J. Cent. South Univ. Technol. (2009) 16: 0931-0935
DOI: 10.1007/s11771-009-0155-7
![]()
Chemical component and antimicrobial activity of volatile oil of
Calycopteris floribunda
LIU Jia-jia(刘佳佳)1, YANG Dong-liang(杨栋梁)1, ZHANG Yan(张 艳)1, YUAN Yao(袁 遥)1,
CAO Fu-xiang(曹福祥)2, ZHAO Jian-ming(赵建明)3, PENG Xiao-bo(彭潇波)1
(1. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. School of Biotechnology, Central South University of Forestry and Technology, Changsha 410004, China;
3. Botanical Garden of Rare and Endangered Plants, Ruili 678600, China)
Abstract:
The volatile oil of leaves and barks of Calycopteris floribunda was examined by gas chromatography-mass spectrometry (GC-MS). 52 volatile chemical components in leaves were identified. The antimicrobial assay of oils in the leaves and barks was carried out by disk diffusion method in vitro. The major components (mass fraction) in leaves are caryophyllene oxide (13.79%), n-hexadecanoic acid (11.91%) and β-caryophyllene (10.45%). Ten constituents are identified accounting for about 99.98% of the total volatile oil in the bark. Among these components, n-hexadecanoic acid (59.18%), linolic acid (12.70%) and butyl octyl phthalate (8.21%) are the major constituents. The oils exhibit strong antimicrobial activity and display more potent against bacteria than fungi.
Key words:
Calycopteris floribunda; volatile oil; antimicrobial activity; GC-MS;
1 Introduction
The traditional Asian medicinal plant C. floribunda, the single member of Calycopteris Lam. included in Combretaceae, is an endemic evergreen liana species to Asian tropic mountains. It only occurs in ecosystems typical of the highlands and Indian Ocean warm current of west Yunnan Province that is characterized by acidic lateritic soil and Shorea assamica Dyer vegetation. C. floribunda has usage in the Asian traditional medicine including Ayurveda, folk and Unani medicine. Several therapeutic properties are assigned to Combretaceae species, such as invigorating the spleen, piperazine citrate, anti-enterobiasis, and dispeling the stasis. The extract of stem-leaf is used in dysentery, fever, emesis and roborans tonic. The fruit is used in the treatment of jaundice and relieving pruritus[1-2]. Moreover, since this plant is one of the endangered plants in China, it has significant value in Chinese flora and medical study[3].
Rabbits and calves fed with fresh C. floribunda leaves showed morbidity and mortality with premonitory clinical signs like depression, downer status, and polyuria[4]. The phytochemical screening revealed the presence of flavonoids, alkaloids, tannins and saponins[5]. 6″-demethoxyneocalycopterone, calyflo- renone C, 6″-epi-calyflorenone B, 6″-epicalyflorenone C, calyflorenone D[6], Neocalycopterone, methyl-neocaly- copterone, calyflorenones A, calyflorenones B, penduletin[7], calycopterone, isocalycopterone, 4- demethylcalycopterone[8] and Calycopterin[9] were isolated from organic extract of C. floribunda leaves. Calycopterone, isocalycopterone and 4-demethyl- calycopterone showed a wide range activity against solid tumor cell lines[8]. The main flavanoid Calycopterin[9] has anthelmintic, antiviral(inhibition of polio virus in vitro)[6] and anticancer activity (anti-proliferative and anti-aromatase)[8, 10].
Except the previous reports of phenolic biflavonoid compounds like calycopterone, calyflore-none and neocalycopterone in ethanol extract[7-8], nearly no published information is available on the phytochemistry of C. floribunda. To provide information for further utilization of this plant, gas chromatography-mass spectrometry (GC-MS) and peak-area-normalization method were applied to analyzing the chemical ingredient of its volatile oils. The pure gas chromatography spectra and mass spectra were obtained according to the retention time and MS, and the similar searching and qualitative analysis were carried out and then the method of peak-area-normalization was used for quantitative analysis. Additionally, the antibacterial and antifungal activities of volatile oils were studied by disk diffusion method in vitro as well.
