
Synthesis and microstructure of zirconia nanopowders by glycothermal processing
Jun-Seop KIM1, Dong-Hae LEE1, Sung KANG1, Dong-Sik BAE1, Hoy-Yul PARK2, Moon-Kyeung NA2
1. School of Nano & Advanced Material Engineering, College of Engineering, Changwon National University,
Gyeongnam 641-773, Korea;
2. Advanced Materials and Application Research Laboratory, Korea Electro Technology Research Institute,
Changwon 641-600, Korea
Received 2 March 2009; accepted 30 May 2009
Abstract:
ZrO2 nanoparticles were prepared under high temperature and high pressure conditions by precipitation from metal nitrates with aqueous potassium hydroxide. The effects of synthesis parameters, such as the concentration of starting solution, pH of starting solution, reaction temperature and time, were discussed. The results show that the ZrO2 nanoparticles are obtained at 230-270 ℃. The average size and size distribution of the synthesized particles are below 10 nm and narrow, respectively. The XRD pattern shows that the synthesized particles are composed of crystalline. The synthesis of ZrO2 nanosized crystalline particles is possible under glycothermal conditions in ethylene glycol solution.
Key words:
ZrO2 nanoparticles; glycothermal processing; particle size; microstructure;
1 Introduction
Today, one of the most rapidly developing fields of knowledge is nanotechnology. One of the parts of this broad field is nanomaterials, i.e. materials with one or more dimensions in the range of 1-100 nm (powders, layers or bulk materials). Properties of such materials can be significantly different from the behaviour of the ordinary ones[1]. Inorganic nanopowders are among the most important factors in many fields of materials such as ceramics, catalysts, medicines and food[2].
Zirconia is an important material because of its use in different fields of chemistry such as ceramics and catalysis[3]. Nano-zirconia ceramics are of great interest for their obvious improvement in strength and toughness[4], which change mechanical property, electrical performance, thermal performance, magnetic performance and optical performance of ceramic components[5]. Furthermore, nano-zirconia has interesting catalytic actions for isomerization of olefins [6] and epoxides[7], dehydration of alcohols[8-9], and so on[10-11]. Practical synthesis methods are still under development in order to obtain zirconia with controlled particle size and general properties. A common synthesis route consists of the calcinations of the hydrous zirconia obtained from hydrolysis of zirconium salts in various media. It was found that the properties of zirconia obtained by hydrolysis depend on several chemical parameters[3]. CHUAH et al[12-14] reported that the reaction time and temperature and the pH of reaction medium are important factors that determine the surface area of zirconia. The influence of reaction is explained in terms of the enhanced agglomeration of primary particles during reaction and the strengthening of the network structure. This led to higher thermal stabilities[3].
Many different methods of producing nanosize powders were described in the literature, such as sol-gel processing, hydrothermal processing, and ion exchange resin manufacture methods[15]. Among these methods listed above, hydrothermal synthesis meets the increasing demand for the direct preparation of crystalline ceramic powders and offers a low temperature alternative to conventional powder synthesis technique in the production of anhydrous oxide powders. This technique can produce fine, high purity and stoichiometric particles of single and multi-component metal oxides[16]. Furthermore, if the process conditions such as solution pH, solute concentration, reaction temperature, reaction time, seed materials, and the type of solvent are carefully controlled, the ceramic particles with desired shape and size can be produced[17].
The objective of this study is to prepare the nanosized zirconia powders by a glycothermal process, and to investigate the effects of the processing conditions on the formation, morphology, and phase of the powders.
2 Experimental
The preparation flow of zirconia powder is schematically illustrated in Fig.1. Zirconia precursors were precipitated from 0.1 mol/L ZrCl2O?8H2O solution by slowly adding NH4OH water with rapid stirring, in which the pH of starting solutions varied between 7 and 11. The precipitated zirconia precursors were washed by respected cycles of centrifugation and re-dispersion in water. Washing was performed for a minimum of five times in ethanol. Excess solution was decanted after the final washing and the wet precursor was re-dispersed in 200 mL ethylene glycol under vigorous stirring. The resulting suspension was placed in a 1 L stainless steel pressure vessel. The vessel was then heated to 270 ℃ at a rate of 10 ℃/min for 6 h. The characterization of the synthesized particles was observed using transmission electron microscope (TEM, JEM 2100F), X-ray diffractometer (XRD, X’pert MPD 3040), and thermal analyzer system(TA 5000/SDT 2960).
