
Preparation and properties of scintillating glass doped with organic activators
ZHU Dong-mei (朱冬梅), LUO Fa (罗 发), ZHAO Hong-sheng (赵宏生), ZHOU Wan-cheng (周万城)
State Key Laboratory of Solidification Processing, Northwestern Polytechnical University, Xi’an 710072, China
Received 10 April 2006; accepted 25 April 2006
Abstract:
A series of scintillating glasses were developed by doping organic activators into low melting temperature glasses according to different ratios. The fluorescence spectra and the transmission spectra of some scintillating glasses were explored and the actual concentration organic in scintillating glass was estimated. The results show that it is feasible to prepare the scintillating glass by doing organic scintillating activators into the low-melting glasses. There are two main reasons for the weak optical properties of the scintillation glasses: one is that the actual concentration of organic activators doped in the glasses is very low, and the other is the existence of lots of defects formed in the scintillating glasses due to the evaporation of organic activator, lowering the transmission of glasses. The fluorescence emission peaks of the glasses move to a longer wavelength compared with those in organic matrixes. To increase the light output of the glass, the optical transmittance of the glasses must be improved and the concentration of activators in the glasses must be increased.
Key words:
scintillating glass; organic activator; doping;
1 Introduction
Nowadays, more and more attention is paid to the preparation of scintillators with high density, short decay time, good radiation hardness, and low cost[1-9]. NOGUES et al[4] developed a fast, radiation-hard scintillating silica glass through sol-gel method, providing a new idea doping organic materials in glass to prepare the scintillating glass. The development of low-melting[2] glass makes it possible to dope organic scintillating materials into bulk inorganic glass. The following investigations[5-7] of doping organic laser-dye in the lead-tin-fluorophosphate glass are also the evidence that organic materials can be doped into the glass.
In this study, a series of scintillating glasses are developed using low-melting tin-fluorophosphate glass as the matrix through doping orgnic activators p-TP and/or POPOP (1,4-bis(5-phenyloxazol-2-yl) benzene).( p-TP and POPOP are often used as the activators in scintillating plastic and POPO P is believed to be the wavelength shifter in the plastic.[8,9])The fluorescence spectra, transmittance of scintillating glass are explored and the actual concentration of organic material in glass is estimated.
2 Experimental
The raw materials were mixed well according to a certain ratio and melted in an alumina crucible at 500 ℃ for 30 min. Then took out the crucible and introduced the organic activator in the melt at about 300 ℃. After stirred the melt for a few minute, poured out the melt in a carbon steel mold and quenched the sample at 10 ℃ above the glass transition temperature (tg).
The transmission spectra of samples are studied in ultraviolet and visible region by using a UV-VIS spectrophotometer with the error bar of 0.3% in transmission and ±0.3 nm in wavelength. The fluorescence spectra are measured with a Hitachi-850 fluorescence spectrophotometer. The concentration of organic activator doped in the glass is determined by comparing the intensity of fluorescence spectrum of standard solution and the solution dissolving the glass
using same solvent. The sample to measure is crushed into powder first and then dissolved in the dense muriatic acid completely. Then the solution is extracted using chloroform and the fluorescence spectrum of the extracted solution is measured and compared with the standard chloroform solution to estimate the concentration of organic activator in scintillating glass.
3 Results and discussion
Table 1 lists the appearance, density, and glass transition temperature (tg) of the undoped glass and doped glass. The results show that the density of the undoped glass is about 3.31 g/cm3, glass transition temperature is about 96 ℃, which is consistent with the data reported in Ref.[10]. We can also see that the density, appearance, and glass transition temperature of doped glass are, essentially, the same as those of the undoped glass, indicating that the doping of organic activators does not bring obvious change on these properties of glasses.
