
Separation of silver from silver-manganese ore with cellulose as reductant
ZHANG Xiao-yun(张小云), TIAN Xue-da(田学达), ZHANG Dong-fang(张东方)
College of Chemical Engineering, Xiangtan University, Xiangtan 411105, China
Received 21 November 2005; accepted 3 April 2006
Abstract:
The silver in some silver-manganese ore with a grade of 3.15×10-4 was concentrated by a combined beneficiation technique including magnetic separation, flotation, reducing leaching and gravity desliming. The major silver contained in manganese ore as isomorphism was concentrated by magnetic separation, while around 8.50% of the silver individual minerals were separated by flotation. The manganese in the mixed concentrate of both magnetic separation and flotation was dissolved in a reducing leaching, in which some cellulose reductant named CMK was used. Part of the slime contained in leach residue was removed by a laboratory desliming equipment. A silver concentrate with a grade of 4.96×10-3 Ag and a recovery of 84.25% were obtained.
Key words:
silver; silver-manganese ore; magnetic separation; flotation; reducing leaching; desliming;
1 Introduction
Silver-manganese ore has been found in Inner Mongolia, Shanxi, Hebei, Hunan and Guangxi, so it is an important silver source in China. Generally, the beneficiability of the silver in this ore is different from in other silver minerals due to its special property. The mineral composition analysis showed that silver presents mainly in the manganese oxidized minerals such as psilomelane and pyrolusite through isomorphism, only a small fraction of the silver presents in individual minerals[1].
Method to recover silver from silver-manganese ore considerably depends on the grades of Ag and Mn, grindability, chemical and physical property, association and dissemination size of minerals. There are various approaches[2, 3], which can be classified into two broad categories, i.e. flotation and magnetic separation[4]. Silver individual minerals of good floatability can be easily separated by flotation[5]. Magnetic separation can effectively recover silver contained in manganese minerals[6,7]. Rough silver-manganese concentrate can be obtained by magnetic separation, then silver is separated from manganese by some other methods such as reducing roasting, reduction by reducing agent, bacterial heap leaching as well as chloridizing roasting[8-13].
Located in Inner Mongolia of China, Errentaolegai Silver Mine is a typical silver-manganese mine, totally containing more than 2 300 t silver. Neither cyanide leaching nor flotation is an effective process to recover silver from this ore, because nearly 91.50% of the silver is disseminated in psilomelane and pyrolusite, and remained 8.50% is in silver individual minerals such as native silver, cerargyrite and orite.
A combined beneficiation technique was developed to process this silver mineral. In this technique, the purpose of magnetic separation and flotation is to recover silver as more as possible, reducing leaching with some cellulose as a reductant is to separate silver from manganese, and desliming is to remove slime, resulting in a rather high grade of silver concentrate.
2 Experimental
The silver-manganese ore was from Errentaoleigai Silver Mine in Inner Mongolia. After being crushed to a certain size range, the manganese minerals containing silver were separated by magnetic separation in a double- roll high-intensity magnetic separator with a magnetic intensity of about 875 kA/m. The tailing of magnetic separation was ground to be <0.074 mm, and then the silver individual minerals were floated in a FXD flotation cell with a live volume of 1 L. The magnetic concentrate and flotation concentrate were mixed together. This mixed concentrate was dissolved in H2SO4 solution with some cellulose as a reductant. The elemental analysis of the ore sample tested is listed in Table 1.
Table 1 Element analysis of silver-manganese ore

The cellulose that was used as reductant, named as CMK in this study, was prepared by a biochemical reaction with an organic by-product of some process as major material. In H2SO4 solution this cellulose CMK is able to convert to polysaccharides[14-16]. The main components of the cellulose is listed in Table 2.
Table 2 Main components of cellulose reductant CMK(%)

3 Results and discussion
3.1 Magnetic separation test
The silver mainly exists in manganese minerals through isomorphism, so it is necessary to separate manganese minerals from other gangues. Considering that manganese minerals, such as psilomelane and pyrolusite, are weakly magnetic minerals with a specific magnetization coefficient of 0.1×10-6 to 7.5×10-6, the high-intensity magnetic separation is a commonly used technique. The grade and recovery of the magnetic concentrate depend mainly upon the grinding fineness of the ore, as shown in Fig.1.