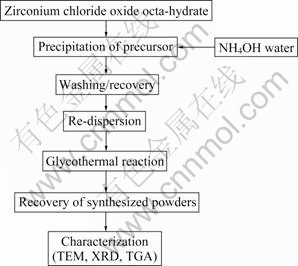
Fig.1 Experimental flow chart of synthesized zirconia powders by glycothermal processing
3 Results and discussion
Glycothermal processing conditions such as pH, reaction temperature and time, have significant effects on the formation, phase component, morphology and particle size of zirconia powders. Fig.2 shows the TEM images of the synthesized particles at different pH values. The average size of the synthesized particles increases with increasing pH value. But it is difficult to distinguish the average size of the synthesized particles at different pH values. It can be seen from Fig.2 that the average size of the synthesized zirconia powders is in the range of 5-10 nm. And with increasing pH, the synthesized particles are aggregated.
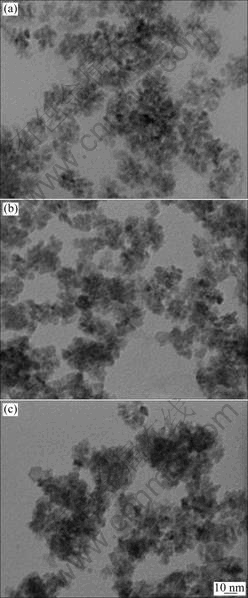
Fig.2 TEM images of synthesized zirconia powders at different pH values: (a) pH=7; (b) pH=9; (c) pH=11
Fig.3 and Fig.4 show the XRD patterns of the synthesized particles by glycothermal processing. Fig.3 shows the XRD patterns of the synthesized powders reacted at 230 ℃ for 6 h with different pH values. The crystalline phase of the synthesized powders is amorphous. As shown in Fig.4, when the reaction temperature increases from 230 ℃ to 270 ℃, the XRD patterns show that the synthesized particles have a crystalline phase. Fig.5 shows the TG-DTA curves of the synthesized particles with pH 9. Except for a water/ solvent vaporization peak(endothermic) between room temperature and 140 ℃, the synthesized particles result in a sharp exothermic peak at 300 ℃, which corresponds to the crystallization of zirconia[18-20].
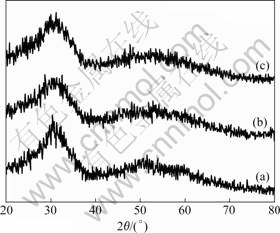
Fig.3 XRD patterns of synthesized zirconia powders reacted at 230 ℃ with different pH values: (a) pH=7; (b) pH=9; (c) pH=11
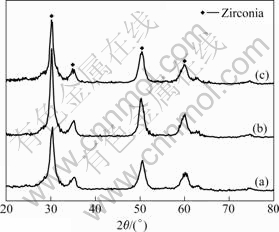
Fig.4 XRD patterns of synthesized zirconia powders reacted at 270 ℃ with different pH values: (a) pH=7; (b) pH=9; (c) pH=11
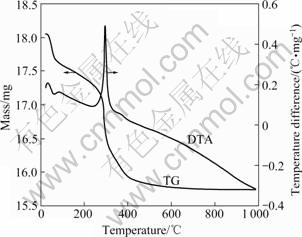
Fig.5 TG-DTA curves of synthesized zirconia powders reacted at 270 ℃ for 6 h(pH=9)
4 Conclusions
1) Zirconia nanoparticles were obtained in ethylene glycol solution reacted (glycothermal processing) at 270 ℃ for 6 h.
2) The average particle size and distribution of the synthesized zirconia powders are in the range of 5-10 nm and narrow, respectively.
3) With increasing pH, all samples are aggregated.
4) The crystalline phase of the synthesized powders is zirconia.
5) Crystallization and thermal behavior of synthesized powders were investigated by XRD/TG- DTA methods.
Acknowledgment
This research was financially supported by the Ministry of Education, Science Technology (MEST) and Korea Industrial Technology Foundation (KOTEF) through the Human Resource Training Project for Regional Innovation.
References
[1] ZYCH ?, HABERKO K. Some observations on filter pressing and sintering of yttria-stabilized zirconia nanopowder [J]. European Ceramic Society, 2007, 27: 867-871.
