Trans. Nonferrous Met. Soc. China 29(2019) 2213-2221
Selective removal of As from arsenic-bearing dust rich in Pb and Sb
Xue-yi GUO1,2, Lei ZHANG1,2, Qing-hua TIAN1,2, Da-wei YU1,2, Jing SHI1,2, Yu YI1,2
1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. Cleaner Metallurgical Engineering Research Center, China Nonferrous Metals Industry Association, Changsha 410083, China
Received 20 December 2018; accepted 1 July 2019
Abstract:
The selective removal of arsenic from arsenic-bearing dust containing Pb and Sb in alkaline solution was studied. The influence of NaOH concentration, temperature, leaching time, liquid to solid ratio, and the presence of elemental sulfur on the dissolution of As, Sb and Pb in NaOH solution was investigated. The results indicate that the presence of elemental sulfur can effectively prevent leaching of lead and antimony from arsenic. The Sb2O3, As2O3 and Pb5(AsO4)3OH in the raw material convert to NaSb(OH)6 and PbS in the leaching residue, while arsenic is leached out as As(III) or As(V) ions in the leaching solution. Arsenic leaching efficiency of 99.84% can be achieved under the optimized conditions, while 97.82% of Sb and 99.97% of Pb remain in the leach residue with the arsenic concentration of less than 0.1%. A novel route is presented for the selective removal of arsenic and potential recycle of lead and antimony from the arsenic-bearing dust leached by NaOH solutions with the addition of elemental sulfur.
Key words:
arsenic removal; sulfur; arsenic-bearing dust; alkaline leaching; lead; antimony;
1 Introduction
Arsenic is a harmful chemical element to both humans and animals [1], which may hurt the gastrointestinal tract, skin, hematopoietic system, cardiovascular system and nervous system after chronical exposure [2]. However, most of the nonferrous metallurgical industries are facing the arsenic pollution problem [3], due to the generation of arsenic-bearing wastes during production, such as smelting slag, dust, and anode slime [4]. Among these arsenic-bearing wastes, arsenic-bearing dust is the most common contaminant obtained in nonferrous pyrometallurgical processes at 600-800 °C, in which the trivalent arsenic (As2O3) vapor forms [5,6]. Moreover, the arsenic-bearing dusts still contain certain useful metal elements, such as lead, antimony, copper and indium [7]. Unfortunately, the arsenic-bearing dust cannot be recycled back to the smelting process, and is usually treated with special landfill as a hazardous waste, resulting in environment pollution and resource waste [8,9]. Therefore, it is meaningful to develop an effective way to comprehensively process the arsenic-bearing dust in an environmentally-sound manner [10].
Both pyrometallurgical and hydrometallurgical processes can be used for arsenic removal [11]. In the pyrometallurgical process, arsenic will sublimate before collection in the form of arsenic oxide [12]. But, there are disadvantages associated with the pyrometallurgical process [13], for example, the incomplete collection of arsenic-bearing dust and high energy consumption [14,15]. Therefore, many researchers conducted investigations on arsenic removal with hydrometallurgical methods using dilute H2SO4 leaching [16], NaHS-NaOH leaching [17], pressure leaching with H2SO4 [18] or NaOH solutions [19], butyl alcohol/heptane leaching, etc. Nevertheless, most metallic elements in arsenic-bearing materials will be dissolved in the leaching solutions, leading to increasing difficulty in the subsequent separation of metallic elements during subsequent processing [20]. Thus, it is important to develop a novel technology for selective extraction of arsenic and recovery of the valuable metal elements. With the presence of sulfide ions [21], the heavy metals in arsenic-bearing materials cannot be dissolved in the alkaline solution, which implies the potential application of mixed NaOH-Na2S or NaOH-NaHS solutions to treat the arsenic-bearing dust. TONGAMP et al [16] used NaOH-NaHS to selectively leach arsenic from enargite (Cu3AsS4). However, they did not study the behaviors of metal impurities during leaching. These leaching media are unsuitable for the treatment of Sb(III)-containing arsenic-bearing dust, because Sb(III) easily transforms to dissolved  and
and  in alkaline solutions containing S2- [22].
in alkaline solutions containing S2- [22].
The arsenic-bearing dust generated from treating jamesonite in the pyrometallurgical process usually contains a considerable amount of lead, arsenic and antimony. In our previous work [8], the mixed NaOH-Na2S leaching agent was used to treat the arsenic-bearing dust. It was found that the lead could be restrained in the residue with a high leaching efficiency of antimony. Therefore, a new and effective method was proposed in this work for the selective removal of arsenic from arsenic-bearing dust using NaOH solutions with the addition of elemental sulfur. The effects of leaching parameters including NaOH concentration, liquid to solid ratio (L/S), time, temperature, and elemental sulfur on extraction of lead, antimony and arsenic were systematically investigated. Additionally, the leaching mechanism of the arsenic-bearing dust in NaOH solutions with the addition of elemental sulfur was explained for the better understanding of the leaching process [9].