2 Experimental
2.1 Sample extraction
The leaves and barks of C. floribunda were collected from Yingjiang County, Yunnan Province, China, and the sample was authenticated by ZHAO Jian-ming of the Botanical Garden of Rare and Endangered Plants in Ruili. The volatile oil was extracted respectively from 50 g of previously air-dried and powdered (particle size 0.83 mm) leaves and barks. The extraction was carried out by steam distillation in Clevenger apparatus for 5 h. The oil obtained was taken with 1 mL hexane and the solutions were stored in a freezer for GC-MS analysis and biological test.
2.2 Gas chromatography-mass spectrometry analysis
Analysis of the oil involved injection of 1 ?L of the hexane solution to a gas chromatograph HP 5890 ser. II plus coupled with a mass spectrometer HP 5989B station operating in the electron impact mode at 70 eV, electron multiplier energy 1.6 kV and ion source temperature 200 ℃. For gas chromatographic analysis, a HP-5MS capillary column of fused silica (30 m×0.30 mm) and helium as carrier gas at a flow rate of 1.0 mL/min were used. Temperatures of the column were programmed starting at 100 ℃ for 1 min and raising to 280 ℃ at a rate of 8 ℃/min. Identification of the compounds was based on comparison of the corresponding mass spectra with data from the libraries Wiley-275 and Wiley/NBS. Relative contents of the constituents in the volatile oil were assumed to be proportional to the areas under the corresponding chromatogram peaks.
2.3 Antimicrobial assay
2.3.1Bacterial and fungal strains
Antimicrobial efficacy assay of the volatile oil was carried out in pestri dishes by disk diffussion method. Four pathogenic bacteria: Staphylococcus aureus, Bacillus subtilis (gram-positive), Escherichia coli, Pseudomonas aeruginosa (gram-negative) and four pathogenic fungi: Candida albicans, Trichophyton rubrum, Aspergillus niger, Penicillium citrinum were used as test organisms, with each representing a major order of pathogen. All the microbial strains were provided by Institute of Microbial Technology, Central South University, China.
2.3.2Antimicrobial investigations
Bacteria were cultured in Mueller-Hinton agar culture medium and incubated in biochemical incubator at 37 ℃. Fungi were cultured in potato dextrose agar culture medium and incubated in diurnal growth incubator at 28 ℃.
Stock cultures were preserved at 4 ℃ on the nutrient agar slant. Active cultures for experiments were prepared by transferring one loopful of cells from stock cultures to fresh nutrient agar medium and incubated without agitation for 24 h respectively. The microbial strains were subcultured three times, and transferred one loopful of the third subcultured cells from solid medium to liquid medium, then shaken for 24 h. The cultures were diluted to 106-107 mL-1 colony forming units by sterilized water.
In order to determine the antimicrobial activity of the volatile oils, disk diffusion method was carried out. Dilutions of the inoculant were cultured on solid agar medium by spreading 0.1 mL inoculum suspension uniformly. Sterilized paper discs (6 mm in diameter) soaked with the required doses of undiluted volatile oil (1, 2, 4 and 6 ?L) were placed on the surface of inoculated plates. The plates were incubated at 37 ℃ for 24 h (for bacteria) and at 28 ℃ for 48 h (for fungi) respectively. The diameters of the inhibition zones were then measured by vernier calliper.
3 Results and discussion
3.1 Chemical analysis
The oil obtained from leaves and barks of C. floribunda were quantitatively analyzed by GC-MS[11]. The total ion current chromatograms of the volatile oil from C. floribunda’s leaves and barks are shown in Figs.1 and 2, respectively. Additionally, the main constituents of the studied volatile oil and relative contents are listed in Tables 1 and 2, respectively.
The leaves produced green-yellow oil with a high yield (1.11%, on dry mass basis). 52 components that account for 96.43% of the chromatographied oil were clearly identified. The oil is composed of 71.72% of sesquiterpenes while the rest are mainly monoterpenes. The dominant components in the oil from leaves are
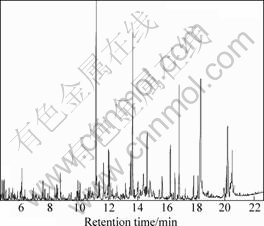
Fig.1 Total ion current chromatogram of volatile oil from leaves of C. floribunda
caryophyllene oxide (13.79%), n-hexadecanoic acid (11.91%) and β-caryophyllene (10.45%).
