Trans. Nonferrous Met. Soc. China 22(2012) s100-s104
Facile synthesis of NiO nanowires and their gas sensing performance
ZENG Wen1, 2, MIAO Bin1, LIN Li-yang1, XIE Jin-yue1
1.College of Materials Science and Engineering, Chongqing University, Chongqing 400044, China;
2. State Key Laboratory of Power Transmission Equipment and System Security and New Technology, Chongqing University, Chongqing 400044, China
Received 9 July 2012; accepted 15 August 2012
Abstract:
The NiO nanowires were prepared by a facile PEG assisted hydrothermal method using NiC2O4·2H2O as a precursor compound. The microstructure of the samples was characterized by SEM and XRD. The gas sensing properties of the NiO nanowires toward ethanol was also investigated. The results show that PEG plays a key role in the synthesis of wire-like NiO. The NiO nanowires show excellent sensing performances to ethanol gas. This morphology holds substantial promise for applying NiO as a potential gas sensing material for future sensor application.
Key words:
NiO nanowires; NiC2O4·2H2O; hydrothermal reaction; gas sensing property;
1 Introduction
As a semiconductor, nickel oxide (NiO) has attracted a wide range of attention because its physical properties are important for many promising applications in the field of catalyst, magnetic and gas sensor [1-4]. In applications, the performance of NiO depends on its crystalline phase state, dimension and morphology [5,6]. In recent years, there have been many efforts in the fabrication of nanostructure NiO with various morphologies to improve its performance [7-14]. Compared to the traditional nanosized NiO, such as nanospheres, and one-dimensional (1-D) nanostructures, such as nanobelts, nanowires and nanotube would exhibit much more excellent performance in catalyst and gas sensor due to their high specific surface area, at the same time, have a lower charge carrier recombination rate [15,16]. Technologies in fabrication of 1-D nanostructures provide us a promising method to explore new materials enable to enhance their performances, i.e., gas sensing functionality.
In this work, the hydrothermal reaction method was employed to prepare NiO nanowires and the gas sensing performance of the obtained nanowires was investigated. The main purpose was to develop a reliable and practical sensing material. It was found that NiO nanowires show excellent gas sensing performances towards ethanol, thereby holding technological promise for fabricating ethanol gas sensor.
2 Experimental
2.1 Preparation of NiO nanowires
In a typical hydrothermal procedure, 3 mmol of NiCl2·6H2O was dissolved into 22.5 mL distilled water to form a green clear solution in a beaker of 70 mL capacity, and then 1 mmol of NaC2O4, 0.15 g of PEG (relative molecular mass 6000) and 40 mL of EG were added to the beaker. The mixture was magnet stirred for 30 min to give a transparent solution and transferred into a Teflon-lined stainless steel autoclave, sealed and maintained at 180 °C for 20 h. After the reaction was completed, the autoclave was cooled to the room temperature naturally. The blue-green products were harvested by pursuing centrifugation, washing with distilled water and ethanol more than 10 times, respectively, to remove unexpected ions, and then dried at 60 °C in vacuum. The powder was heated to 400 °C with a heating rate of 2.0 °C/min and then annealed at 400 °C for 2 h.
2.2 Microstructure characterization
The crystal structure and morphology of the samples were characterized by X-ray diffraction (XRD)and field emission scanning electron microscopy (FE-SEM), respectively. XRD analysis was conducted using RigakuD/Max-1200X diffractometer with the Cu Kα radiation operated at 30 kV and 100 mA. The morphology was characterized with a Nova 400 Nano FE-SEM.
2.3 Fabrication of gas sensor and gas sensing measurement
Before sensors fabrication, the as-obtained precipitate was annealed at 100 °C for 2.5 h in air to remove crystal water for stabilizing. A proper amount of the final powder was mixed with distilled water to form a paste, which was then coated onto an alumina tube with a pair of Au electrodes and four Pt wires. A Ni-Cr alloy filament which inserted into the tube was used as heater. The temperature of coated tube could be controlled by regulating voltage. Different voltages were corresponded to various temperatures. The gas-sensors were dried at 120 °C for 8 h in air to evaporate the water molecules existed possibly. Finally, the sensor was aged for 2 d before measurement.