2 Experimental
2.1 Materials
The arsenic-bearing dust samples were generated from blast furnace smelting of copper dross in a lead smelter located in Guangxi Province, China. The arsenic-bearing dust samples were dried, ground, and sieved with a 100 mesh sieve. Their XRD pattern and chemical composition are shown in Fig. 1 and Table 1, respectively. The major elements of the sample are lead,arsenic and antimony with their phase compositions of PbS, As2O3, Sb2O3, Pb3As2O8 and Pb5(AsO4)3OH.
Sodium hydroxide (NaOH, 95% in purity) and sulfur (analytically pure) used in the experiments were produced by Sinopharm Chemical Reagent Co., Ltd. Deionized water was used throughout the experiments.
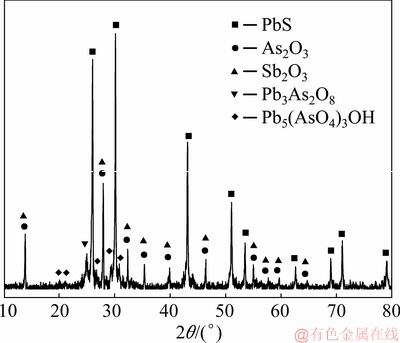
Fig. 1 XRD pattern of arsenic-bearing dust sample
Table 1 Chemical composition of arsenic-bearing dust (wt.%)

2.2 Procedure
The experiment was carried out in a 1200 mL four-neck round-bottom flask, which was placed in a water bath with precise temperature control (±1.0 °C). A certain volume of NaOH solution was put into the flask, and heated to a preset temperature. Subsequently, 100 g arsenic-bearing dust was added into the flask, stirred by a mechanical stirrer with three parallel blades at 450 r/min. The water-cooled condensate reflux was installed at the top of the flask to prevent the evaporation of solution. After a certain duration, the slurry was filtered using vacuum filtration. The obtained filtrate was analyzed to determine the leaching efficiencies of arsenic, lead and antimony. The residue was washed four times with deionized water, and then was dried, pulverized before analysis for its phases and chemical composition.
2.3 Characterization
The solid samples were analyzed by X-ray diffractometer (XRD, Japanese Rigaku Corporation) using Cu Kα radiation with a step size of 0.01° and a scanning rate of 9 (°)/min. The arsenic content was detected by an atomic fluorescence spectrometry (AFS-2201E, Hagang Corp., Shenzhen, China). The contents of other elements were detected by inductively coupled plasma optical emission spectrometer (ICP-OES, Intrepid II XSP, Thermo Elemental Corporation, America).
3 Results and discussion
3.1 Leaching experiments
3.1.1 Effect of NaOH concentration
The effects of NaOH concentration on the leaching of arsenic, antimony and lead are shown in Fig. 2. The leaching efficiencies of metal elements increase with increasing sodium hydroxide concentrations up to 3 mol/L. With further increase of the sodium hydroxide concentration, the leaching efficiency of arsenic levels off, while the leaching efficiencies of antimony and lead still increase. At a NaOH concentration of 3 mol/L, the leaching efficiencies are 88.36%, 21.36% and 14.32% for As, Sb and Pb, respectively. The possible reason is that arsenite is the main As-bearing phase in the arsenic- bearing dust, whose solubility increases rapidly with the increase of NaOH concentration [23]. Meanwhile, the hydroxide of Pb(II) and Sb(III) form hydroxyl complexes including 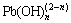 and
and  in concentrated alkaline solutions, resulting in high leaching efficiencies of lead and antimony [24,25]. The chemical reactions taking place in the leaching process could be expressed as Eqs. (1)-(4). Because of the separation of the solid and liquid phases becomes very difficult when the NaOH concentration keeps at a high level [26], 3 mol/L NaOH solution is adopted in the following experiments.
in concentrated alkaline solutions, resulting in high leaching efficiencies of lead and antimony [24,25]. The chemical reactions taking place in the leaching process could be expressed as Eqs. (1)-(4). Because of the separation of the solid and liquid phases becomes very difficult when the NaOH concentration keeps at a high level [26], 3 mol/L NaOH solution is adopted in the following experiments.
As2O3+2OH-= +H2O (1)
+H2O (1)
Pb3As2O8+6OH-= +3Pb(OH)2 (2)
+3Pb(OH)2 (2)
Pb(OH)2+(n-2)OH-=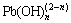 (3)
(3)
Sb2O3+2(m-3)OH-+3H2O= (4)
(4)
(n, m=1-4)

Fig. 2 Effect of NaOH concentration on leaching efficiencies of arsenic, antimony and lead (L/S=8, 80 °C, 2 h)
3.1.2 Effect of leaching temperature
The effects of temperature on the leaching efficiencies of arsenic, antimony and lead are presented in Fig. 3. The leaching efficiency of arsenic increases from 63.92% to 81.62% with the increase of temperature from 50 to 100 °C. This observation is consistent with the work by BALAZ et al [27]. However, temperature has little impact on the leaching rate of lead, which is different from that of arsenic. The leaching of antimony remains relatively constant at low temperatures, and then increases rapidly at temperatures over 90 °C, due to easier dissolution of Sb2O3 in hot NaOH solutions [28]. Based on these results, 90 °C is chosen for selective removal of arsenic from the arsenic-bearing dust.
