
Performance and mechanism of Prussian blue (PB) modified carbon felt electrode
XUE Fang-qin (薛方勤), ZHANG Hong-tao(张鸿涛), WU Chun-xu(吴春旭), NING Tao(宁 涛), XU Xuan(徐 璇)
Department of Environmental Science and Engineering, Tsinghua University, Beijing 100084, China
Received 10 August 2009; accepted 15 September 2009
Abstract:
Prussian blue (PB) modified carbon felt electrodes were prepared. The electrochemical behavior was investigated by cyclic voltammetry, electrochemical impedance spectroscopy and charge-discharge experiments. In order to distinguish the mechanism of the PB modified carbon felt electrode, the electrochemical quartz crystal microbalance (EQCM) was employed. The results of cyclic voltammetry show that the modification can improve the reversibility and the suitable PB deposition is the amount of 10 circles deposition. PB modified carbon felt electrode can effectively decrease the charge transfer resistance. The voltage efficiency of VRB employing PB modified carbon felt electrode can be increased by 12%. The mass change of the PB modified Pt crystal electrode in the process of the potential scan is obvious. The reaction of substitution of VO2+ for high-spin Fe ion in PB is probable to happen and the possible reaction equation is given. The preliminary exploration shows that PB modified carbon felt is electrochemically promising for redox flow battery.
Key words:
porous electrode; carbon felt; redox flow battery (RFB); Prussian blue; electrochemical quartz crystal microbalance;
1 Introduction
The global economy development demands a more effective energy system. Developing new type reproducible energy is an inevitable trend since the reserves of fossil, such as coal, petroleum and natural gas, are limited. Renewable energy sources such as solar, wind and tide energy suffer from fluctuation and dispersed property and therefore need to be equipped with storage facilities, for example, secondary rechargeable batteries. Great efforts were made to develop new types of redox flow battery (RFB)[1-3].
The most promising system in RFB is all-vanadium redox flow battery (VRB)[4-6]. In order to obtain a higher current density suitable for the high power electric appliances, many carbon felt electrodes have been increasingly widely used in VRB. Carbon felt is a common porous electrode with a larger surface area of the electrochemical activity and good mechanical strength. However, carbon felt still has some defects, for example, poor kinetics reversibility. Much attention has been paid to the modification of the carbon felt electrodes to improve the reversibility. The chemical and electrochemical modification on carbon felt electrode are important ideas to improve the reversibility, and many modification methods have been reported, among them, one effective way is via electrodepositing of metals on carbon fibres. There are many ions and compounds to be used in modification, such as Nafion, Pt4+, Pd2+, Au4+, TiO2, MnO2, Bi3+, Pb2+ and Co2+.
Prussian blue (PB) film has a good electrochemical reversibility and high stability, thus PB has been widely used in electro-catalysis and secondary battery. Recently, some electrochemical properties of PB modified carbon felt electrode have been investigated [7-8]. But farther mechanism study of PB modified carbon felt electrode has not been reported.
In this work, PB modified carbon felt electrode was prepared. Then, electrochemical behaviors were investigated by cyclic voltammetry, electrochemical impedance spectroscopy and constant current charge- discharge experiments. Moreover, the mass change in the process of the PB modification was also inspected by way of electrochemical quartz crystal microbalance (EQCM) and the oxidation and reduction mechanism ofPB in the vanadium sulfate solution was discussed.
2 ExperimentalThe electrolytes used were composed of vanadium sulfate, sulfuric acid, anhydrous ferric chloride and potassium ferrocyanide. All chemicals used were of reagent grade without further purification and deionized water was used throughout. All tests were carried out at room temperature.
Polyacrylonitrile(PAN) based carbon felts obtained from Lanzhou Carbon Works were used in this work. All carbon felts were divided into two types according to different treatment methods. The first kind was called heat treated carbon felt which was treated as follows: carbon felts were immerged into dense sulfuric acid for 5 h, then washed to neutral, dried to anhydrous and heat-treated at 450 ℃ for 2 h in tube furnace.
