极底物,以驯化后的活性污泥混合菌为阳极接种微生物,以Cu2+为阴极电子受体可成功启动无膜折流板微生物燃料电池,最大功率密度为31.3 mW/m2,开路电压最高为678.0 mV,Cu2+去除率为99.6%;经XRD分析,阴极板上的沉积物主要为单质铜,双室无膜折流板微生物燃料电池可有效处理含铜废水并回收铜。
关键词:
文章编号:1004-0609(2017)-03-0648-07 中图分类号:X703 文献标志码:A
含铜废水主要来自电镀加工、矿山开采、金属冶炼、印刷电路板制造等行业。含铜重金属废水对环境会产生巨大危害,含铜重金属废水的处理及铜的回收利用已引起广泛关注。生物法处理含铜废水具有费用低、适应性强、无二次污染、可回收再利用等特点。罗丽卉等[1]利用硫酸盐还原菌处理含铜废水,Cu2+去除率达到99.9%。KHAN等[2]采用人工湿地法去除工业废水中的重金属离子,Cu2+去除率为48.3%。电解法是一种较为成熟的含铜废水处理技术,流程简单、操作方便、可直接回收金属铜,但主要缺点是能耗高,高浓度的含铜废水经电解后,铜含量仍可能超过排放标准[3],在低浓度含铜废水的处理中受到一定限制[4]。GAIKWAD等[5-6]利用离子交换法处理矿山含铜酸性废水。CORUH等[7]利用斜发沸石处理低浓度含铜废水,Cu2+去除率可达98.0%~99.0%;van NGHIEM等[8]采用Dowex G-26树脂处理铜浓度为500~700 mg/L的含铜废水,铜的吸附率达99.6%。崔春花等[9]利用中空纤维更新液膜技术处理模拟含铜电镀废水,出水中Cu2+的含量低于1.0 mg/L,Cu2+的去除率为99.0%。AZIZ等[10]以粗石灰石作滤床处理含Cd、Cu和Ni等重金属离子废水,去除率90.0%。ROMERO等[11]利用钙质页岩中的方解石作为中和剂,处理矿山酸性废水中的重金属。LI等[12]利用二乙基二硫代氨基甲酸钠(DDTC)作为重金属捕获剂,当DDTC与Cu的质量比为0.8~1.2时,Cu2+的去除率99.6%。龙来寿等[13]采用间歇式固定床真空热解装置对废线路板进行真空热解前处理,然后利用剪切破碎和气流分选方法对真空热解渣中的金属铜进行回收,铜的总回收率为99.86%。LIANG等[14]研究高位阻β-二酮从氨性蚀刻废液中萃取铜。仉丽娟等[15]利用生物浸出的方法实现废覆铜板分选渣中残留铜的资源化,铜浸出率95%以上,生物法反应周期长,且在反应过程中要严格控制外界的因素对微生物的影响。
微生物燃料电池(Microbial fuel cell,MFC)利用微生物的新陈代谢作用将化学能转化为电能,目前的研究更多关注MFC的产电能力[16-18]。牟姝君等[19]研究了铜离子对MFC电能输出的影响。ZHANG等[20]研究了由质子交换膜构成的双室MFC对含铜废水处理的影响。目前MFC处理重金属废水的报道还不多,LI等[21]发现:光照条件下,26 h内Cr6+的去除率达到97%。ZHANG等[22]利用双室MFC处理含V5+和Cr6+的废水,最大输出功率970.2 mW/m2,反应240 h后V5+和Cr6+的去除率分别为67.9%和75.4%。张永娟等[23]构建了一个双瓶式MFC,最大电压417.0 mV,最大输出功率密度44.2 mW/m2,Cu2+去除率为59.8%。梁敏等[24]构建了双室MFC处理模拟含铜废水,最大体积输出功率为536.0 mW/m3,Cu2+的去除率达到97.8%,阴极板上沉积物是Cu2O和Cu4(OH)6SO4,没有实现单质铜的回收。HEIJINE等[25]调节含铜溶液pH为3,MFC的最大体积输出功率430.0 mW/m3,Cu2+的去除率99.8%。