3.1.3 Effect of liquid to solid ratio

Fig. 3 Effect of temperature on leaching efficiencies of arsenic, antimony and lead (3 mol/L NaOH, L/S=8, 2 h)

Fig. 4 Effect of L/S ratio on leaching efficiencies of arsenic, antimony and lead (3 mol/L NaOH, 90 °C, 2 h)
As shown in Fig. 4, the effect of liquid to solid ratio (from 3 to 10) on the leaching efficiencies of arsenic, antimony and lead indicates that the leaching efficiencies of arsenic, antimony and lead increase from 70.46%, 9.61% and 1.41%, to 91.14%, 22.39% and 15.03%, respectively, with the increase of L/S ratio from 3 to 10. It is the fact that the mass transfer within the liquid-solid interface becomes faster at a higher L/S ratio [29]. Considering the consumption of water and the leaching efficiencies of arsenic, antimony and lead, the optimum L/S ratio of 8 is determined.
3.1.4 Effect of leaching time
The effects of leach time on the leaching efficiencies of arsenic, antimony and lead in NaOH solutions are presented in Fig. 5. The result shows that the leaching efficiency of arsenic increases gradually with reaction time, whereas the leaching efficiencies of antimony and lead decrease rapidly. The leaching efficiency of arsenic raises from 66.30% to 81.48%, with the increase of leaching time from 10 to 90 min, However, with the continuous increase of reaction time beyond 90 min, the leaching of arsenic is barely changed. The leaching efficiencies of antimony and lead at 10 min reach 46% and 28%, respectively. This can be explained by the easy dissolution of Sb(III) and Pb(II) in NaOH solution as  and
and 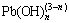 , respectively. Furthermore, according to the study by OZGE [23], Sb(III) can be easily oxidized at ESb(V)/Sb(III)<-450 mV when pH>13, so part of antimony is expected to be oxidized by the dissolved oxygen in the system and then precipitates as Na[Sb(OH)6] (Ksp=8.89×10-6). Moreover, the concentration of NaOH decreases gradually with its continuous consumption, resulting in de-hydrolyzation of
, respectively. Furthermore, according to the study by OZGE [23], Sb(III) can be easily oxidized at ESb(V)/Sb(III)<-450 mV when pH>13, so part of antimony is expected to be oxidized by the dissolved oxygen in the system and then precipitates as Na[Sb(OH)6] (Ksp=8.89×10-6). Moreover, the concentration of NaOH decreases gradually with its continuous consumption, resulting in de-hydrolyzation of 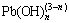 forming PbO, followed by its precipitation in the form of 3PbO·Sb2O4. Therefore, the leaching efficiencies of antimony and lead reduce gradually with prolonged reaction time. In order to ensure high arsenic extraction, low antimony and lead loss, and low energy consumption, 1.5 h is determined to be the optimum reaction time.
forming PbO, followed by its precipitation in the form of 3PbO·Sb2O4. Therefore, the leaching efficiencies of antimony and lead reduce gradually with prolonged reaction time. In order to ensure high arsenic extraction, low antimony and lead loss, and low energy consumption, 1.5 h is determined to be the optimum reaction time.

Fig. 5 Effect of leaching time on leaching efficiencies of arsenic, antimony and lead (3 mol/L NaOH, L/S=8, 90 °C)
3.1.5 Effect of addition of elemental sulfur
According to the above results, it is difficult to achieve the effective extraction of arsenic while maintaining low extractions of antimony and lead. Decreasing the dissolutions of antimony and lead in the solution is key to the selective removal of arsenic from the dust, which may be achieved by forming the metal sulfide precipitates. The NaOH-S leaching system provides an opportunity for the low dissolution of antimony and lead, resulting from the formation of sodium sulfide (Na2S) and sodium polysulfide (Na2Sx) in the solution, according to the reaction equations (5) and (6) [30]. The solubility of Sb(V) is lower than Sb(III) in aqueous solution, and the Sb(III) can be oxidized by sodium polysulfide (Na2Sx) to Sb(V) in NaOH-S system [31,32]:
4S0+6OH-= +2S2-+3H2O (5)
+2S2-+3H2O (5)
S2-+(x-1)S0= (x=2-5) (6)
(x=2-5) (6)
Sulfur in arsenic-bearing dust is mainly found in lead sulfide and arsenic sulfide, and the leaching behavior of sulfur in NaOH-S leaching system is further discussed. Eh–pH diagrams reflecting the experimental conditions used in this research were calculated using thermodynamic data taken from relevant literatures [32]. The diagrams were constructed using molarities, since representative activity coefficients were not readily available due to the complex nature of the solutions in this system.
