
Nickel electrodeposition from novel citrate bath
LI Chao-qun(李超群), LI Xin-hai(李新海), WANG Zhi-xin(王志兴), GUO Hua-jun(郭华军)
School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
Received 15 July 2007; accepted 10 September 2007
Abstract:
A new type of electroplating bath suitable for nickel electrodeposition was developed. Trisodium citrate was used as a complexing agent and a buffer in the bath. The buffering capacity between trisodium citrate and boric acid were compared. The effects were investigated under different conditions of bath composition, current density, pH and temperature on the potentiodynamic cathodic polarization curves, cathodic current efficiency and throwing index, as well as the electrical conductivity of these baths. The optimum conditions for producing sound and satisfactory nickel deposits were:
Key words:
citrate bath; nickel electrodeposition; throwing index; buffering capacity; cathodic current efficiency;
1 Introduction
Electrodeposited nickel has been used for various applications such as transport, and service apparatus, and to give the decorative and functionally suitable metal coatings[1].
The selection of an electroplating bath depends primarily on the required characteristics of the nickel deposit. A sulfamate bath is an important bath. Nickel deposit from sulfamate bath has low stress and good mechanical properties[2], but this coating can be produced expensively and it is commercially undesirable for economic considerations. A Watt bath is a most popular nickel electroplating bath and boric acid is an essential ingredient for controlling a bath pH and forming smooth and ductile deposits[3]. It is worth noticing that waste bath containing boron is harmful and is restricted dumping. Recently, strict environmental protection regulations restricting dumping of waste containing boron were imposed in Japan[4]. Therefore, it becomes necessary to find more environmentally friendly alternatives to boric acid for nickel electro- deposition.
Citric acid and citrate are the reagents used chiefly as pH butter agent and a complexing agent for electroless nickel plating[5] and electroplating iron-alloy[6], Ni-W alloy[7], Cu-Ni alloy[8] baths. Citrate ions in the citrate bath react with nickel ions to form nickel citrate complexes, and the complexes are adsorbed on the surface of a cathode. This contributes to the electrodeposition of nickel and the crystallization of the deposits. Citrate ion is also adsorbed on the surface of the cathode, resulting in the inhibition of the hydrogen evolution reaction. So citric acid and citrate are possible substitution agents of boric acid.
The bath concentration investigated in Refs.[9-11] is generally lower. The compositions of the plating baths are listed in Table 1.
In this study, nickel electrodeposition in the industrial concentration levels from citrate bath was investigated under different operating conditions, such as bath composition, pH, plating current density, temperature and plating time to find the optimum conditions for producing sound and satisfactory deposits. In all cases, the results produced by the citrate bath were
Table 1 Characteristics of nickel-citrate plating baths investigated in Refs. and in present work
compared with those produced by traditional Watts bath under the same plating conditions.
2 Experimental
All baths used were freshly prepared with deionized water and analytical grade chemicals. The pH of the plating bath was adjusted with diluted sulfuric acid or basic nickel carbonate.
The plating cell containing 1000 mL electrolyte was a rectangular Perspex trough (100 mm×100 mm×120 mm). The electrolyzed-nickel was used as anode (60 mm×80 mm). The cathodes were made of low-carbon steel sheet with dimensions of 40 mm×30 mm×0.08 mm. Its composition (mass fraction, %) was C 0.05-0.11; Si≤0.03; Mn 0.25-0.50; P≤0.035; S≤0.035; Ni≤0.25; Cr≤0.1; Cu≤0.25 and balance iron(Fe). Steel sheet was mechanically polished with different grade emery papers: 600, 800 and 1 000, respectively. After degreasing by electrochemical method, the activation treatment, carried out before the electrodeposition, represented etching in 7%HCl solution. Deposition was conducted in galvanostatic mode without agitation. The cathode current efficiency(CCE) was calculated based on the difference of mass of specimen before and after the plating (η=mexp/mtheo). Where mexp is the mass of the deposit obtained experimentally and mtheo is the theoretically mass of the deposit determined by calculation of the quality of the electricity passing through the cell with a copper coulometer according to Faraday’s law.
The crystal structure of deposit was examined by X-ray diffraction analysis (Cu kα radiation, D/max 2 550 vb+18 kW). The surface morphology of the deposits was observed by SEM (JEOL JSM6380).
The throwing index(TI) of the solution was measured using a Haring-Blum rectangular perplex cell fitted with one foam nickel anode (50 mm×50 mm×2 mm) between two parallel copper sheet cathodes (70 mm×50 mm).
For examining the pH buffer characteristics of the plating bath, a plating solution was sampled by taking 100 mL and titrated by using a 1.085 mol/L NaOH standard solution, to determine pH titration curves. The solution was well stirred and the pH of the clear solution was measured with a glass electrode (E-201-C) using pHS-25 digital pH meter.