双室MFC利用微生物降解阳极室的有机废水,将化学能转成电能的同时,在阴极处理含铜重金属废水,不仅实现了同时处理了两种不同类型的废水,而且获得了电能,利用MFC产生的电流代替电解法处理含铜废水技术中的传统电源,在MFC阴极室实现Cu2+的还原,并回收金属铜。双室MFC膜的使用增加了MFC的成本,基于此,本文作者构建了双室无膜折流板MFC,以乙酸钠为阳极底物,以驯化后的活性污泥混合菌为阳极接种微生物,以Cu2+为阴极电子受体,以石墨棒为电极,研究利用MFC产生的电能代替外部电源,在阴极处理模拟含铜废水并回收铜,探讨无膜折流板MFC的产电性能及其对含铜废水处理和铜回收的影响。
1 实验
1.1 实验装置
实验构建了双室无膜折流板式MFC,实验装置如图1所示。
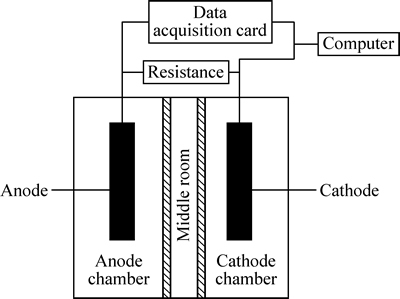
图1 双室无膜折流板MFC装置示意图
Fig. 1 Schematic diagram of baffled membraneless dual chamber MFC
双室无膜MFC反应器的阳极室和阴极室通过折流板构成的中间室相连,阳极室和阴极室的尺寸均为10 cm×10 cm×5 cm,用石墨棒作电极材料,用铜导线连接阳极和阴极,并接入0~9999 Ω电阻值可调的负载电阻箱。使用数据采集卡实时记录MFC不同负载下的电压和电流。
1.2 实验原料与过程
厌氧活性污泥:接种污泥取自污水处理厂的二沉池,将其过滤、沉淀后保存在厌氧环境下。通过显微镜观察,厌氧活性污泥中的细菌主要有菌胶团和丝状细菌,它们构成了活性污泥的骨架,此外污泥中还有原生动物和后生动物等微型动物。细菌、微型动物以及其他微生物加上水中的悬浮物和一些溶解性物质混在一起,形成了具有分解有机物能力的絮凝体,即活性污泥。取回的厌氧活性污泥用培养液进行驯化,培养液的组成:蔗糖、磷酸二氢钾、磷酸氢二钾、硫酸铝、柠檬酸三铵、氯化钠、氯化铵、氯化镁、氯化钙。以驯化后的活性污泥混合菌为阳极接种微生物。
MFC启动期底物组成:4000 mg/L CH3COONa,1500 mg/L K2HPO4,1500 mg/L KH2PO4,450 mg/L NH4Cl,300 mg/L MgCl2,50 mg/L C4H6O4Zn·2H2O,20 mg/L CaCl2,底物与驯化后的厌氧活性污泥的体积比为1:1,阴极液为1000 mg/L的乙酸钠溶液,启动完成并稳定后,阴极液更换为模拟含铜废水。
1.3 评价方法
采用稳态放电法测得MFC的极化曲线,在欧姆极化区拟合相应直线,得到的斜率即为MFC的内阻。在不同外阻下得到的功率密度和电流密度做图即得到功率密度曲线。MFC的电流密度Jan(单位为mA/m2)和功率密度Pan(单位为mW/m2)分别为
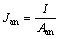 (1)
(1)
 (2)
(2)
式中:I为电流值(mA);Aan为阳极有效面积(m2);Rext为外电路电阻(Ω)。
采用ICP-AES分析阴极液中铜离子的浓度,采用X射线衍射对阴极板上沉积物进行分析,采用扫描电镜观察阴极铜粉的形貌。
2 结果与分析
2.1 MFC的启动
将底物与驯化后的厌氧活性污泥按体积比1:1加入阳极室,阴极室为1000 mg/L的乙酸钠溶液,阳极液和阴极液的总量均为300 mL。电压降至100 mV以下时,认为本次周期结束,此时从阳极室排出100 mL阳极液,利用催化密闭消解法测定其COD值,并计算COD去除率,同时加入100 mL新的营养液,开始下一个周期,如此反复运行几个周期使开路电压达到稳定值,每一周期结束后阳极液的COD去除率如表1所列。
表1 COD去除率
Table 1 Removal rate of COD
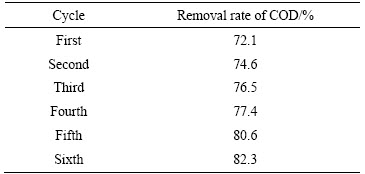