Table 1 Appearance, density, and glass transition temperature of some glasses
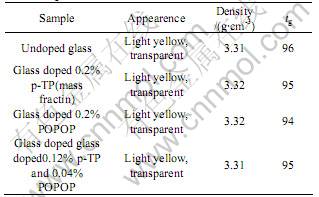
Fig.1 shows the fluorescence peak of glass doped with 0.2% POPOP. Table 2 lists the absorption peak and emission peak of POPOP in different matrix. The absorption peak of the glass is at about 367 nm, as shown in Fig.1, which is almost the same as that of toluene solution dissolving some POPOP shown in Table 1. There are two peaks in the emission spectrum at 420nm and 540 nm, respectively. The peak at 420 nm is very close to that of pure POPOP (Table 2) and its fluorescence intensity is weaker than that of the peak at 540 nm. The Stoke shift between the absorption and

Fig.1 Fluorescence spectra of glass doped with 0.2%POPOP
emission spectra is about 177 nm.
The fluorescence spectra of glass doped with 0.2% p-TP are given in Fig.2. It can be found that the fluorescent intensity of absorption and emission spectra of this glass are much weaker than that of glass doped with 0.2% POPOP. Two peaks appear in the absorption spectrum of the glass: one is at 290 nm with very weak fluorescence intensity, while the other peak is at 355 nm with relatively strong fluorescence intensity. There is only one peak for the emission spectrum (about 610 nm). It can be seen that the glass exhibits a large STOKES shift (about 255 nm) between the absorption spectrum and emission peak. Compared with the emission peak of p-TP in SiO2 glass[4] prepared by sol-gel method (about 360 nm) and that in polythene plastic (about 340 nm)[8], the emission peak of sample 2 move obviously toward the longer wavelength.

Fig.2 Fluorescence spectra of glass doped with 0.2%p-TP
Table 2 Emission maximum ,absorption maximum and decay time of POPOP in different matrixes

Fig.3 gives the fluorescence spectra of glass doped with 0.12% p-TP and 0.04% POPOP. There are two peaks for the absorption spectrum. One peak (about 290 nm) is very close to the first absorption peak of glass only doped with 0.2% p-TP, therefore, it is believed that a certain amount of p-TP exists in the glass. The other peak at about 370 nm, which is almost the same as the only absorption peak of glass only doped with 0.2% POPOP, maybe corresponds to the existence of POPOP in the glass. It can also be seen that the emission spectrum is almost the same as that of glass only doped POPOP, as shown in Fig.1. Although the actual concentrations of the organic activators are unknown because of the evaporation of organics during preparation of sample. It is believed that there are some p-TP and POPOP doped in the glass, which produce the fluorescence together when excited. POPOP is not the wavelength shifter in the glass any more, because the emission peak of p-TP and POPOP both move toward the longer wavelength in the scintillating glass compared to that of p-TP and POPOP in scintillating plastic.
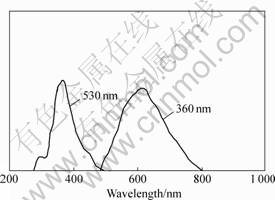
Fig.3 Fluorescence spectra of glass doped with 0.12% p-TP and 0.04% POPOP
In summary, all the scintillating glass doped with organic activators p-TP and POPOP exhibit a relatively large Stoke shift between absorption and emission spectra. And the strongest absorption and emission peak for each glass both shift to longer wavelength, which is attributed to the interaction between the organics and matrix glass [10,11].
The transmission spectrum of one doped glass was measured and given in Fig.4. The UV-cutoff wavelength of the glass is at about 318 nm. The result shows that the glass has relatively weak transmission property. The transmittance of the glass is very low and can only reach about 50% at 500 nm. It is mainly due to the existence of defects in the sample, most of which are bubbles formed during preparation of sample. Although the organic activators were doped in the glass at low temperature, there is still some amount of organics evaporated during doping. The evaporated organics will form bubbles in the melt and some of them will be embedded in the glass after quenching. The existence of these defects not only decreases the transmittance of glasses, but also weakens the other optical properties of glass, such as fluorescence intensity and light yield of scintillation glasses. Therefore, how to avoid the evaporation of organics and the formation of defects are the most important points in the following investigation.