The barks produced yellowish oil in a yield of 0.60% (on dry mass basis). Ten constituents were identified, accounting for 99.98% of the total oil. Among them, 91.41% constituents belong to the class of sesquiterpenes. The major constituents are n-hexadecanoic acid (59.18%), linolic acid (12.70%) and butyl octyl phthalate (8.21%).
It seems that the composition of the volatile oil from the leaves is more complex than that from the barks with 52 components in the leaves but 10 components in the latter. That is partially due to their different biosynthetic paths and living environment such as light illumination, temperature and osmotic pressure. Volatile oils of leaves and barks are somewhat similar in compositions, that is,
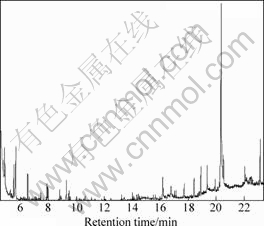
Fig.2 Total ion current chromatogram of volatile oil from barks of C. floribunda
Table 1 Analysis of volatile oil from leaves of C. floribunda
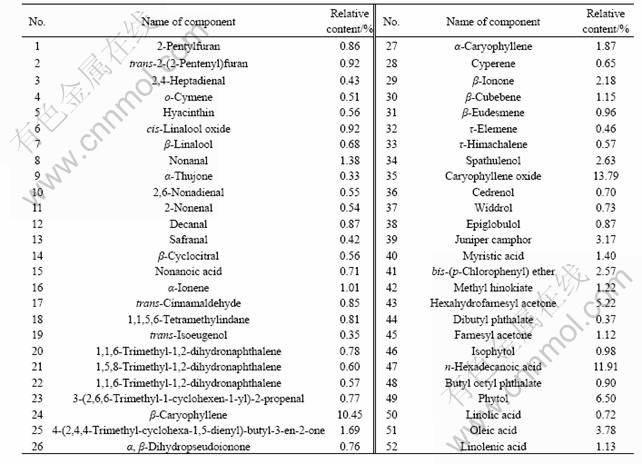
Table 2 Analysis of volatile oil from barks of C. floribunda
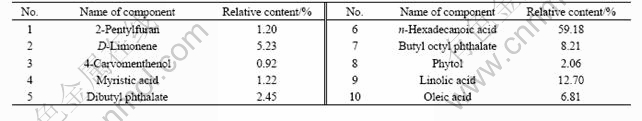
the contents of n-hexadecanoic acid are high in both volatile oils, especially in bark oil. Moreover, the occurrence of sesquiterpenes as the predominant volatile oil constituents is another chemical characteristic of C. floribunda. Anyway, the volatile oil of leaves differs from that of the barks in its high content of caryophyllenes.
The main constituents in the volatile oil are sesquiterpenes and monoterpenes. Since terpene is an important parental hydrocarbon of some drugs that have antivirus, anti inflammatory and antitumor property[12], cytotoxic nature of these ingredients may be responsible for cell specific damage observed in vital organs like kidney, cardiac, and nervous system with the manifestation of related disturbances such as ataxia, forelimb paresis, cachexia and polyuria[8].
The constituent difference between the two kinds of volatile oils provides some information for medical, genetic, and botanical research of C. floribunda. So far, due to the particularity of C. floribunda, its certain taxon in combretaceae has been investigated by some researchers. In fact, GOUTSIOU et al[13] showed that chemical profiles in each population are under genetic control, which strengthens HARBORNE and TURNER’s point that volatile terpenoids may be useful for studies of plant population structure[14]. The above comments of similarities and differences between volatile oils of leave and bark as well as taxonomic potentialities need to be taken with caution, because the possibility of infraspecific variation is often noted in distributions of volatile terpenoids. This is in favor of further investigateing the chemotaxonomic occurrence of C. floribunda.
3.2 Antimicrobial activities
The volatile oils of the leave and bark of C. floribunda were further studied regarding some of their biological activities. Both kinds of oils were tested for bactericidal and fungicidal effects. The results are shown in Table 3.