从表1看出,COD的去除率随着MFC运行时间的增加不断增加,到第五周期后稳定在80%以上。由于厌氧菌接种到阳极室后,经过一段适应期在新的环境中生长,并吸收营养物质,在这个过程中,有机物被缓慢降解,随着微生物不断适应环境,细菌的生长繁殖速率不断增加,MFC对有机物的降解能力不断增强。
MFC启动期输出电压如图2所示。从图2中可以看出,反应开始时,由于微生物接触新的环境,好氧菌在厌氧环境下逐渐被淘汰,产电菌开始适应环境,MFC输出电压较低,最高为240 mV。在第二周期更换了一次营养液之后,开路电压小幅增加,最大为290 mV;MFC运行的第三周期,开路电压最高达到380 mV。继续更换阳极基质,在MFC运行的第四周期,开路电压达到560 mV,电压上升较快;MFC运行到第五周期时,开路电压达到620 mV;随着反应的进行,到第六周期,MFC的最大开路电压基本稳定在620 mV左右,MFC完成了启动过程。

图2 MFC启动期输出电压
Fig. 2 Output voltage of MFC during start-up period
2.2 产电性能
MFC启动完成后,将阴极液中的乙酸钠溶液更换为5000 mg/L的Cu(NO3)2、CuCl2和CuSO4溶液,对应的MFC装置分别命名为M1、M2、M3,研究以Cu2+为电子受体时MFC的产电性能,MFC输出电压随时间变化情况如图3所示。

图3 MFC电压变化规律
Fig. 3 Voltage variation of MFC
MFC启动后,阴极液更换为含铜模拟废水,由于营养物质足以供给微生物生长繁殖,细胞代谢活力增强,生长旺盛,细菌生长速率不断增大, MFC产电迅速,电压上升较快。从图3看出,M1、M2和M3分别经过120、108和72 h的反应,电压达到最大值,分别为678、670和391 mV。反应持续一段时间后,由于底物被逐渐降解,代谢产物大量积累,产电菌群营养物被逐渐消耗,电压逐渐减小,产电性能逐渐下降。图3表明,M1和M2的电压变化规律接近,M3的电压最低,说明不同的阴极液会影响MFC的产电能力。其原因与不同条件下MFC对有机物COD的去除能力不同有关,通过测定COD值,经计算得到的M1、M2和M3对应的有机物COD去除率分别为80.9%、79.8%和77.3%,M1和M2的COD去除率很接近,M1的去除率最高,M2的略小。说明M1和M2微生物降解有机物的能力较强,更容易将底物的化学能转化成电能,产生的电子与质子更多,传递到阴极室,与阴极室的Cu(NO3)2和CuCl2溶液发生反应,产生较高的电压。M3的COD去除率最低,说明相比M1和M2,以CuSO4为阴极液时,微生物更难降解有机物,将化学能转化成电能的效率较低,产生的电子和质子较少,导致电压偏低。
对MFC进行稳态放电试验,测定其极化曲线,如图4所示,将极化曲线的欧姆极化区数据进行线性拟合,所得斜率即为MFC的内阻。根据图4的极化曲线,计算得出M1、M2和M3的内阻分别为2975.2、5696.4和2762.1 Ω。M3的内阻最小,而欧姆内阻小,整个系统阻力越低,输出功率就越高,这点从图5可以得到证实,从图5的功率密度曲线看出,M3的功率密度高于M1和M2的,M3的最大功率密度为31.3 mW/m2,是M1的1.9倍,M2的2.1倍。

图4 MFC极化曲线
Fig. 4 Polarization curves of MFC
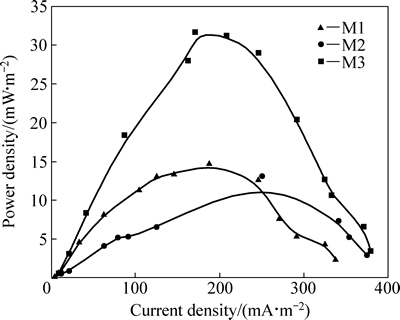
图5 MFC功率密度曲线
Fig. 5 Power density curves of MFC
2.3 Cu2+去除与铜回收
当MFC运行结束后,考察了阴极液中Cu2+的浓度和Cu2+去除率,结果如表2所列。
表2 阴极Cu2+浓度及去除率
Table 2 Cathode Cu2 + concentration and removal rate