The Eh-pH diagram for the S-H2O system is shown in Fig. 6. The sulfur activity is 0.1. It is found that H2S, S, 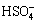 ,
,  , HS- and S2- are stable in the H2O-stable region (i.e., the region between lines a and b). With the increase of pH value, the area where sulfur is stable decreases gradually. When the pH value of the system is higher than 8.5, sulfur is mainly present in the form of
, HS- and S2- are stable in the H2O-stable region (i.e., the region between lines a and b). With the increase of pH value, the area where sulfur is stable decreases gradually. When the pH value of the system is higher than 8.5, sulfur is mainly present in the form of  , HS- and S2-.
, HS- and S2-.

Fig. 6 Eh-pH diagram of S-H2O system at 298 K (Sulfur activity is 0.1)
The distribution coefficient of sulfur at different acidic values is studied by thermodynamic analysis, according to Eqs. (7) and (8):
S2-+H2O=HS-+OH- (7)
HS-+H2O=H2S+OH- (8)
Figure 7 shows the distribution coefficient of sulfur. It is apparent that as the acidity of the system decreases, the distribution coefficient of H2S decreases gradually. When the pH value is larger than 13, HS- distribution coefficient decreases while S2- distribution coefficient increases. The sulfur in a highly alkaline solution occurs generally in the form of S2-.
The possible leaching reactions are discussed as follows. Thermodynamic data for the various species expected to be present in this system at 298 K are presented in Table 2. Table 3 shows that  for all of these reactions are negative and the
for all of these reactions are negative and the  values are very large. The thermodynamic calculation results indicate that these reaction equations are susceptible to take place.
values are very large. The thermodynamic calculation results indicate that these reaction equations are susceptible to take place.
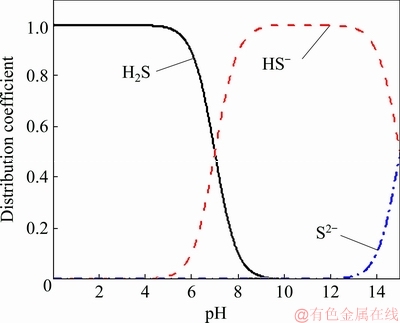
Fig. 7 Evolution of various sulfur-bearing species as function of pH at constant temperature of 298 K
From the above analysis on the thermodynamics of the NaOH-S system, it is apparent that its chemical reactions are very complex. It is generally accepted that the disproportionation reaction of sulfur would take place in an alkaline solution, resulting in the formation of polysulfide (see Reactions (5) and (6)). At the same time, it should be noted that the Sb(III) can be oxidized by the newly-formed sodium polysulfide (Na2Sx) to Sb(V) in the NaOH-S system. Subsequently, Sb(V) can be hydrolyzed and form NaSb(OH)6 easily. The solubility of NaSb(OH)6 in the solution is very low under alkaline condition, and most antimony in the leaching solution enters the residue in the form of NaSb(OH)6. Besides, Na2S reacts with  and
and  in the leaching solution to produce lead sulfide and zinc sulfide that are difficult to dissolve in water, which can be represented by Eqs. (9) and (10). The concentrations of
in the leaching solution to produce lead sulfide and zinc sulfide that are difficult to dissolve in water, which can be represented by Eqs. (9) and (10). The concentrations of  and
and  ions in the leaching solution decrease significantly, and the equilibrium of the leaching reaction of lead sulfide and zinc sulfide as shown in Eqs. (11), (12) and (13) shifts to the right, which promotes the leaching of arsenic in lead sulfide and zinc sulfide, and improves the leaching rate of arsenic. At the same time, lead and zinc remain in the leach residue in the form of sulfides, minimizing the dissolution of lead and zinc in the leaching process. In summary, with the presence of elemental sulfur, arsenic can be effectively leached out as As(III) or As(V) ions. And the Sb2O3, As2O3 and Pb5(AsO4)3OH in the raw material are converted to NaSb(OH)6 and PbS in the leaching residue.
ions in the leaching solution decrease significantly, and the equilibrium of the leaching reaction of lead sulfide and zinc sulfide as shown in Eqs. (11), (12) and (13) shifts to the right, which promotes the leaching of arsenic in lead sulfide and zinc sulfide, and improves the leaching rate of arsenic. At the same time, lead and zinc remain in the leach residue in the form of sulfides, minimizing the dissolution of lead and zinc in the leaching process. In summary, with the presence of elemental sulfur, arsenic can be effectively leached out as As(III) or As(V) ions. And the Sb2O3, As2O3 and Pb5(AsO4)3OH in the raw material are converted to NaSb(OH)6 and PbS in the leaching residue.