A cathodic polarization curve was measured by the potential scanning method (scanning speed: 0.1 mV/S), using CHI660A electrochemical work station (produced by Shanghai Chenhua Instrument Company). The experiments were conducted in a three-electrode cell provided with a steel cathode (10 mm×10 mm), a platinum plate (20 mm×20 mm) as counter electrode and a saturated calomel electrode(SCE) as a reference electrode. The latter was connected to the cell with a luggin capillary, filled with solution to minimize the iR drop of the solution and contamination. All potentials were measured and recorded vs the saturated calomel electrode. The electrolytes were purged with nitrogen at least 10 min prior to the experiment. Working electrode was mechanically polished with different grade emery papers: 600# and 1000#, then rinsed with deionized water and washed with acetone.
3 Results and discussion
3.1 pH titration curves
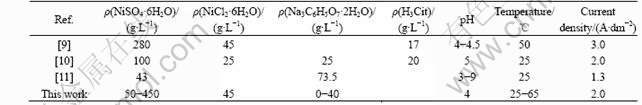
Curve 1 in Fig.1 shows the results of titration of a bath not containing buffer. The buffer-free bath shows no pH buffering capacity. For comparison, the addition of boric acid to the above solution increases the buffering
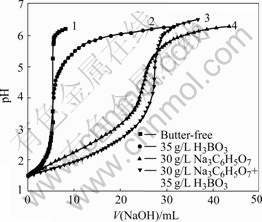
Fig.1 pH titration curves in variation of bath pH as function of amount of titration with 1 mol/L NaOH: 1 NiSO4?6H2O 350 g/L; NiCl2?6H2O 45 g/L; 2 NiSO4?6H2O 350 g/L, NiCl2?6H2O 45 g/L, H3BO3 35 g/L; 3 NiSO4?6H2O 350 g/L, NiCl2?6H2O 45 g/L, Na3C6H5O7 30 g/L; 4 NiSO4?6H2O 350 g/L, NiCl2?6H2O 45 g/L, Na3C6H5O7 30 g/L, H3BO3 35 g/L
capacity (curve 2). On the other hand, the citrate bath shows a strong pH buffer capacity in a bath pH region of 3.5 or less (curve 3), whereas the pH buffer capacity decreases remarkably in a pH region of 3.5 to 5.5. In view of the pH titration curve, both the free citric acid (not forming complexes with nickel ions) and the NiCit- complex which can supply H+ to OH- derived from added NaOH exists in the bath pH region where the pH buffer capacity increases. Curve 4 illustrates the effect of addition of both boric acid and trisodium citrate together to the above bath. This reveals that the presence of citrate and boric acid further increases the buffering capacity.
In the bath pH region where the pH buffer capacity decreases remarkably, the NiCit- complex exists predominantly. Since all protons in three carboxylic groups of citric acid have been dissociated, the bath loses the pH buffer capacity. This confirms the result of the calculation for the chemical species because the forms of citric acid determined by the calculation agrees with the bath pH buffering characteristics actually demonstrated by the pH titration curve.
3.2 Throwing index
The throwing index is an another way expressing the throwing power of the plating bath. There are several advantages associated with expressing TP in the form of TI. For example, a single number is obtained, characteristic of a range of linear ratios. In addition, TI is obtained from several experimental points and thus minimizes errors in measurement of any one point. The metal distribution ratio(M) is therefore plotted vs the linear ratio(K), within the range from K=1 to K=5, as a function of various deposition conditions. Some representative results are shown in Fig.2, which shows reasonable linear plots passing through the points represented by M=1 and K=1. The TI value obtained form citrate bath is about 0.783. This is approximately
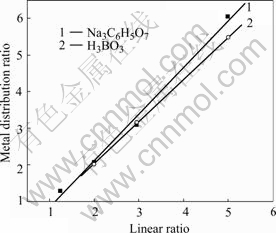
Fig.2 Metal distribution ratio vs linear ratio (NiSO4?6H2O 350 g/L, NiCl2?6H2O 45 g/L at pH=4, 55 ℃ and 1 A)
equal to that from boric acid bath (0.861).
In general, the results of the throwing power and the throwing index study could be explained in terms of the two main factors that control this property. Firstly, an increase of the cathodic polarization has an equalizing effect on the primary current distribution and so increases the throwing power. Secondly increase in electrolytic conductivity results in improved throwing power. So it is advantageous to improve the throwing index for increasing cathodic polarisation and electrolytic conductivity
3.3 Cathodic current efficiency
The effect of Ni content in the bath on the current efficiency during nickel electrodeposition is shown in Fig.3. The data indicate that the CCE depends strongly on Ni content in the bath. At a given Na3C6H5O7 concentration, the CCE increases markedly with the increase of nickel content in the bath.
Fig.3 Effect of NiSO4 concentration on cathodic current efficiency of nickel electrodeposition from bath containing NiCl2?6H2O 45 g/L, Na3C6H5O7 30 g/L at pH=4, 55 ℃ and 1 A
Fig.4 shows the influence of pH on the current efficiency in the pH range from 2.0 to 5.5. It is clear that the current efficiency of nickel deposition increases with increasing pH value of the bath from 2 to 4 and then decreases slightly. The CCE of the citrate bath reaches about 98% at a bath pH of 4. This finding implies that hydrogen evolution reaction tends to occur easily as the bath pH decreases and this lowers the CCE. These agree well with the results that the shift in cathodic polarisation curves towards the less negative direction with decreasing pH is related mainly to decrease in the overpotential of the hydrogen evolution reaction.