从表2可以看出,MFC能有效去除阴极液中的Cu2+,在微生物燃料电池中,MFC阴极的主要作用是接受来自阳极释放的电子和质子,其性能的好坏直接决定MFC的输出效率。传统的MFC中,主要以氧作为MFC的电子受体,着重于提高氧化剂接受质子和电子的能力,以乙酸盐为阳极底物,阴极以Cu2+为电子受体构成微生物燃料电池,阳极反应为
CH3COO-+3H2O → HCO3-+CO2+8H++8e,
φan=-289 mV (3)
阴极反应为
Cu2++2e→Cu,φca=340 mV (4)
若以这两种物质分别作为MFC的阳极室和阴极室中的反应物,则理论电压为629 mV,根据电化学原理,反应可自发进行。可见以乙酸盐为阳极底物时,Cu2+为MFC阴极电子受体时,不需外加电源就可实现铜的还原,大大降低电解法处理含铜废水的运行成本。
MFC运行结束后,取出阴极石墨棒,可观察到MFC阴极表面红褐色沉积物,如图6所示。

图6 阴极表面沉积物
Fig. 6 Sediment of cathode surface
将阴极沉积物进行XRD分析,结果如图7所示。从图7可以看到,谱线在2θ为36.4°、61.4°处出现衍射峰,经计算机检索与标准卡Cu2O的特征峰一致,表明阴极还原产物中有Cu2O的存在。谱线在2θ为43.3°、50.4°和74.1°处有尖锐的衍射峰,经计算机检索与标准卡单质铜的特征峰一致,从衍射峰分布看,阴极上的沉积物主要是单质铜和少量Cu2O的混合物。经化学分析,阴极沉积物中单质铜的含量为98.3%,和电解法获得的铜粉质量相比,铜含量偏低,主要是铜粉中还含有少量Cu2O所致。
通过扫描电镜观察,阴极回收的铜粉形貌如图8所示。由图8可看出,铜粉是一种薄片状的集合体,表面光滑致密。

图7 MFC阴极表面沉积物XRD谱
Fig. 7 XRD pattern of MFC cathode surface sediment

图8 阴极铜粉的SEM像
Fig. 8 SEM image of cathode copper powder
3 结论
1) 以Cu2+为电子受体的双室无膜折流板MFC的最大功率密度为31.3 mW/m2,开路电压最高为678 mV,CuSO4溶液为阴极液的MFC内阻最小。
2) 不需要外部电源,直接利用双室无膜折流板MFC产生的电能,可处理阴极的含铜溶液并回收铜。双室无膜折流板式MFC对阴极液中Cu2+去除率98%以上,阴极板上的沉积物经XRD检测,主要为单质铜。
REFERENCES
[1] 罗丽卉, 谢翼飞, 李旭东. 生物硫铁复合材料处理含铜废水及机理研究[J]. 中国环境科学, 2012, 32(2): 249-253.
LUO Li-hui, XIE Yi-fei, LI Xu-dong. Biological iron sulfide composites in the treatment of copper-contaminated wastewater and its mechanism[J]. China Environmental Science, 2012, 32(2): 249-253.
[2] KHAN S, AHMAD I, SHAH M T, REHMAN S, KHALIQ A. Use of constructed wetland for the removal of heavy metals from industrial wastewater[J]. Journal of Environmental Management, 2009, 90: 3451-3457.
[3] 肖书虎, 张国芳, 宋永会, 曾 萍, 李 辉. 电化学双极法处理高浓度含铜黄连素制药废水[J]. 环境工程技术学报, 2011, 1(4): 295-299.