In general, the oils exhibite strong antimicrobial activity. It is notable that a complete inhibition against Candida albicans of leaves oil was observed only at 1 ?L, and an obvious inhibition against Staphylococcus aureus, Bacillus subtilis, Escherichia coli and Trichophyton rubrum was observed at 2 ?L. But it was found to have no suppression against Penicillium citrinum. Though the bark oil showed inhibition against all the tested microbial strains, it occurred only when the content of oil was high; additionally, no inhibition was obtained against the growth of Trichophyton rubrum and Aspergillus niger.
The leave oil exhibited higher inhibition activity against most microbes and broader antibacterial spectrum than those of the bark oil. Both kinds of volatile oils exhibited stronger acitivity against the tested bacteria than the fungi.
Concerning the biological activities of essential oil of C. floribunda, some of the sesquiterpenes found in leave oil, such as caryophyllene oxide, showed to have anti-fungal, as well as insecticide and antifeedant activities[15]. Although detected in minor amounts, the terpenes: o-cymene, β-linalool, safranal, β-cyclocitral, α-ionene, cyperene, β-cubebene, β-eudesmene, τ-elemene, τ-himachalene, spathulenol, cedrenol, epiglobulol and myristic acid, were exclusively found in leave oil. At present, however, the mode of action of terpenic constituents on microorganisms is not fully understood. In view of their hydrophobilicity, it is generally considered that they are involved in such mechanisms as cytoplasmic membrane disturbance, coagulation of cell contents and disruption of the proton motive force[16]. Based on the literature reports, spathulenol acts as cytotoxic, and cymene and safranal are well known chemicals with pronounced antimicrobial effects[17]. Additionally, the minor compounds in the
Table 3 Antimicrobial activities of leave and bark volatile oils of C. floribunda
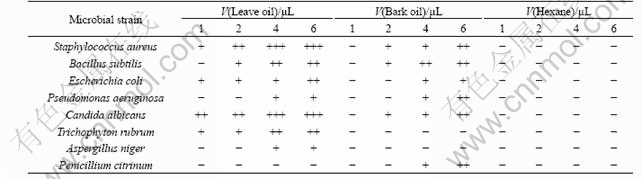
Note: Antimicrobial activities were designated: not detected (-) for diameters less than 6 mm; little sensitive (+) for diameters 8-10 mm; sensitive (++) for diameters 10-15 mm; very sensitive (+++) for diameters more than 15 mm. All experiments were performed in triplicate.
oils might also play a critical role in antimicrobial activity, possibly by producing a synergistic effect with other components.
Since the volatile oil from C. floribunda leaves has strong suppression activity against various pathogens such as Candida albicans, it may be the protective substance in plant. So the elucidation of active constituents in the volatile oil may provide useful lead to the development of new and effective antimicrobial agents. In order to find an alternative approach for discovery of medicinal components, further studies are needed for more extensive evaluations of the biological properties of the volatile oils of C. floribunda.
4 Conclusions
(1) The volatile oils of the leaves and barks of C. floribunda are extracted by steam distillation, analyzed by GC-MS. The comparison between their composition and relative contents is made. Leaves produce volatile oil with a higher yield than barks. More volatile fragrant substances with different properties and antimicrobial effects are favored in leaves than in barks.
(2) In antimicrobial assay, the antibacterial and antifungal activities of leave and bark oil are investigated by disk diffusion method. The leave oil shows a strong inhibitory effect against the growth of Staphylococcus aureus, Bacillus subtilis, Escherichia coli, Candida albicans and Trichophyton rubrum. A moderate effect is observed for Pseudomonas aeruginosa and Aspergillus niger, and no effect against Penicillium citrinum. The bark oil shows a strong inhibitory effect against the growth of Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa, Candida albicans and Penicillium citrinum, a moderate effect against Escherichia coli, and no effect against Trichophyton rubrum and Aspergillus niger.
AcknowledgementsThe authors thank the Microbial Type Culture Collection, Institute of Microbial Technology, Central South University for providing microbial strains.