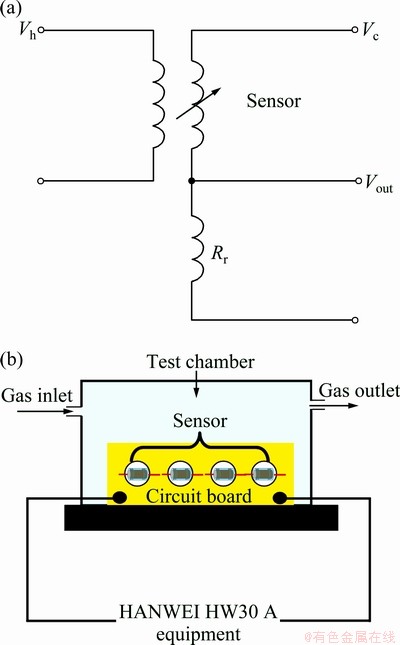
Fig. 1 Schematic diagram of electric circuit (a) and gas-sensing measurement system (b)
Gas sensing properties were measured using a static system that was controlled by a computer (HW-30A, Hanwei Electronics Co., China). The schematic for the gas response measurement is shown in Fig. 1(a). The sensors are operated using a simple circuit voltage (Vc), which was applied to allow a measurement of the output voltage (Vout) across. The gas inlet and the air admittance valves are made at the base plate in order to inject the test gas and, air as shown in Fig. 1(b). When air and ppm-level target gases flowed through the test chamber, the corresponding resistances of the sensor in air (Ra) and in the presence of the target gas in air (Rg) were measured by monitoring the Vout. The operating temperature of the sensor was controlled by varying the current flow through the heater. Ethanol gases were injected inside the bell jar through a needle valve. Gas sensing studies were carried out under laboratory condition at room temperature of 27 °C and relative humidity of 40%. Gas response in this work was defined as S=Ra/Rt, where Ra and Rt are the resistance of the sensor in air and in ethanol, respectively [17].
3 Result and discussion
The samples obtained via the hydrothermal process at 180 °C for 12 h were characterized with SEM and XRD, respectively. The results are shown in Fig. 2. Figure 2(a) shows a low SEM image of the samples, which indicates that the samples show the wire-like structure. Figures 2(b) and (c) show magnified SEM images of the samples. It can be seen that the samples show a wire-like structure. The length and diameter of the nanowires are 2-4 μm and 80-100 nm, respectively. The XRD patterns of the samples are shown in Fig. 2(d). It can be seen that all peaks in the XRD patterns are consistent with the JCPDS (21-1272) data of the NiC2O4·2H2O with a monoclinic phase. Moreover, no NiO related peak was detected in this sample obtained from hydrothermal process, which verifies a pure phase of NiC2O4·2H2O products.
The pervious references addressed that the NiC2O4·2H2O nanowire is unstable that they will transform quickly from single crystal into NiO polycrystals. Thereby, the as-prepared wire-like NiC2O4·2H2O precursors were annealed at 400 °C for 2h in air, and the resultant products were characterized using SEM and XRD, respectively.
Figure 3(a) shows the SEM image of the samples obtained after annealing the as-prepared NiC2O4·2H2O precursors. The SEM image indicates that the sample keeps the geometrical morphology of wire-like structures. The length and diameter of the nanowires are 2-3 μm and 85-100 nm, respectively. The corresponding XRD patterns are shown in Fig. 3(b). Three orientation peaks are observed from the patterns where 2θ is 37°, 43° and 62°, respectively. According to JCPDS card No.04-0835, the obtained samples after annealing the NiC2O4·2H2O precursors are cubic NiO, and the three peaks are assigned to (111), (200) and (110), respectively. So, it is suggested that the NiC2O4·2H2O will transform into NiO phase, while the corresponding nanowires structure change slightly.