Table 2 Thermodynamic data of relevant species

Table 3 Thermodynamic calculation results of relavant reaction equations


Fig. 8 Effect of elemental sulfur on leaching efficiency of arsenic (3 mol/L NaOH, L/S=8, 90 °C)
Na2PbO2+Na2S+2H2O=PbS+4NaOH (9)
Na2ZnO2+Na2S+2H2O=ZnS+4NaOH (10)
Pb5(AsO4)3OH+19NaOH =5Na2PbO2+3Na3AsO4+10H2O (11)
Pb2As2O7+10NaOH=2Na2PbO2+2Na3AsO4+5H2O (12)
As2O3+2NaOH= 2NaAsO2+H2O (13)
Zn3(AsO4)2+12NaOH =3Na2ZnO2 +2Na3AsO4+6H2O (14)
The effects of the addition of elemental sulfur on the leaching efficiencies of arsenic are presented in Fig. 8. The result in Fig. 8 indicates that the leaching of arsenic increases with the addition of elemental sulfur, with the arsenic leaching efficiency of about 99.59% within 60 min at sulfur addition of 10 wt.%. The addition of sulfur promotes arsenic extraction, likely due to dissolution of elemental sulfur in the solution which acted as a lixiviant for the dissolution of arsenic [31], as represented by Eq. (15) and (16) [30]. Thus, the highly- efficient leaching of arsenic can be achieved in NaOH-S solutions.
As2O3(s, in dust)+6S2-=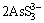 +6OH- (15)
+6OH- (15)
As(V)(s, As-Pb compounds in dust)+S2-= (16)
(16)
As shown in Figs. 9 and 10, the leaching efficiencies of antimony and lead decrease rapidly with the leaching time with the addition of elemental sulfur. The leaching rate of antimony is barely changed with 3.75 wt.% sulfur addition, while lead is nearly unextracted with the addition of 3.75 wt.% sulfur after 60 min. This indicates that sulfur addition favors the precipitation of lead prior to the precipitate of antimony. Furthermore, the dissolution of antimony apparently can be prevented with the addition of 7.5 and 10 wt.% of sulfur after 60 min. With the gradual precipitation of antimony and lead in the leaching solution, the amount of sodium sulfide residue in the leaching solution increases gradually. However, with the prolonging of the leaching time, the equilibrium of the Na3SbS4 hydrolysis reaction moves to the left, resulting in the partial re-dissolution of NaSb(OH)6 from the residue into the solution. During the experiment, the concentration of alkali in the leaching solution gradually increases from 1.32 to 1.61 mol/L. Due to the leaching of arsenic, antimony and lead, the optimal conditions are 10% sulfur addition and leaching time of 1.5 h.

Fig. 9 Effect of elemental sulfur on leaching efficiency of antimony (3 mol/L NaOH, L/S=8, 90 °C)

Fig. 10 Effect of elemental sulfur on leaching efficiency of lead (3 mol/L NaOH, L/S=8, 90 °C)
Figure 11 shows a schematic diagram for the reaction sequence of lead and antimony in the NaOH-S solution. The lead and antimony are firstly dissolved as 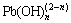 and
and  , respectively. Then, the lead precipitates as PbS by reacting with S2-, and the antimony precipitates as Na[Sb(OH)6] by the processes of oxidation, hydrolyzation and precipitation. The relevant reactions are listed as follows.
, respectively. Then, the lead precipitates as PbS by reacting with S2-, and the antimony precipitates as Na[Sb(OH)6] by the processes of oxidation, hydrolyzation and precipitation. The relevant reactions are listed as follows.