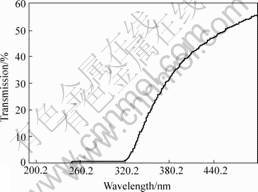
Fig.4 Transmission spectrum of glass doped with 0.2% p-TP
Fig.5 gives the comparison of emission spectra for extracted solution C from glass doped with POPOP and two standard solutions A and C. The concentration of POPOP in standard solution A is 10-5 mol/L and that in solution C is 10-6 mol/L. As shown in Fig.5, the concentration of POPOP in solution does not change the location of emission peak in the spectra, but the intensity of fluorescence. The fluorescence intensity of extracted solution is between those of standard solution, indicating that the concentration of POPOP in the extracted solution is between those of standard solution. The calculated results show that the concentration of POPOP in extracted solution is about 1.7?10-6 mol/L, and the calculated volume concentration of POPOP in glass is about 1.1?1018 molecules/cm3, means that there are about 1.1?1018 molecules doped in every cubic-centimeters scintillating glass. Although the concentration is lower than that reported in Ref.[11], it is in the same order as that reported. Compared with the concentration of organic activators in scintillating plastic[8], the actual concentration of organic in scintillating glass is much lower and the lower concentration of organics in glass

Fig.5 Comparison of emission spectra for extracted solution (B) from sample 5 and standard solutions A (10-5 mol/L) and C (10-6 mol/L)
will weaken the ability of glass to yield light. Therefore, how to increase the concentration of organic activators in the scintillating glass is the second key point to prepare scintillating glass with better properties.
4 ConclusionsIt is feasible to prepare the scintillating glass by doping organic activators into low melting glass. Compared with the scintillating plastic, the emission peak of scintillation glass doped with organics moved toward the longer wavelength. POPOP in the glass is not the wavelength shifter any more and produces fluorescence directly. Due to the evaporation of organics during preparation of glasses, however, the actual concentration of organic activators in glass is very low, which is the main reason for the weak optical properties of the as-obtained glasses. To get better scintillating glass, it is critical to increase the concentration of organics in glass and avoid the evaporation of organics during preparation of glasses.
References
[1] TICK P A. Water durable glasses with ultra low melting tmperature[J]. Physics and Chemistry of Glasses, 1984, 25(6): 149-154.
[2] IM H J, WILLIS C, SAENGKERDSUB S. Scintillators for alpha and neutron radiations synthesized by room temperature sol-gel processing [J]. Journal of Sol-Gel Science and Technology, 2004, 32 (1-3): 117-123.
[3] RODOVA A, CIHLAR A, KNIZEK K. Preparation and properties of Ce-doped Na-Gd phosphate glasses[J]. Radiation Measurements, 2004,38 (4-6): 489-492.
[4] NOGUES J L, MAJEWSKI S, WALKER J K. Fast, radiation-hard scintillating detector: A potential application for sol-gel glass[J]. J Am Ceram Soc, 1988, 71(12): 1159-63.
[5] TOMPKIN W R, BOYD R W, HALL D W, TICK P A. Nonlinear-optical properties of lead tin fluorophospate glass containing acridine dyes[J]. J Opt Soc Am, 1987, 4(6)
[6] GAPOINENKO S V, GRIBKOVSKII V P, .ZIMIN L G. Nonlinear phenomena of acridine orange in inorganic glass at nanosecond scale[J]. Optical Materials, 1993, 104(12): 53-58.
[7] ZHAO H, ZHOU W, ZHU D,WU J. Synthesis and characterization of low melting scintillating glass doped with organic activator[J]. Nuclear Instruments and Methods in Physics Research A,2000, 448(3): 39-42.
[8] AMBROSIO C D, LEUTZ H, TAILHARDAT S, TAUFER M. Organic scintillators with large stokes shifts dissolved in polystyrene[J]. Nuclear Instruments and Methods in Physics Research, 1991, A307: 430-435.
[9] BROSS A D, PLA-DALMAU A. New fluorescent compounds for plastic scintillator applicatins, nuclear instruments and methods in physics research[J]. 1993, A325: 168-175.
[10] XU X J, DAY D E. Properties and structure of Sn-P-O-F glasses[J]. Phys Chem Glasses, 1990, 31 (5): 183-187.
[11] HE K X, BRUANT W, VENKATESWARLU P. Nonlinear optical effects on the surface of acridine yellow-doped lead-tin- fluorophosphate glasses[J]. Appl Phys Lett, 1991, 59(16): 1935-1937.
Corresponding author: ZHU Dong-mei, PhD; Tel: +86-29-88494574; E-mail: dzhunwpu@nwpu.edu.cn