The other kind was called PB modified carbon felt which was prepared by two steps. Firstly, the carbon felts were immerged in the dense sulfuric acid for 2 h, and then washed to neutral. Then the carbon felts were electrodeposited as following: 0.005 mol/L anhydrous ferric chloride and potassium ferrocyanide were prepared freshly, respectively. Then the two solutions of equal bulk were mixed and the pH value was adjusted to 2. Then the carbon felts was electrodeposited by cyclic voltammetry, which CV potential scan range was from 0 to 0.5 V and the scanning rate was 20 mV/s.
Some electrochemical experiments, such as cyclic voltammetry, electrochemical impedance spectroscopy and charge-discharge experiments were performed with a VMP2 multichannel potentiostat (Princeton Applied Research, USA). A conventional three-electrode cell was used with a carbon felt as the working electrode, a saturated calomel electrode as the reference electrode and a large area graphite plate as the counter electrode. The electrochemical quantz crystal microbalance was achieved with a 2273 Potentiostat (Princeton Applied Research, USA). QCA 922 quartz crystal analyzer was employed and controlled by Powersuite software. The base frequency of the quartz crystal electrode was 9 MHz.
The performance of a test battery employing V(Ⅳ)/V(Ⅴ) as anolyte active species and V(Ⅱ)/V(Ⅲ) as catholyte ones was evaluated with constant-current charge-discharge experiment. The membrane used in test battery was Nafion 117 (Dupont, USA) and the electrode area was 10 cm2. The anolyte was 20 mL 1.2 mol/L VOSO4 and 3 mol/L H2SO4 medium while the catholyte was 20 mL 1.2 mol/L V3+ and 3 mol/L H2SO4 medium. The current density in charge-discharge experiments was 20 mA/cm2 and the flow rate for the electrolytes was 6 mL/min. Because V(Ⅱ) is inclined to be oxidized, the catholytes were deaerated by bubbling with argon before and during the experiment.
3 Results and discussion3.1 Cyclic voltammetry comparison between heat treated and PB modified carbon felt
Cyclic voltammograms for 0.3 mol/L VOSO4 in 3 mol/L H2SO4 on different carbon felt, including heat treated felt and PB modified felt, are shown in Fig.1. The electrochemical data, including peak potentials separation and peak current ratio are summarized in Table 1. All the current densities were calculated by the projected area of the carbon felt.
It can be concluded from Fig.1 that, compared with the heat treated carbon felt, the peak shape of the cyclic voltammetry of PB modified carbon felt electrode is more symmetrical and the peak currents of anodic and cathodic are closer. Moreover, the peak potentials separation after the modification decreases significantly. From Table 1, it can be seen that the peak potential
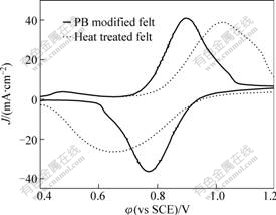
Fig.1 Cyclic voltammograms for 0.3 mol/L VOSO4 in 3 mol/L H2SO4 at scanning rate of 2 mV/s on heat treated and PB modified carbon felt electrodes
Table 1 Cyclic voltammogram data for 0.3 mol/L VOSO4 in 3 mol/L H2SO4 on heat treated and PB modified carbon felt electrodes
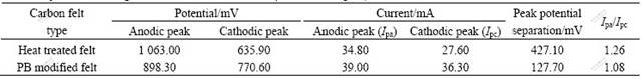
separation of heat treated carbon felt is 427.1 mV and that of PB modified carbon felt is 127.7 mV. At the same time, the ratio of peak current (Ipa/Ipc), PB modified carbon felt electrode also decreases obviously compared with that of heat treated carbon felt, which indicates the improvements of the redox reversibility. The reversibility of carbon felt after PB modification is improved obviously.