1) The reaction sequence of lead
Extraction:
Pb(II)(s, As-Pb compounds in dust)+nOH-=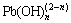 (17)
(17)
Precipitation:
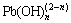 +S2-=PbS(s)+nOH- (18)
+S2-=PbS(s)+nOH- (18)
2) The reaction sequence of antimony [30,31]
Extraction:
Sb2O3+2(m-3)OH-+3H2O= (19)
(19)
 +3S2-=
+3S2-= +mOH- (20)
+mOH- (20)
Oxidation:
 +
+ =
= +S2- (21)
+S2- (21)
Hydrolyzation:
 +6OH-=
+6OH-= +4S2-+3H2O (22)
+4S2-+3H2O (22)
 +3H2O=
+3H2O= (23)
(23)
Precipitation:
 +Na+=Na[Sb(OH)6](s) (24)
+Na+=Na[Sb(OH)6](s) (24)
Therefore, the overall reaction between elemental S and Sb2O3 in NaOH solution appears as follows:
(x-1)Sb2O3(s)+2(x+1)S+6xNaOH+3(x-2)H2O=2(x-1)Na[Sb(OH)6](s)+2xNa2S+Na2S2O3 (x=2-5) (25)
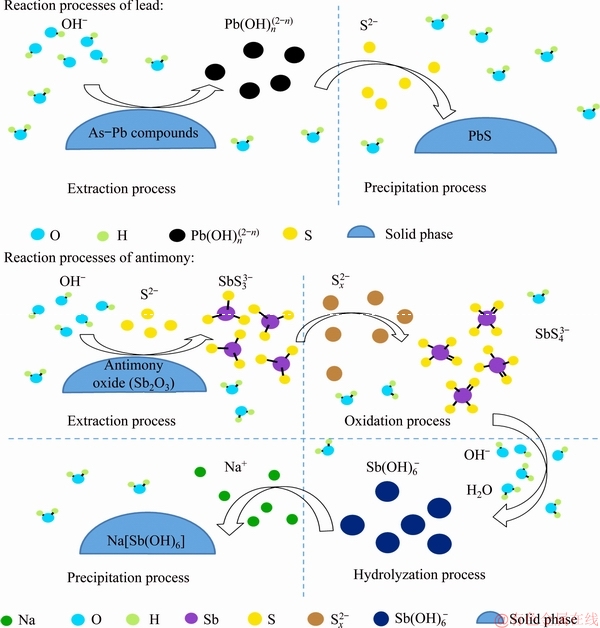
Fig. 11 Schematic diagram demonstrating reaction sequence of lead and antimony in NaOH-S solutions
3.2 Leaching of main metals in leaching process
The leaching efficiencies of main elements from the dust in the leaching process are listed in Table 4. The result indicates that the addition of elemental sulfur obviously prevents the extraction of antimony, tin and zinc, while improves the leaching efficiency of arsenic. Meanwhile, the enhanced leaching efficiency of sulfur in leachate proves the dissolution of sulfur in NaOH solutions, as shown in Eqs. (5) and (6).
Table 4 Leaching efficiencies of main elements from arsenic-bearing dust in leaching process (L/S=8, 3 mol/L NaOH, 90 °C, 1.5 h)

Table 5 Chemical compositions of leaching residue (3 mol/L NaOH, L/S=8, 10% of sulfur, 90 °C, 1.5 h) (wt.%)
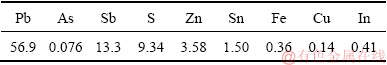

Fig. 12 XRD patterns of solid samples before and after leaching
The results of chemical and XRD analyses for the leaching residue are presented in Table 5 and Fig. 12. The main elements in the leaching residue are Pb, Sb and S, with the compounds of PbS, NaSb(OH)6 when elemental sulfur is added in the leaching process. However, the characteristic peaks of Pb5(AsO4)3OH and 3PbO·Sb2O5 still exist for the leaching process without sulfur addition, which can be eliminated with the addition of elemental sulfur. All of these prove the effectiveness of selective removal of arsenic by using NaOH-S solutions.
The optimum conditions for the removal of arsenic from arsenic-bearing dust are 10% sulfur addition with L/S ratio of 8 in 3 mol/L NaOH solution at 90 °C for 1.5 h. Under these conditions, the arsenic leaching efficiency of 99.84% is achieved with little dissolution of antimony and lead, resided in the leaching residue.
4 Conclusions
(1) A novel process for the selective removal of arsenic and potential recycle of lead and antimony from the arsenic-bearing dust leached by NaOH solutions with the addition of elemental sulfur is proposed.
(2) With the presence of elemental sulfur, arsenic can be effectively leached out as As(III) or As(V) ions in the leaching solution. And the Sb2O3, As2O3 and Pb5(AsO4)3OH in the raw material convert to NaSb(OH)6 and PbS in the leaching residue. Under the optimum conditions of 10 wt.% sulfur addition with L/S ratio of 8 in 3 mol/L NaOH solution at 90 °C for 1.5 h, the arsenic leaching efficiency of 99.84% can be achieved, while 97.82% of Sb and 99.97% of Pb remain in the leach residue with the arsenic content of about 0.076%.
(3) Leaching of arsenic-bearing dust by NaOH-S solutions can be utilized for the selective removal of arsenic with potential recycle of lead and antimony from arsenic-bearing dust.
References
[1] TAN Cheng, LI Lei, ZHONG Da-peng, WANG Hua, LI Kong-zhai. Separation of arsenic and antimony from dust with high content of arsenic by a selective sulfidation roasting process using sulfur [J]. Transactions of Nonferrous Metals Society of China, 2018, 28(5): 1027-1035.
[2] YANG Jin-qing, CHAI Li-yuan, LI Qing-zhu, SHU Yu-de. Redox behavior and chemical species of arsenic in acidic aqueous system [J]. Transactions of Nonferrous Metals Society of China, 2017, 27(9): 2063-2072.
[3] TIAN Qing-hua, ZHANG Zhen, LI Xiao-jing, LI Dong, GUO Xue-yi. High arsenic in copper electrolyte vortex electric product to take off the noise [J]. Transactions of Nonferrous Metals Society of China, 2018, 28(8): 1637-1644.