The results indicate that a satisfactory grade and recovery can be obtained when the grinding fineness is 60%<1.0 mm. When the ore is crushed or ground to be 20%<1.0 mm, the concentrate is contaminated by gangues and silver grade decreases. When the grinding fineness is 80%<1.0 mm, the magnetic separation becomes difficult and silver recovery is rather low. When the grinding fineness is 60%<1.0 mm, a concentrate is gained with grade of 8.42×10-4 Ag and 38.50% Mn, and the recovery of Ag is 83.0%.
3.2 Flotation test
The normal technique to recover silver individual minerals with a low silver grade is flotation, usually with sodium sulphide as an activator, butyl xanthate and ammonium dibutyl dithiophosphate as collectors. Considering that silver individual minerals are only 8.50% of total silver minerals, the flotation condition is determined as follows: sodium sulphide 500 g/t, butyl xanthate 8 g/t, and ammonium dibutyl dithiophosphate 10 g/t, the flotation time was 12 min, and the pulp density is around 35%. A flotation concentrate is obtained with a grade of 5.65×10-4 Ag and a recovery of 7.23 %.

Fig.1 Effect of grinding fineness on magnetic separation
3.3 Separation test of silver from manganese
Magnetic separation and flotation are the basic stages to concentrate silver in the ore. Combining the magnetic concentrate with the flotation concentrate, a mixed concentrate is formed with a grade of 8.10×10-4 Ag, containing 34.28% Mn. The total recovery of Ag is 90.23%. This mixed concentrate was ground to be <0.074 mm before Mn leaching test.
It is impossible to separate silver from manganese minerals by physical methods. Chemical methods are commonly effective method. Some cellulose CMK is used as a reductant in this study due to its strong reducing ability to MnO2 in H2SO4 solution. After the dissolution of manganese minerals, the grade of Ag is enhanced and the recovery of silver is achieved.
The mechanism of this cellulose CMK reducing MnO2 is that it can produce polysaccharides in H2SO4 solution, and the chemical reactions are as follows:
n(C6H10O5)+nH2SO4→n(C6H11O5)HSO4 (1)
n(C6H11O5)HSO4+nH2O→n(C6H12O6)+nH2SO4 (2)
The polysaccharides produced in above reactions can react with MnO2 , so MnO2 is reduced to Mn2+:
MnO2+n(C6H10O6)+H2SO4→MnSO4+CO2+H2O (3)
3.3.1 Selection of grinding fineness of mixed concentrate
Tests were carried out under the conditions of 500 g mixed concentrate, 350 g H2SO4, 20 g CMK, 30% pulp density, pulp temperature 95 ℃ and leaching time 60 min. The effect of grinding fineness of mixed concentrate on Mn leaching rate is shown in Fig.2.
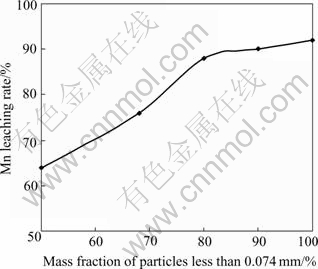
Fig.2 Effect of grinding fineness on Mn leaching rate
It can be seen from Fig.2 that when the grinding fineness is 80%<0.074 mm, the Mn leaching rate reaches 88.0%, and the Ag grade in the leaching residue is improved to 16.10×10-4 from 8.10×10-4 in the mixed concentrate. A smaller grinding fineness brings out a little addition of Mn leaching rate.
A screening analysis was made for the leaching residue when Mn leaching finished. The distribution of Ag in different size range of the residue is shown in Table 3.
Table 3 Screening analysis of Mn leaching residue

The results show that more than 78.00 % of Ag distributes in the size range of 0.15 mm to 0.074 mm. If the grinding fineness is 80%<0.074 mm, it will be very hard to separate silver from slime in the desliming process, and the Ag grade in concentrate will be lower than 4.00×10-3. Therefore, a suitable grinding fineness should be <0.074 mm, that is 100%<0.074 mm, rather than 80%<0.074 mm.