3.2 Stability of PB modified carbon felt electrode
Fig.2 shows the various circles of cyclic voltammograms for 0.3 mol/L VOSO4 in 3 mol/L H2SO4 solution at scanning rate of 2 mV/s on a PB modified carbon felt electrode. Table 2 lists the potential and current data from 1st to 10th cycles, in which the peak potential increases a little while the peak current decreases also a little. The stability of PB modified carbon felt electrode is good.
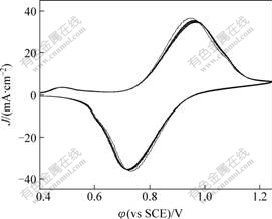
Fig.2 Cyclic voltammograms for 0.3 mol/L VOSO4 in 3 mol/L H2SO4 solution at scanning rate of 2mV/s on PB modified carbon felt electrode at various scanning circles
3.3 Influence of deposition amount on peak potential separation
Because the Prussian blue was deposited by way of CV scan process, the number of the CV scan circle can judge the PB deposition amount. Fig.3 shows the cyclic voltammograms on a PB modified carbon felt electrode with various PB deposition amount. It can be concluded that the peak potential separation decreases with increasing PB deposition amount before 10 cycles. However, the peak potential separation increases obviously after 10 cycles. The reason is possible that, with increasing PB deposition amount, the conductivity of PB modified carbon felt electrode decreases and the impedance of V(Ⅳ)→V(Ⅴ) electrochemical reaction increases. Accordingly, the reversibility decreases, and therefore, the deposition amount of 10-15 cycles is suitable for preparing the PB modified carbon felt electrode.
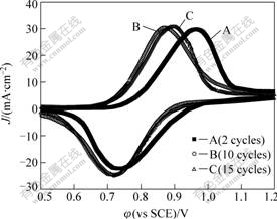
Fig.3 Cyclic voltammograms for 0.3 mol/L VOSO4 in 3 mol/L H2SO4 solution at scanning rate of 2 mV/s on PB modified carbon felt electrode with various PB deposition amounts
3.4 EIS analyses
Electrochemical impedance spectroscopy was galvanostatically measured by applying an Ac voltage of 20 mV over the frequency ranging from 10-3 Hz to 105 Hz. The results are shown in Figs.4 and 5.
It can be seen from Figs.4 and 5 that, both two
Table 2 Cathodic and anodic peak current and potential obtained on PB modified electrode at various scan circles
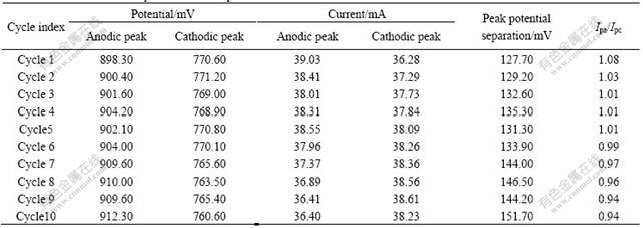

Fig.4 EIS plot for 0.3 mol/L VOSO4 in 3 mol/L H2SO4 on heat treated carbon felt electrode
Fig.5 EIS plot for 0.3 mol/L VOSO4 in 3 mol/L H2SO4 on PB modified carbon felt electrode
charts have common characteristics, which show a line at low frequency and a semicircle at high frequency. These results indicate that both two electrochemical processes are together controlled by electrochemical reaction and diffusion step. Equivalent circuit model is also proposed in Fig.6. In the equivalent circuit, R1 stands for the electrolyte resistance and Cdl stands for the capacity of the Prussian blue film. R2 stands for the resistance of the Prussian blue film and CPE stands for the constant phase element of interface between the electrode and electrolyte. R3 stands for the resistance between the liquid film of solid phase and solid phase main body. C stands for the electric double layer of the electrochemical reaction, R4 stands for the charge transfer resistance in the electrochemical process and W stands for the Warburg resistance of diffusion step. From Figs.4 and 5, it can also be concluded that PB modified carbon felt electrode can effectively decrease the charge transfer resistance and electrochemical reaction impedance, and this is the reason why the reversibility of carbon felt after PB modification is significantly improved.