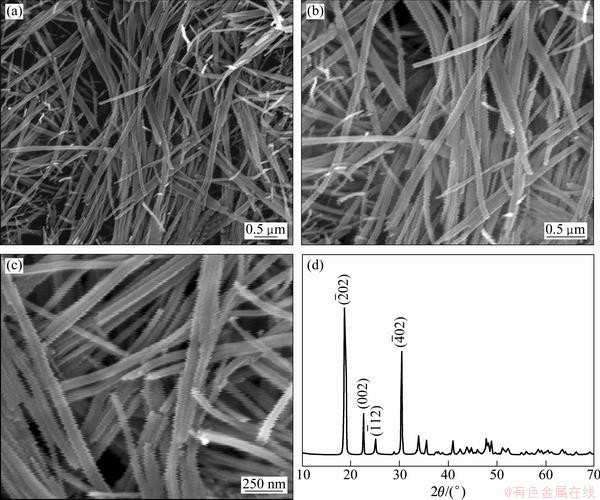
Fig. 2 SEM images (a-c) and XRD pattern (d) of samples obtained from hydrothermal process
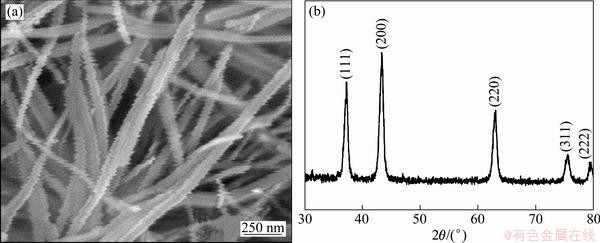
Fig. 3 SEM image (a) and XRD pattern (b) of sample obtained after annealing as-prepared NiC2O4·2H2O precursors.
The possible formation mechanism of NiO and the chemical reactions to form NiO nanowires are [18]:
Cl2+ NiC2O4=NiC2O4·2H2O+2NiCl (1)
NiC2O4·2H2O=NiC2O4+2H2O (2)
2NiC2O4+O2=2NiO+4CO2 (3)
In the hydrothermal procession, NiCl2 reacted with NiC2O4 and H2O in a high temperature and high press atmosphere, and ultimately formed the NiC2O4·2H2O nanowires. Then the NiC2O4·2H2O precursors were annealed in air, NiC2O4·2H2O decomposed into NiC2O4 and H2O. Subsequently, NiC2O4 reacted with O2 to NiO and CO2.
Gas sensing properties of NiO and NiC2O4·2H2O nanowires were investigated in this work. Figure 4(a) illustrates the structure of the gas sensor and Fig. 4(b) presents the gas response of the sensors to ethanol at operating temperatures of 300 °C with the gas volume fraction of 5×10-4. It can be seen that the resistances in both two cases increase when gas is in and return to their original state while gas is out. The major difference between the two cases is that the increasing extent of resistance for the NiO is substantially larger than that for the solid solutions at working stage. That means the NiO nanowires sensor exhibits higher sensing signal toward ethanol than NiC2O4·2H2O nanowires sensor.