3.3.2 Effect of CMK fineness on Mn leaching
The fineness of CMK is one of the important factors affecting Mn leaching rate. According to the theoretical calculation from the reducing reaction, 500 g silver- manganese mixed concentrate with 34.28% Mn and 8.10×10-4 Ag needs 20 g CMK, so the test was done in the following conditions: 500 g mixed concentrate, 350 g H2SO4, 20 g CMK, 30% pulp density, pulp temperature 95 ℃ and leaching time 60 min. The effect of CMK fineness on the Mn leaching rate is shown in Fig.3.

Fig.3 Effect of CMK fineness on Mn leaching rate
It can be seen from Fig.3 that when the size of CMK is larger than 0.5 mm, the Mn leaching rate is lower than 70% at a certain leaching time; when the fineness of CMK is 0.1 mm, the Mn leaching rate reaches up to 90%. Therefore, the fineness of CMK should be ground to be <0.1 mm.
3.3.3 Effect of CMK dosage on Mn leaching rate
To improve the Mn leaching rate, it is necessary to increase the applied dosage of reducing agent on basis of theoretical dosage (20 g CMK for 500 g mixed concen- trate, theoretically). Tests were made to investigate the effect of CMK dosage on the Mn leaching rate, and the results are shown in Fig.4. Meanwhile, considering that oxalic acid is known as an effective reducing agent to MnO2, comparison tests between CMK and oxalic acid were also made. Oxalic acid will not be applied in industrial process, of course, because it is always expensive.
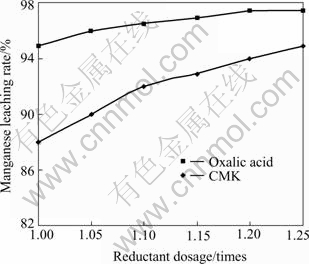
Fig.4 Effect of CMK dosage on Mn leaching rate
It can be seen from Fig.4 that CMK presents a strong reducing ability to MnO2. With the increase of CMK dosage, the Mn leaching rate increases apparently. When CMK dosage is 1.2 times more than the theoretical dosage, the Mn leaching rate reaches up to 94%, almost equals to that of using oxalic acid as a reductant.
3.4 Separation of silver from slime
When the leaching process is finished, the pulp contains a large amount of slime, MnSO4 and a small fraction of other sulfates. With a density more than 1 g/cm3, these sulfates are rather convenient for the separation of Ag from slime. Involved in the leach residue, Ag and Ag minerals are retained at the bottom of the desliming equipment, and the slime and sulfates overflow, hence a Ag concentrate, the leach residue, is obtained. The grade of Ag concentrate mainly depends on the yield of leach residue, as shown in Table 4.
Table 4 Results of Ag separation in desliming process

Generally, as a product in market, the demand for Ag grade of Ag concentrate is higher than 4.00×10-3. From Table 3 it can be seen that when the yield of leach residue is controlled at about 5.40 %, Ag concentrate with a satisfactory grade of 4.96×10-3 Ag and recovery of 84.25% can be obtained.
4 Conclusions
1) A combined beneficiation technique including magnetic separation, flotation, reducing leaching and desliming is used to concentrate Ag in some silver- manganese ore with a grade of 3.15×10-4 Ag. By this technique, a silver concentrate with a grade of 4.96×10-3 Ag and a recovery of 84.25% are obtained.
2) When the ore is ground to 60%<1.0 mm, a magnetic concentrate with a grade of 8.42×10-4 Ag and recovery of 83% is gained in a double-roll high-intensity magnetic separator with a magnetic field strength of about 875 kA/m.
3) Silver individual minerals are recovered by flotation, obtaining a flotation concentrate with a grade of 5.65×10-4 Ag and a recovery of 7.23%.
4) The cellulose CMK is an effective reductant for the leaching of Mn in the silver-manganese ore. To get the leaching rate of Mn as high as 94.0%, the fineness of CMK should be <0.1 mm, and the CMK dosage should be 1.2 times more than the theoretical dosage.
5) When the leaching process is finished, Ag grade can further be enhanced by removing slime.