[2] SOMIYA S, ROY R. Hydrothermal synthesis of fine oxide powders [J]. Bull Mater Sci, 2000, 23(6): 453-460.
[3] INOUE M, SATO K, NAKAMURA T, INUI T. Glycothermal synthesis of zirconia-rare earth oxide solid solutions [J]. Catal Lett, 2000, 65: 79-83.
[4] UHLMANN E, SPUR G. Surface formation in creep feed grinding of advanced ceramics with and without ultrasonic assistance [J]. CIRP Annals-Manufacturing Technology, 1998, 47(1): 1, 249-252.
[5] GAO G F, ZHAO B, XIANG D H, KONG Q H. Research on the surface characteristics in ultrasonic grinding nano-zirconia ceramics [J]. Materials Processing Technology, 2009, 209: 32-37.
[6] NAKANO Y, IIZUKA T, HATTORI H, TANABE K. Surface properties of zirconium oxide and its catalytic activity for isomerization of 1-butene [J]. Catal, 1979, 57: 1-10.
[7] ARATA K, KATO K, TANABE K. Epoxide rearrangement (Ⅳ): Isomerization of cyclohexene and 1-methyl-cyclohexene oxides over solid acids and bases in gas phase bull [J]. Chem Soc Jpn, 1976, 49: 563-564.
[8] YAMAGUCHI T, SASAKI H, TANABE K. High selectivities of zirconium oxide catalyst for isomerization of 1-butene and dehydration of sec-butanol [J]. Chem Lett, 1973, 9: 1017-1018.
[9] DAVIS B H, GANESAN P. Catalytic conversion of alcohols Ⅱ: Influence of preparation and pretreatment on the selectivity of zirconia [J]. Ind Eng Chem Prod Res Dev, 1979, 18: 191-198.
[10] XU B Q, SETOYAMA T, TANABE K. Characteristic action of zirconium dioxide in the decomposition of alkylamines [J]. Appl Catal, 1990, 64: 41-45.
[11] FENG Z, POSTULA W S, PHILIP C V, AKGERMAN A, ANTHONY R G. Selective formation of isobutane and isobutene from synthesis gas over zirconia catalysts prepared by a modified sol-gel method [J]. Catal, 1994, 148: 84-90.
[12] CHUAH G K, JAENICKE S, CHEANG S A, CHAN K S. The influence of preparation conditions on the surface area of zirconia [J]. Appl Catal, 1996, A145: 267-284.
[13] CHUAH G K, JAENICKE S, PONG B K. The preparation of high-surface-area zirconia: Ⅱ. Influence of precipitating agent and digestion on the morphology and microstructure of hydrous zirconia [J]. Catal, 1998, 175: 80-92.
[14] CHUAH G K, JAENICKE S. The preparation of high surface area zirconia—Influence of precipitating agent and digestion [J]. Applied Catalysis A—General, 1997, 163: 261-273.
[15] BAE D S, KIM E J, PARK S W, HAN K S. Synthesis and characterization of nanosized ZnxMn1-xFe2O4 powders by glycothermal process [J]. Mater Sci Foru, 2005, 486: 436-439.
[16] BAE D S, HAN K S, CHO S B, CHOI S H. Synthesis and characterization of the ultrafine ZnFe2O4 powder by glycothermal [J]. Kor Assoi Crys Grow, 1997, 1: 167-173.
[17] CHO S B, VENIGALLA S, ADAIR J H. Morphological control of α-Al2O3 in 1,4-butanediol solution [C]// Science, Technology, and Applications of Colloidal Suspensions. Westerville, OH: American Ceramic Society, 1995: 139-150..
[18] ZENG H C, QUIN M. Diffusion of transition metals (Co, Ni) and its effects on sol–gel derived ZrO2 polymorphic stabilities [J]. Mater Chem, 1996, 6: 435-442.
[19] ZENG H C, TUNGS K. Synthesis of lithium niobate gels using a metal alkoxide-metal nitrate precursor [J]. Chem Mater, 1996, 8: 2667-2672.
[20] ZHAN Z, ZENG H C. Metastability of tetragonal ZrO2 derived from Zr-n-propoxide-acetylacetone-water-isopropyl alcohol [J]. J Mat Res, 1998, 13: 2174-218.
Corresponding author: Dong-Sik BAE; Tel: +82-55-213-3715; E-mail: dsbae7@changwon.ac.kr
(Edited by LI Xiang-qun)