Fig.6 Equivalent circuit
3.5 Charge-discharge circles performance of all vanadium redox flow battery
In order to demonstrate whether PB modified carbon felt electrode could be applied in VRB, the performance of a test battery employing V(Ⅳ)/V(Ⅴ) as anolyte active species and V(Ⅱ) /V(Ⅲ), which has a standard reduction potential of -0.255 V, as catholyte ones were evaluated with constant current charge-discharge experiments. In this experiment, the test batteries were divided into two types according to different positive electrodes. In sample group, PB modified carbon felt was employed as positive electrode and heat treated felt was selected as negative electrode. While in the control group, both positive and negative electrodes were heat treated carbon felt. Coulombic efficiency, voltage efficiency and energy efficiency of two types of all vanadium redox flow battery are shown in Table 3.
It can be seen from Table 3 that, when the current density is 20 mA/cm2, the coulombic efficiency of battery employing PB modified carbon felt electrode is not higher than that adopting heat treated felt. This is mainly because that the impact factors on the coulombic efficiency are side reaction and self-discharge due to the membrane penetration. The modification of PB can not effectively decrease the side reaction and membrane penetration. But the reversibility of PB modified carbon felt electrode is improved, therefore, the voltage efficiency is increased by about 12%. Accordingly, the energy efficiency is also improved obviously.
3.6 Mechanism study by electrochemical quartz crystal microbalance
Electrochemical quartz crystal microbalance (EQCM)[9-17] is a kind of method to prepare many processes relevant to the mass change, such as electrochemical crystallization, electrode modification, electrochemical biosensors. The principle of the electrochemical quartz crystal microbalance is Sauerbrey equation, which indicates that the increase of the minor mass could bring the obvious decrease of the resonance frequency.
Sauerbrey equation is displayed as follows:
Δf= -2.26×10-6αBf 2 ΔM/A=-ΔM (1)
where f stands for the base frequency and Δf stands for
Table 3 Charge-discharge performance of two types of all vanadium redox flow battery employing heat treated carbon felt electrode and PB modified carbon electrode

the frequency decrease; A is the quartz crystal electrode area and ΔM stands for the adsorbent mass; α stands for the surface number that the quartz crystal contact the liquid and B is the frequency distribution of the quartz crystal. The minus symbol indicates that with increasing mass, the frequency decreases.
Fig.7 shows the cyclic voltammograms and mass change on blank Pt crystal electrode. It can be concluded that, the oxidation and reduction peak is not obvious and the reversibility is poor. Furthermore, the values of the oxidation and reduction peak current have a large difference and the shape is lack of symmetry. As for the mass change, the auxiliary input of the Pt crystal electrode is only 10 mV, which indicates that the mass changes a little. The reason is that in the oxidation and reduction process, both reactant and product, VO2+ and VO2+ are soluble and the mass varies a little. Fig.8 shows the cyclic voltammograms and mass change on PB modified Pt crystal electrode. It can be concluded that the reversibility is improved significantly. Furthermore, the value of the oxidation is close to the reduction peak current and the shape is symmetrical. As for the mass change, the auxiliary input is about 600 mV, which indicates that the mass varies remarkably. Because the reactant and product, such as VO2+ and VO2+ are soluble, the mass change must be attributed to Prussian blue.
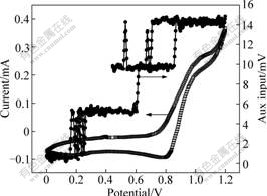
Fig.7 Cyclic voltammograms and mass change for 0.3 mol/L VOSO4 in 3 mol/L H2SO4 solution at scanning rate of 2 mV/s on blank Pt crystal electrode

Fig.8 Cyclic voltammograms and mass change for 0.3 mol/L VOSO4 in 3 mol/L H2SO4 solution at scanning rate of 2 mV/s on PB modified Pt crystal electrode
Therefore, some actions involving PB mass change could happen.