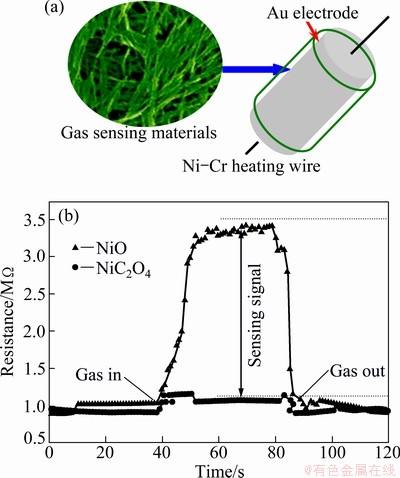
Fig. 4 Schematic diagram of gas sensor (a) and gas response-recovery characteristics of sensor (b)
It is well known that the NiO belongs to p-type semiconductor sensor. When the NiO sensors are exposed to air, oxygen molecules will be adsorbed on the surface of NiO in the form of O- and O2-. The high coverage with adsorbed oxygen causes an increase in the electrical holes of the NiO sensing materials, and thus increases its conductivity as indicated in the region (Fig. 4(b)) before introducing ethanol. When the sensors are exposed to ethanol, the ethanol reacts with the adsorbed ionic oxygen owing to its reducing property, and thus injects electrons in the NiO sensing material, resulting in a decrease the concentration of holes, that is the electrical conductivity will decrease. The gas sensing reaction may be described as:
C2H5OH(gas)+O2-(ads)→C2H5O-(gas)+OH-(ads) (4)
C2H5O-(gas)→(C2H5)2O(ads)+O-(ads)+e- (5)
2C2H5OH(gas)+O2-(ads)→2C2H4O-(ads)+2H2O (6)
C2H4O-(ads)→CH3CHO(ads)+e- (7)
CH3CHO(ads)+5O2-(ads)→4CO2+4H2O+10e- (8)
As the sensing investigation above, even the similar morphology of NiO and NiC2O4·2H2O, they will also show completely different sensing performances. Further understanding of the mechanism requires more advanced measurement technique and high-precision theoretical calculations.
4 Conclusions
1) NiO nanowires are prepared through hydrothermal method and their microstructures and gas sensing properties are investigated. The results show that NiO nanowires exhibit excellent gas sensing performance toward ethanol.
2) The results suggest that the gas sensing properties of nanocrystals can be significantly improved by tailoring the shape and the surface structure of nanocrystals, which provides a new concept for rational design and development of high performance sensing materials.
References
[1] TIWARI S D, RAJEEV K P. Magnetic properties of NiO nanoparticles [J]. Thin Solid Films, 2006, 505(1-2): 113-117.
[2] SONG X C, LU J, ZHANG T S, MA J. Sintering behavior and mechanisms of NiO-doped 8 mol% yttria stabilized zirconia [J]. Journal of the European Ceramic Society, 2011, 31(14): 2621-2627.
[3] JANG W L, LU Y M, HWANG W S, CHEN W C. Electrical properties of Li-doped NiO films [J]. Journal of the European Ceramic Society, 2010, 30(2): 503-508.
[4] ANANDAN K, RAJENDRAN V. Morphological and size effects of NiO nanoparticles via solvothermal process and their optical properties [J]. Materials Science in Semiconductor Processing, 2011, 14(1): 43-47.
[5] XIA Q X, HUI K S, HUI K N, HWANG D H, LEE S K, ZHOU W, CHO Y R, KWON S H, WANG Q M, SON Y G. A facile synthesis method of hierarchically porous NiO nanosheets [J]. Materials Letters, 2012, 69(2): 69-71.
[6] WANG L L, DENG J N, FEI T, ZHANG T. Template-free synthesized hollow NiO-SnO2 nanospheres with high gas sensing perfprmance [J]. Sensors and Actuators B: Chemical, 2012, 164(1): 90-95.
[7] STEINEBACH H, KANNAN S, RIETH L, SOLZBACHER F. H2 gas sensor performance of NiO at high temperatures in gas mixtures [J]. Sensors and Actuators B: Chemical, 2010, 151(1): 162-168.
[8] LEE C Y, CHIANG C M, WANG Y H, MA R H. A self-heating gas sensor with integrated NiO thin-film for formaldehyde detection. [J]. Sensors and Actuators B: Chemical, 2007, 122(2): 503-510.
[9] ASLANI A, OROOJPOUR V, FALLAHI M. Sonochemical synthesis, size controlling and gas sensing properties of NiO nanoparticles [J]. Applied Surface Science, 2011, 25(9): 4056-4061.
[10] ZHENG Y G, WANG J, YAO P J. Formaldehyde sensing properties of electrospun NiO-doped SnO2 nanofibers [J]. Sensors and Actuators B: Chemical, 2011, 156(2): 723-730.