XIAO Shu-hu1, ZHANG Guo-fang, SONG Yong-hui, ZENG Ping, LI Hui. Treatment of berberine pharmaceutical wastewater containing copper by bipolar-electrochemical process[J]. Journal of Environmental Engineering Technology, 2011, 1(4): 295-299.
[4] FALAYI T, NTULI F. Removal of heavy metals and neutralisation of acid mine drainage with un-activated attapulgite[J]. Journal of Industrial and Engineering Chemistry, 2014, 20 (4): 1285-1292.
[5] GAIKWAD R W, MISAL S A, DHIRENDR A, GUPTA D V. Removal of copper ions from acid mine drainage (AMD) by ion exchange resins: Indion 820 and Indion 850[J]. Journal of Applied Sciences in Environmental Sanitation, 2009, 4(2): 133-140.
[6] GAIKWAD R W, SAPKAL V S, SAPKAL R S. Ion exchange system design for removal of heavy metals from acid mine drainage waste water[J]. Acta Montanistica Slovaca, 2010, 15(4): 298-304.
[7] CORNH S, TURAN G, AKDEMIR A, ERGUN O N. The influence of chemical conditioning on the removal of copper ions from aqueous solutions by using clinoptilolite[J]. Environmental Progress and Sustainable Energy, 2009, 28(2): 202-211.
[8] van NGHIEM N, LEE J C, JHA M K, YOO K, JEONG J. Copper recovery from low concentration waste solution using Dowex G-26 resin[J]. Hydrometallurgy, 2009, 97(3/4): 237-242.
[9] 崔春花, 张卫东, 任钟旗, 刘君腾, 孟慧琳. 中空纤维更新液膜技术处理模拟含铜电镀废水[J]. 电镀与涂饰, 2009, 28(3): 31-33.
CUI Chun-hua, ZHANG Wei-dong, REN Zhong-qi, LIU Jun-teng, MENG Hui-lin. Treatment of simulated electroplating wastewater containing copper(II) using hollow fiber renewal liquid membrane[J]. Electroplating & Finishing, 2009, 28(3): 31-33.
[10] AZIZ H A, ADLAN M N, ARIFFIN K S. Heavy metals (Cd, Pb, Zn, Ni, Cu and Cr(III)) removal from water in Malaysia: Post treatment by high quality limestone[J]. Bioresource Technology, 2008, 99(6): 1578-l583.
[11] ROMERO F M, NUNEZ L, GUTIERREZ M E, ARMIENTA M A, CENICEROS-GOME A E. Evaluation of the potential of indigenous calcareous shale for neutralization and removal of arsenic and heavy metals from acid mine drainage in the Taxco mining area, Mexico[J]. Archives of Environmental Contamination and Toxicology, 2011, 60(2): 191-203.
[12] LI Y J, ZENG X P, LIU Y F, YAN S S, HU Z H, NI Y M. Study on the treatment of copper-electroplating wastewater by chemical trapping and flocculation[J]. Separation and Purification Technology, 2003, 31(1): 91-95.
[13] 龙来寿, 孙水裕, 钟 胜, 刘敬勇, 邓 丰, 李红军. 真空热解预处理对回收废线路板中铜的影响[J]. 中国有色金属学报, 2010, 20(4): 795-800.
LONG Lai-shou, SUN Shui-yu, ZHONG Sheng, LIU Jing-yong, DENG Feng, LI Hong-jun. Effect of vacuum pyrolysis pretreatment on recovery of copper from scrap printed circuit boards[J]. The Chinese Journal of Nonferrous Metals, 2010, 20(4): 795-800.
[14] LIANG Qi-wen, HU Hui-ping, FU Weng, YE Ting, CHEN Qi-yuan. Recovery of copper from simulated ammoniacal spent etchant using sterically hindered beta-diketone[J]. Transactions of Nonferrous Metals Society of China, 2011, 21(8): 1840-1846.
[15] 仉丽娟, 刘晓文, 康 鑫, 陈岩贽, 周文根, 史美玉, 周文博, 温 勇, 王 炜, 周洪波. 嗜酸铁氧化富集物高效浸提废覆铜板分选残渣中的铜[J]. 中国有色金属学报, 2015, 25(10): 2936-2944.