Refs.[9-13] reported the mechanism and the structure of Prussian blue in the potassium chloride solution. It is well-known that because of similar Pauling ionic radii, Ni, Cd, etc, can replace high-spin Fe ion of PB in an electrochemically induced substitution reaction. According to the above analysis based on the references, the Pauling ionic radius of VO2+ is 0.61 ?, which is close to the Pauling ionic radii of the Fe and Ni. The reaction of substitution of VO2+ for high-spin Fe ion of PB is probable to happen. In order to find the clear evidence of this substitution, the steady polarization curve of 0.3 mol/L VOSO4 in 3 mol/L H2SO4 is adopted and the corresponding mass change is also recorded as shown in Fig.9.
From above study of the references, it can be concluded that in the vanadium sulfate solution, the VO2+ can probably substitute the high-spin Fe ion in PB as follows:
![]() (2)
(2)
According to the above formula, VO2+ and Fe3+ are
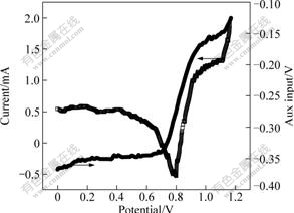
Fig.9 Steady polarization curve and mass change for 0.3 mol/L VOSO4 in 3 mol/L H2SO4 solution at scanning rate of 2 mV/s on PB modified Pt crystal electrode
soluble but (VO)x![]() and
and ![]() are insoluble. Because the relative molecular mass are different, the PB film mass will change with the process of the potential change. By the means of mass change before and after the potential scan process, x can be calculated and the degree of the substitution of VO2+ for Fe3+ can be evaluated quantitatively. The amount of VO2+ is more than
are insoluble. Because the relative molecular mass are different, the PB film mass will change with the process of the potential change. By the means of mass change before and after the potential scan process, x can be calculated and the degree of the substitution of VO2+ for Fe3+ can be evaluated quantitatively. The amount of VO2+ is more than ![]() , so all the
, so all the ![]() can be consumed. The relative molecular mass of the
can be consumed. The relative molecular mass of the ![]() is 860 and the relative molecular mass of the
is 860 and the relative molecular mass of the ![]() is 67x+56(4-x)+636. It is concluded that the mass change is 0.001 5 mg according to Fig.9. Then x is calculated to be 1.55. Therefore, in the potential scan process, the substitution of VO2+ for Fe3+can happen as follows:
is 67x+56(4-x)+636. It is concluded that the mass change is 0.001 5 mg according to Fig.9. Then x is calculated to be 1.55. Therefore, in the potential scan process, the substitution of VO2+ for Fe3+can happen as follows:
![]()
![]() (3)
(3)
1) Prussian blue modified carbon felt electrode was prepared and the electrochemical behavior was investigated by cyclic voltammetry, electrochemical impedance spectroscopy and charge-discharge experiments. The results of the cyclic voltammetry indicate that, the modification can improve the reversibility and the suitable PB deposition is the amount of 10 circles cyclic deposition. The results of the electrochemical impedance spectroscopy indicate that, PB modified carbon felt electrode can effectively decrease the charge transfer resistance. The voltage efficiency of VRB employing PB modified carbon felt electrode can be increased by 12%.
2) The results of EQCM show that, the mass change of the PB modified Pt crystal electrode in the process of the potential scan is obvious. The analysis of mass change demonstrates that the reaction of substitution of VO2+ for high-spin Fe ion in PB is probable to happen.
References[1] RYCHCIK M, SKALLAS K M. Evaluation of electrode materials for vanadium redox cell [J]. J Power Sources, 1987, 19(1): 45-54.
[2] PONCE C, FERRER A F, GARC?A J G, SZ?NTO D A, WALSH F C. Redox flow cells for energy conversion [J]. J Power Sources, 2006, 160(1): 716-732.
[3] ZHOU H, ZHANG H M, ZHAO P, YI B L. A comparative study of carbon felt and activated carbon based electrodes for sodium polysulfide/bromine redox flow battery [J]. Electrochim Acta, 2006, 51(28): 6304-6312.