[11] WEI Fei, WU Ye-fan, LUO Ling-hong, SHI Ji-jun, CHENG Liang, MIAO Li-feng. Synthesis and characterization of flower-like NiO nanoarchitectures [J]. Journal of the Chinese Ceramic Society, 2009, 37(12): 1975-1981.
[12] LIU Wei-xing, YAO Su-wei, ZHANG Wei-guo, HAN Yu-xing. Preparation and photoelectric property of nickel oxide nanowires [J]. Electroplating & Finishing, 2006, 25(12): 14-17.
[13] TANG Hong-wei, WANG Jiang-liang, CHANG Zhao-rong, MIAO Wang, SUN Dong. Sol-gel template synthesis of NiO nanowires [J]. Surface Technology, 2007, 36(4): 15-19.
[14] WANG Jing, WU Na, WU Feng-qing, XIE Yong-ping. Preparation and characteristics of nanocrystalline NiO gas sensing material [J]. Chinese Journal of Scientific Instrument, 2004, 25(4): 249-250.
[15] WANG L L, LOU Z, FEI T, ZHANG T. Ehanced acetone sensing performances of hierarchical hollow Au-loaded NiO hybrid structures [J]. Sensors and Actuators B: Chemical, 2012, 161(1): 178-183.
[16] CHO N G, HWANG I S, KIM H G, LEE J H, KIM I D. Gas sensing properties of p-type hollow NiO hemispheres prepared by polymeric colloidal templating method [J]. Sensors and Actuators B: Chemical, 2011, 155(1): 366-371.
[17] ZENG W, LIU T M, LIU D J, HAN E J. Hydrogen sensing and mechanism of M-doped SnO2 (M=Cr3+, Cu2+ and Pd2+) nanocomposite [J]. Sensors and Actuators B: Chemical, 2011, 160(1): 455-462.
[18] LIU B, YANG H Q, ZHAO H, AN L J, ZHANG L H, SHI R Y, WANG L, BAO L, CHEN Y. Synthesis and enhanced gas sensing properties of ultralong NiO nanowires assembled with NiO nanocrystals [J]. Sensors and Actuators B: Chemical, 2011, 156(1): 251-262.
镍纳米线的简易合成及其气敏性能
曾 文1, 2,苗 斌1,林栎阳1,谢锦岳1
1. 重庆大学 材料科学与工程学院,重庆 400044;
2. 重庆大学 输配电装备及系统安全与新技术国家重点实验室,重庆 400044
摘 要:采用水热法,以聚乙二醇(PEG)作为辅助剂,通过先驱物NiC2O4·2H2O合成氧化镍纳米线,并通过SEM和XRD对样品的微观结构进行分析。实验结果显示:PEG对镍线的合成起重要作用,镍纳米线表现出良好的气敏性能。该形态使得氧化镍(NiO)可作为传感器的气敏材料得到应用。
关键词:镍纳米材料;NiC2O4·2H2O;水热反应;气敏特性
(Edited by DENG Lü-xiang)
Foundation item: Project (2012M511904) supported by China Postdoctoral Science Foundation; Project (CDJZR12110051) supported by Fundamental Research Funds for the Central Universities, China; Porject (1110611007) supported by National Innovation Experiment Program for University Students, China
Corresponding author: ZENG Wen; Tel: +86-23-65102465; E-mail: wenzeng@cqu.edu.cn
Abstract: The NiO nanowires were prepared by a facile PEG assisted hydrothermal method using NiC2O4·2H2O as a precursor compound. The microstructure of the samples was characterized by SEM and XRD. The gas sensing properties of the NiO nanowires toward ethanol was also investigated. The results show that PEG plays a key role in the synthesis of wire-like NiO. The NiO nanowires show excellent sensing performances to ethanol gas. This morphology holds substantial promise for applying NiO as a potential gas sensing material for future sensor application.