ZHANG Li-juan, LIU Xiao-wen, KANG Xin, CHEN Yan-zhi, ZHOU Wen-gen, SHI Mei-yu, ZHOU Wen-bo, WEN Yong, WANG Wei, ZHOU Hong-bo. High extraction of copper from flotation tailings of waste copper-clad laminates by acidophilic iron-oxidizing enrichment[J]. The Chinese Journal of Nonferrous Metals, 2015, 25(10): 2936-2944.
[16] RICHTER H, NEVIN K P, JIA H F, LOWY D A, LOVILEY D R. Cyclic voltammetry of biofilms of wild type and mutant Geobacter sulfurreducens on fuel cell anodes indicates possible roles of OmcB, OmcZ, type IV pili and protons in extracellular electron transfer[J]. Energy and Environmental Science, 2009, 2: 506-516.
[17] HUANG J S, YANG P, GUO Y, LI X F, LUO M. Start-up of a continuous flow MFC and bio-particles formation[J]. Journal of Chemical Engineering of Chinese Universities, 2010, 24(4): 694-699.
[18] 王艳芳, 刘百仓, 郑 哲, 郑雪艳. 电极面积和电极间距对立方体型MFCs产电能力的影响[J]. 可再生能源, 2013, 31(8): 68-74.
WANG Yan-fang, LIU Bai-cang, ZHENG Zhe, ZHENG Xue-yan. Effects of the electrode area and electrode spacing on the electricity generation capacity of MFCs[J]. Renewable Energy Resources, 2013, 31(8): 68-74.
[19] 牟姝君, 李秀芬, 任月萍, 王新华. 铜离子对双室微生物燃料电池电能输出的影响研究[J]. 环境科学, 2014, 35(7): 2791-2797.
MU Shu-jun, LI Xiu-fen, REN Yue-ping, WANG Xin-hua. Effect of Cu2+ on the power output of dual-chamber microbial fuel cell[J]. Environmental Science, 2014, 35(7): 2791-2797.
[20] ZHANG L J, TAO H C, WEI X Y, LEI T, LI J B, WANG A J, WU W M. Bioelectrochemical recovery of ammonia-copper(II) complexes from wastewater using a dual chamber microbial fuel cell[J]. Chemosphere, 2012, 89(10): 1177-1182.
[21] LI Y, LU A H, DING H R, JIN R, YAN Y H, WANG C Q, ZEN C P, WANG X. Cr(VI) reduction at rutile-catalyzed cathode in microbial fuel cells[J]. Electrochemistry Communications, 2009, 11(7): 1496-1499.
[22] ZHANG B G, FENG C P, NI J R, ZHANG J, HUANG W L. Simultaneous reduction of vanadium (Ⅴ) and chromium (Ⅵ) with enhanced energy recovery based on microbial fuel cell technology[J]. Journal of Power Sources, 2012, 204: 34-39.
[23] 张永娟, 李永峰, 刘春研, 王艺璇, 李 龙, 王耔人, 董义兴. 利用双室微生物燃料电池处理模拟废水的产电特性研究[J]. 环境科学, 2012, 33(7): 2427-2431.
ZHANG Yong-juan, LI Yong-feng, LIU Chun-yan, WANG Yi-xuan, LI Long, WANG Zi-ren, DONG Yi-xing. Electricity generation performance of two-chamber microbial full cell in the treatment of simulated wastewater[J]. Environmental Science, 2012, 33(7): 2427-2431.
[24] 梁 敏, 陶虎春, 李绍峰, 李 伟, 张丽娟, 倪晋仁. 剩余污泥为底物的微生物燃料电池处理含铜废水[J]. 环境科学, 2011, 32(1): 179-185.
LIANG Min, TAO Hu-chun, LI Shao-feng, LI Wei, ZHANG Li-juan, NI Jin-ren. Treatment of Cu2+-containing wastewater by microbial fuel cell with excess sludge as anodic substrate[J]. Environmental Science, 2011, 32(1): 179-185.
[25] HEIJNE A T, LIU F, WEIJDEN R V D, WEIJMA J, BUISMAN C J N, HAMELERS H V M. Copper recovery combined with electricity production in a microbial fuel cell[J]. Environmental Science and Technology, 2010, 44(11): 4376-4381.