References
[1] LU Zhi-cheng, ZHANG Pei-ping, DUAN Guo-zheng. Study on manganese minerals of E’rentaolegai silver deposit [J]. Mineral Petrol , 2002, 22(3): 1-3.(in Chinese)
[2] LI Wei-tian. Review for recovery technique of silver-manganese oxide ores [J]. Guangxi Geology, 2001, 12(3): 63-66.(in Chinese)
[3] WU Wen-wei. The concentration of silver from oxidative silver-manganese ore with united technologies of beneficiation and metallurgy [J]. Nonferrous Metals (Mineral Processing), 2003, 55(5): 22-24.(in Chinese)
[4] YUE Tie-bing, LI Ying-guo, WEI De-zhou, CAO Jin-cheng, WANG Yan. A technical research on concentrating silver and manganese ores of one lower grade [J]. China’s Manganese Industry, 2004, 22(3): 4-7.(in Chinese)
[5] CHEN Ling, YANG Xian-wan, SI Yun-sen. Technological condition in slurry electrolysis of high-silver galena concentrate [J]. Trans Nonferrous Met Soc China, 2002, 12(2): 344-348.
[6] GUO Xiu-ping, WU Yan-qiu, LI Zhao-hui, QUAN Wen-xin. High intensity magnetic separation of a low grade manganese-silver ore [J]. Multipurpose Utilization of Mineral Resources, 2004, 24(1): 7-9. (in Chinese)
[7] SUN Jing-feng. Recovery of silver from silver-manganite [J]. Hydrometallurgy of China, 2002, 21(1): 25-27. (in Chinese)
[8] WU Wen-wei. Silver extraction from silver-manganese concentrate by roasting-leaching with sulfuric acid [J]. Nonferrous Metals, 2004, 56(1): 48-50. (in Chinese)
[9] YU Li-xiu, YANG Hui-peng, WANG Qiu-xia. Study on new technique of reduction processing Ag-Mn ore with organic reducing agent [J]. Conservation and Utilization of Mineral Resources, 2002(2): 38-40. (in Chinese)
[10] SUN Yan-guang, YU Li-qiu. Study on technique and application of reduction of Mn-Ag ore with organic compound [J]. China’s Manganese Industry, 2004, 22(1): 1-4. (in Chinese)
[11] JIANG Tao, YANG Yong-bin. Simultaneous leaching of manganese and silver from manganese-silver ores at room temperature [J]. Hydrometallurgy, 2003, 69(1-3): 177-186.
[12] ZHANG Bin, CHEN Qi-yuan, JIANG Tao, YANG Yong-bin. A new technological research for simultaneous leaching of manganese and silver from a manganese-silver associated ore [J]. Gold, 2001, 22(7): 26-29. (in Chinese)
[13] ZHOU Yuan-min, MEI Guang-gui. Study on the preparation manganese carbonate or manganese sulphate from leaching silver-manganese ore solution [J]. China’s Manganese Industry, 2003, 21(1): 10-13.(in Chinese)
[14] ZHANG Xiao-yun, TIAN Xue-da, LIU Shu-gen, ZHANG Ping-ping. Utilization technique of ferromanganese ore [J]. The Chinese Journal of Nonferrous Metals, 2005, 15(4): 650-654.
[15] SHI Hua, TIAN Xue-da, ZHANG Xiao-yun, DENG Yi-qiang. Treatment of chromium-containing wastewater by a new reducer [J]. Natural Science Journal of Xiangtan University, 2003, 25(3): 79-81. (in Chinese)
[16] ZHANG Xiao-yun,TIAN Xue-da. Preparation of manganese sulfate from pyrolusite with cellulose as reductant [J]. Fine Chemicals, 2006, 23(2): 195-197.(in Chinese)
Foundation item: Project(03SSY1011) supported by the Key Item of Hunan Science and Technology Department; Project(04C645) supported by Hunan Education Department
Corresponding author: ZHANG Xiao-yun; Tel: +86-732-8292469; E-mail: snowy@xtu.edu.cn
Abstract: The silver in some silver-manganese ore with a grade of 3.15×10-4 was concentrated by a combined beneficiation technique including magnetic separation, flotation, reducing leaching and gravity desliming. The major silver contained in manganese ore as isomorphism was concentrated by magnetic separation, while around 8.50% of the silver individual minerals were separated by flotation. The manganese in the mixed concentrate of both magnetic separation and flotation was dissolved in a reducing leaching, in which some cellulose reductant named CMK was used. Part of the slime contained in leach residue was removed by a laboratory desliming equipment. A silver concentrate with a grade of 4.96×10-3 Ag and a recovery of 84.25% were obtained.