[4] XUE F Q, WANG Y L, WANG W H, WANG X D. Investigation on the electrode process of the Mn(Ⅱ)/Mn(Ⅲ) couple in redox flow battery [J]. Electrochim Acta, 2008, 53(22): 6636-6642.
[5] FABJAN C, GARCHE J, HARRER B, JORISSEN L, KOLBECK C, PHILIPPI F, TOMAZIC G, WAGNER F. The vanadium redox- battery: an efficient storage unit for photovoltaic systems [J]. Electrochim Acta, 2001, 47(5): 825-831.
[6] TSUDA I, NOZAKI K, SAKUTA K, KUROKAWA K. Improvement of performance in redox flow batteries for PV systems [J].Solar Energy Mater Solar Cells, 1997, 47(1/4) : 101-107.
[7] LIU S Q, ZHANG W X, HUANG K L. Study on the electrochemical properties of carbon felt modified by PB and oxalic acid VRB application [J]. Chinese J Power Sources, 2006, 30 (5): 395-397. (in Chinese)
[8] LI H, YAN C W, TIAN B. Electrocatalysis of Prussian Blue for V(Ⅴ)/V(Ⅳ) in vanadium redox cell [J]. Chinese J Power Sources, 2004, 28 (3): 167-169. (in Chinese)
[9] BO A L, LIN X Q. Cyclic voltammetric and in situ Fourier transform infrared spectroelectrochemical studies on the reaction of Cd2+ on the Prussian blue/platinum modified electrode [J]. Chinese Journal of Analytical Chemistry, 1999, 27(4): 392-397. (in Chinese)
[10] DOSTAL A, MEYER B, SCHOLZ F. Electrochemical study of microcrystalline solid prussian blue particles mechanically attached to graphite and gold electrodes: electrochemically induced lattice reconstruction [J]. J Phys Chem, 1995, 99 (7): 2096-2103.
[11] DOSTAL A, SCHRODER U, SCHOLZ F. Electrochemistry of chromium(Ⅱ) hexacyanochromate(Ⅲ) and electrochemically induced isomerization of solid iron(Ⅱ) hexacyanochromate(Ⅲ) mechanically immobilized on the surface of a graphite electrode [J]. Inorg Chem, 1995, 34 (7): 1711-1717.
[12] DOSTAL A, HERMES M, SCHOLZ F. The formation of belayed nickel-iron, cadmium-iron and cadmium-silver hexacyanoferrates by an electrochemically driven insertion-substitution mechanism [J]. J Electroanalytical Chemistry, 1996, 415(1/2): 133-141.
[13] REDDY S J, DOSTAL A, SCHOLZ F. solid state electrochemical studies of mixed nickel- iron hexacyanoferrates with the help of abasive stripping voltammetry [J]. J Electroanalytical Chemistry, 1996, 403(1/2): 209-212.
[14] LIN C L, HO K C. A study on the deposition efficiency, porosity and redox behavior of Prussian blue thin films using an EQCM [J]. J Electroanalytical Chemistry, 2002, 524/525: 286-293.
[15] OGURA K, NAKAYAMA M, NAKAOKA K. Electrochemical quartz crystal microbalance and in situ infrared spectroscopic studies on the redox reaction of Prussian blue [J]. J Electroanalytical Chemistry, 1999, 474 (2): 101-106.
[16] LIAO H, WU X Q, ZHANG Z X. Study on the electrochemical behaviors of Prussian blue film using electrochemical quartz crystal microbalance [J]. Electrochemistry, 2004, 10(3): 293-297.
[17] OH I, LEE H, YANG H, KWAK J. Ion and water transports in prussian blue films investigated with electrochemical quartz crystal microbalance [J]. Electrochemistry Communications, 2001, 3(6): 274-280.
(Edited by LONG Huai-zhong)
Foundation item: Project (2008ZX07313-005) supported by the National Water Pollution Control and Management of Major Special Science and Technology Foundation
Corresponding author: XUE Fang-qin; Tel/Fax: +86-10-62797240; E-mail: xuefangqin@126.com