Copper recovery from copper-containing wastewater through treating membraneless microbial fuel cell and its electricity production
LIU Wei-ping, YIN Xia-fei, LU Juan-juan, LIANG Guo-bin, CHEN Yue-mei
(School of Chemistry and Environment Engineering, Jiangsu University of Technology, Changzhou 213000, China)
Abstract: The baffled membraneless dual chamber microbial fuel cell (MFC) was constructed, the electricity generation capacity of baffled membraneless dual chamber MFC and wastewater treatment were studied, the electricity produced by MFC was used directly to treat cathode simulation copper-containing wastewater and recover copper. The results show that the baffled membraneless dual chamber MFC is started successfully in which sodium acetate is used as anode substrate, complex acclimated microorganism in activated sludge is used as anode inoculating microorganism, Cu2+ is used as cathodic electron acceptor, the maximum power density is 31.3 mW/m2 and the maximum open circuit voltage is 678.0 mV, the removal rate of Cu2+ is 99.6%, sediment on the cathode plate detected by XRD is mainly Cu. The baffled membraneless dual chamber MFC can effectively treat copper-containing wastewater and recover copper.
Key words: microbial fuel cell; copper-contained wastewater; copper recovery; electricity production
Foundation item: Project (BK20131133) supported by Jiangsu Provincial Natural Science Foundation, China
Received date: 2016-03-28; Accepted date: 2016-08-20
Corresponding author: LIU Wei-ping; Tel: +86-519-86953092; E-mail: weiping@jsut.edu.cn
(编辑 李艳红)
基金项目:江苏省自然科学基金资助项目(BK20131133)
收稿日期:2016-03-28;修订日期:2016-08-20
通信作者:刘维平,教授,博士;电话:0519-86953092;E-mail:weiping@jsut.edu.cn
摘 要:构建双室无膜折流板式微生物燃料电池,研究双室无膜折流板微生物燃料电池的产电性能和废水处理,在不需要外部电源的情况下,直接利用微生物燃料电池产生的电能处理阴极模拟含铜废水并回收铜。结果表明:以乙酸钠为阳极底物,以驯化后的活性污泥混合菌为阳极接种微生物,以Cu2+为阴极电子受体可成功启动无膜折流板微生物燃料电池,最大功率密度为31.3 mW/m2,开路电压最高为678.0 mV,Cu2+去除率为99.6%;经XRD分析,阴极板上的沉积物主要为单质铜,双室无膜折流板微生物燃料电池可有效处理含铜废水并回收铜。
[1] 罗丽卉, 谢翼飞, 李旭东. 生物硫铁复合材料处理含铜废水及机理研究[J]. 中国环境科学, 2012, 32(2): 249-253.
[3] 肖书虎, 张国芳, 宋永会, 曾 萍, 李 辉. 电化学双极法处理高浓度含铜黄连素制药废水[J]. 环境工程技术学报, 2011, 1(4): 295-299.
[9] 崔春花, 张卫东, 任钟旗, 刘君腾, 孟慧琳. 中空纤维更新液膜技术处理模拟含铜电镀废水[J]. 电镀与涂饰, 2009, 28(3): 31-33.
[13] 龙来寿, 孙水裕, 钟 胜, 刘敬勇, 邓 丰, 李红军. 真空热解预处理对回收废线路板中铜的影响[J]. 中国有色金属学报, 2010, 20(4): 795-800.
[18] 王艳芳, 刘百仓, 郑 哲, 郑雪艳. 电极面积和电极间距对立方体型MFCs产电能力的影响[J]. 可再生能源, 2013, 31(8): 68-74.
[19] 牟姝君, 李秀芬, 任月萍, 王新华. 铜离子对双室微生物燃料电池电能输出的影响研究[J]. 环境科学, 2014, 35(7): 2791-2797.
[23] 张永娟, 李永峰, 刘春研, 王艺璇, 李 龙, 王耔人, 董义兴. 利用双室微生物燃料电池处理模拟废水的产电特性研究[J]. 环境科学, 2012, 33(7): 2427-2431.
[24] 梁 敏, 陶虎春, 李绍峰, 李 伟, 张丽娟, 倪晋仁. 剩余污泥为底物的微生物燃料电池处理含铜废水[J]. 环境科学, 2011, 32(1): 179-185.