[4] LIU Wei-feng, HUANG Ke-hong, YANG Tian-zu, ZHANG Du-chao, CHEN Lin. Selective leaching of antimony from high arsenic antimony gold ore by wet method [J]. Transactions of Nonferrous Metals Society of China, 2018, 28(1): 205-211.
[5] GOMEZ M A, BECZE L, CELIKIN M, DEMOPOULOS G P. The effect of copper on the precipitation of scorodite (FeAsO4·2H2O) under hydrothermal conditions: Evidence for a hydrated copper bearing ferric arsenate sulfate-short lived intermediate [J]. Journal of Colloid and Interface Science, 2015, 360: 508-518.
[6] DRIEHAUS W, SEITH R, JEKEL M. Oxidation of arsenate(III) with manganese oxides in water treatment [J]. Water Research, 1995, 29: 297-305.
[7] LEIST M, CASEY R J, CARID D. The management of arsenic wastes: Problems and prospects [J]. Journal of Hazardous Materials, 2000, 76: 125-138.
[8] YI Yu, SHI Jing, TIAN Qing-hua, GUO Xue-yi. Arsenic removal from high-arsenic dust by NaOH-Na2S alkaline leaching [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(3): 806-804.
[9] YI Yu, SHI Jing, TIAN Qing-hua, GUO Xue-yi. Novel technology for preparation of sodium pyroantimonate from alkali leaching residue of high arsenic dust [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(1): 241-249.
[10] QIAO Jin-xi, LONG Shuang, MA Ya-lin, QIU Yang, CHEN Jing-yang, MIAO Hua-lei, CHEN Ai-liang. Alkali leaching of cobalt/nickel residue containing arsenic using air [J]. Transactions of Nonferrous Metals Society of China, 2018, 28(11): 2358-2365.
[11] BROSTOW W, GAHUTISHVILI M, GIGAURII R, LOBLAND H, JAPARIDZE S, LEKISHVILI N. Separation of natural trivalent oxides of arsenic and antimony [J]. Chemical Engineering Journal, 2010, 159: 24-26.
[12] FERNANDEZ M A, SEGARRA M, ESPIELL F. Selective leaching of arsenic and antimony contained in the anode slimes from copper refining [J]. Hydrometallurgy, 1996, 41: 255-267.
[13] LI Y, LIU Z, LI Q, ZHAO Z, LIU Z, ZENG L, LI L. Removal of arsenic from arsenate complex contained in secondary zinc oxide [J]. Hydrometallurgy, 2011, 109: 237-244.
[14] NORGATE T E, RANKIN W J. The role of metals in sustainable development [C]// Green Processing. Cairns, QLD, PA: AusIMM, 2002: 49-55.
[15] KASHIWAKURA S, OHNO H, MATSUBAE-YOKOYAMA K, KUMAGAI Y, KUBO H, NAGASAKA T. Removal of arsenic in coal fly ash by acid washing process using dilute H2SO4 solvent [J]. Journal of Hazardous Materials, 2010, 181: 419-425.
[16] TONGAMP W, TAKASAKI Y, SHIBAYAMA A. Selective leaching of arsenic from enargite in NaHS-NaOH media [J]. Hydrometallurgy, 2010, 101: 64-68.
[17] ZHAO Zhan-chong, SHI Yi-feng, ZHU Xing, QI Xian-jin, WANG Xiao-wu, YOU Kai-yun, WANG Hua. Reductive decomposition behavior of arsenic bearing gypsum sludge with coal and arsenic migration rule [J]. Transactions of Nonferrous Metals Society of China, 2017, 27(1): 187-197.
[18] XU Z F, QIANG L I, NIE H P. Pressure leaching technique of smelter dust with high-copper and high-arsenic [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(1): 176-181.
[19] YU G L, ZHANG Y, ZHENG S L, XING Z O, WANG X H, ZHANG Y. Extraction of arsenic from arsenic-bearing cobalt and nickel slag and preparation of arsenic-bearing compounds [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(6): 1918-1927.
[20] LEWIS A E. Review of metal sulphide precipitation [J]. Hydrometallurgy, 2010, 104: 222-234.
[21] PARADA F, JEFFREY M I, ASSELIN E. Leaching kinetics of enargite in alkaline sodium sulphide solutions [J]. Hydrometallurgy, 2014, 146: 48-58.
[22] RASCHMAN P, SMINCAKOVA E. Kinetics of leaching of stibnite by mixed Na2S and NaOH solutions [J]. Hydrometallurgy, 2012, 113: 60-66.
[23] OZGE G. Catalytic production of antimonate through alkaline leaching of stibnite concentrate [J]. Hydrometallurgy, 2014, 149: 68-74.