References[1] KIRTHIKAR K R, BASU B D. Indian medicinal plants[M]. Uttaranchal: Oriental Enterprises, 2001: 1441-1443.
[2] NARAYANA K, KUNDUR P S, USHA N, SHRIDHAR N B. Poisonous and medicinal plants[M]. Bangalore: Jayashri Publications, 2003: 125-126.
[3] YANG Zhi-yun, GONG Xun, ZHANG Qi-tai. A karyomorphological study on Calycopteris floribunda[J]. Acta Botanica Yunnanica, 2002, 24(2): 250-252.
[4] SREEKANTH P, NARAYANA K, SHRIDHAR N B, AVINASH B. Toxicity studies of Calycopteris floribunda Lam. in calf, rabbit and rat[J]. Journal of Ethnopharmacology, 2006, 107(2): 229-233.
[5] HARBONE J B. Phytochemical methods—guide to modern te- chniques of plant analysis[M]. Madras: Chapman & Hall, 1991: 125.
[6] MAYER R. Five biflavonoids from Calycopteris floribunda (Combretaceae)[J]. Phytochemistry, 2004, 65(5): 593-601.
[7] MAYER R. Calycopterones and calyflorenones, novel biflavonoids from Calycopteris floribunda[J]. Journal of Natural Product, 1999, 62(9): 1274-1278.
[8] WALL M E, WANI M C, FULLAS F, OSWALD J B, BROWN D M, SANTISUKT T, REUTRAKUL V, MCPHAIL A T, FARNSWORTH N R, PEZZUTO J M. The calycopterones, a new class of biflavonoids with novel cytotoxicity in a diverse panel of human tumor cell lines[J]. Journal of Medicinal Chemistry, 1994, 37(10): 1465-1470.
[9] RODRIGUEZ E, VANDER V G, MARBY T J. The structure of calycopterin[J]. Phytochemistry, 1972, 11: 2311-2312.
[10] POUGET C, FAGNERE C, BASLY J P, LEVEQUE H, CHULIA A J. Synthesis and structure of flavan-4-ols and 4-methxyflavans as new potential anticancer drugs[J]. Tetrahedron, 2000, 56(32): 6047-6052.
[11] LI Xiao-ru, LAN Zheng-gang, LIANG Yi-zeng. Analysis of olatile chemical components of Radix Paeoniae Rubra by gas chromatography-mass spectrometry and chemometric resolution[J]. Journal of Central South University of Technology, 2007, 14(1): 57-61.
[12] ZHOU Dan, LIU Jia-jia, TANG Ke-wen, HUANG Ke-long. Chiral extraction of ketoprofen enantiomers with chiral selector tartaric esters[J]. Journal of Central South University of Technology, 2007, 14(3): 353-356.
[13] GOUTSIOU P, NAXAKIS G, SKOULA M. Diversity of monoterpenoids of Origanum microphyllum (Labiatae)[J]. Biochemical Systematics and Ecology, 2002, 30(9): 865-879.
[14] HARBORNE J B, TURNER B L. Plant chemosystematics[M]. London: Academic Press, 1984.
[15] DUARTE-ALMEIDA J M, NEGRI G, SALATINO A. Volatile oils in leaves of Bauhinia (Fabaceae Caesalpinioideae)[J]. Biochemical Systematics and Ecology, 2004, 32(8): 747-753.
[16] BURT S. Essential oils: Their antibacterial properties and potential applications in foods a review[J]. International Journal of Food Microbiology, 2004, 94(3): 223-253.
[17] MEVY J P, BESSIERE J M, DHERBOMEZ M, MILLOGO J, VIANO J. Chemical composition and some biological activities of the volatile oils of a chemotype of Lippia chevalieri Moldenke[J]. Food Chemistry, 2007, 101(2): 682-685.
(Edited by YANG You-ping)
Foundation item: Project(2007-1718) supported by Development and Reform Commission of Yunnan Province, China
Received date: 2009-02-26; Accepted date: 2009-05-21
Corresponding author: LIU Jia-jia, Professor; Tel: +86-13974870621; E-mail: liujj0903@163.net
- Chemical component and antimicrobial activity ofvolatile oil of Calycopteris floribunda