[24] KYLE J H, BREUER P L, BUNNEY K G, PLEYSIER R, MAY P M. Review of trace toxic elements (Pb, Cd, Hg, As, Sb, Bi, Se, Te) and their deportment in gold processing. Part 1: Mineralogy, aqueous chemistry and toxicity [J]. Hydrometallurgy, 2011, 107: 91-100.
[25] SAHIN M, ERDEM M. Cleaning of high lead-bearing zinc leaching residue by recovery of lead with alkaline leaching [J]. Hydrometallurgy, 2015, 153: 170-178.
[26] YOUCAI Z, STANFORTH R. Integrated hydrometallurgical process for production of zinc from electric arc furnace dust in alkaline medium [J]. Journal of Hazardous Materials, 2000, 80: 223-240.
[27] BALAZ P, ACHIMOVICOVA M. Mechano-chemical leaching in hydrometallurgy of complex sulphides [J]. Hydrometallurgy, 2006, 84: 60-68.
[28] ZHAO Rui-rong, SHI Xi-chang. The metallurgical physical chemistry of antimony [M]. Changsha: Central South University Press, 2006: 10-15. (in Chinese)
[29] ZHANG S H, LI T, ZHU B C, ZHU Z B. Gas-liquid mass transfer in three-phase mechanical agitated reactor [J]. Chemical and Industrial Engineering, 2005, 56: 200-226.
[30] ANDERSON C G. The metallurgy of antimony [J]. Chemie Der Erde-Geochemistry, 2012, 72: 3-8.
[31] LEI Ting, ZHU Cong-jie, ZHANG Han-ping. Antimony metallurgy [M]. Beijing: Metallurgical Industry Press, 2009: 442-444. (in Chinese)
[32] ANDERSON C G, TWIDWELL L G. The alkaline sulfide hydrometallurgical separation, recovery and fixation of tin, arsenic, antimony, mercury and gold [C]// International Symposium on Lead and Zinc Processing. Durban, South Africa, PA: SAIMM, 2002: 49-55.
[33] DEAN J A, WEI Jun-fa. Lange's handbook of chemistry [M]. Beijing: Science Press, 2003. (in Chinese)
[34] YE Da-lun, HU Jian-hua. Practical inorganic thermodynamics data book [M]. Beijing: Metallurgical Industry Press, 2002. (in Chinese)
[35] LIN Chuan-xian. Handbook of thermodynamic data for minerals and related compounds [M]. Beijing: Science Press, 1985. (in Chinese).
富铅锑含砷烟尘中砷的选择性脱除
郭学益1,2, 张 磊1,2, 田庆华1,2, 于大伟1,2, 石 靖1,2, 易 宇1,2
1. 中南大学 冶金与环境学院,长沙 410083;
2. 中国有色金属工业协会 中国清洁冶金工程研究中心,长沙 410083
摘 要:开展碱性体系选择性脱除富铅锑含砷烟尘中砷的研究,分别考察NaOH浓度、温度、浸出时间、液固比、元素硫对砷、锑、铅溶解的影响。结果表明,砷浸出过程中,元素硫的存在可以有效抑制铅和锑浸出。原料中的Sb2O3、As2O3和Pb5(AsO4)3OH转化为NaSb(OH)6和PbS,存在于渣中,砷以砷(III)或砷(V)离子的形式溶于浸出液中。在最优条件下,砷的浸出效率可达99.84%;97.82%锑和99.97%铅留在渣中,浸出渣中砷的含量低于0.1%。提出一种氢氧化钠体系添加元素硫在选择性脱除含砷烟尘中砷后潜在回收铅、锑的新途径。
关键词:除砷;硫;含砷烟尘;碱性浸出;铅;锑
(Edited by Bing YANG)
Foundation item: Project (51604303) supported by the National Natural Science Foundation of China; Project (2019JJ20031) supported by the Hunan Natural Science Fund for Distinguished Young Scholar, China
Corresponding author: Xue-yi GUO; Tel: +86-13875844958; E-mail: xyguo@csu.edu.cn
DOI: 10.1016/S1003-6326(19)65127-7
Abstract: The selective removal of arsenic from arsenic-bearing dust containing Pb and Sb in alkaline solution was studied. The influence of NaOH concentration, temperature, leaching time, liquid to solid ratio, and the presence of elemental sulfur on the dissolution of As, Sb and Pb in NaOH solution was investigated. The results indicate that the presence of elemental sulfur can effectively prevent leaching of lead and antimony from arsenic. The Sb2O3, As2O3 and Pb5(AsO4)3OH in the raw material convert to NaSb(OH)6 and PbS in the leaching residue, while arsenic is leached out as As(III) or As(V) ions in the leaching solution. Arsenic leaching efficiency of 99.84% can be achieved under the optimized conditions, while 97.82% of Sb and 99.97% of Pb remain in the leach residue with the arsenic concentration of less than 0.1%. A novel route is presented for the selective removal of arsenic and potential recycle of lead and antimony from the arsenic-bearing dust leached by NaOH solutions with the addition of elemental sulfur.