The influence of temperature on CCE for nickel deposition is shown in Fig.5. The data reveal that an increase of temperature from 25 to 65 ℃ has no significant effect on the CCE. This means that increasing
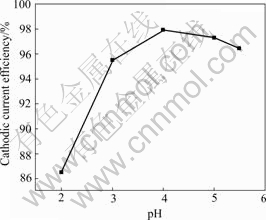
Fig.4 Effect of bath pH on cathodic current efficiency of nickel electrodeposition from bath containing NiSO4?6H2O 350 g/L, NiCl2?6H2O 45 g/L, Na3C6H5O7 30 g/L at 55 ℃ and 1 A
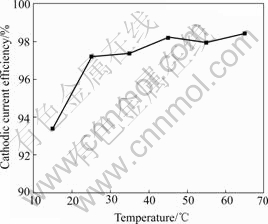
Fig.5 Effect of bath temperature on cathodic current efficiency of nickel electrodeposition from bath containing NiSO4?6H2O 350 g/L, NiCl2?6H2O 45 g/L, Na3C6H5O7 30 g/L at pH=4 and 1 A
bath temperature affects nearly equally both the deposition of nickel and the evolution of hydrogen. The remainder studies are carried out at 55 ℃.
At a given NiSO4 concentration in the bath, the CCE is found to decrease with increasing citrate ion (Fig.6). The result shows that nickel complexes increase with increasing citrate ion while free Ni2+ concentration decreases.
3.4 Cathodic polarisation
The effect of boric acid and sodium citrate on the cathodic polarization for nickel electroplating on to low-carbon steel sheet substrates from sulphate bath is shown in Fig.7. Curve 1 represents the cathodic polarization in bath containing NiSO4?6H2O and NiCl2?6H2O. It is observed that the polarization curve shows a little overpotential. The addition of boric acid to
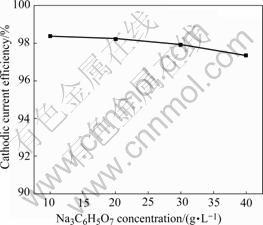
Fig.6 Effect of bath trisodium citrate concentration on cathodic current efficiency of nickel electrodeposition from bath containing NiSO4?6H2O 350 g/L, NiCl2?6H2O 45 g/L at pH=4, 55 ℃ and 1 A
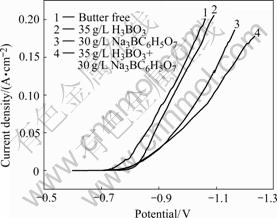
Fig.7 Cathodic polarization curves for electrodeposition of nickel on low carbon steel sheet from various solutions at pH=4, temperature of 55 ℃ from bath containing: 1 NiSO4?6H2O 350 g/L, NiCl2?6H2O 45 g/L; 2 NiSO4?6H2O 350 g/L, NiCl2?6H2O 45 g/L, H3BO3 35 g/L; 3 NiSO4?6H2O 350 g/L, NiCl2?6H2O 45 g/L, Na3C6H5O7 30 g/L; 4 NiSO4?6H2O 350 g/L, NiCl2?6H2O 45 g/L, H3BO3 35 g/L, Na3C6H5O7 30 g/L
the above solution increases slightly the cathodic polarization (curve 2). The effect of boric acid must be considered in terms of blocking the surface sites for nickel deposition. The addition of sodium citrate to the above considered bath shows a further increase in the cathodic polarization. This behaviour could be attributed to the formation of soluble complex species NiCit- (curve 3). Curve 4 illustrates the effect of addition of both boric acid and trisodium citrate together to the above bath. This reveals that the presence of citrate and boric acid further increases the cathodic polarization during nickel deposition. This behaviour is caused by the formation of Ni2+ ions complexes and block effect of boric acid. This inhibition effect of citrate ion could be assigned to the decrease in the concentration of the free Ni2+ as a result of complexation. In addition, citrate ions as such or in the form of metal complexes may adsorb on the cathode surface and block the active sites available for Ni2+ discharge process[12].
The effects of some plating parameters on the cathodic polarization curves for nickel deposition from citrate bath are examined. Fig.8 shows the cathodic polarization curves from different nickel content baths containing citrate. Inspection of the data reveals that the nickel deposition is accompanied by significant cathodic polarization. Increasing the Ni2+ ion concentration in the bath changes the deposition potential of Ni2+ to a less negative potential. Such behavior could be attributed to the decrease in the concentration overpotential.
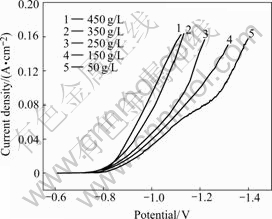
Fig.8 Effect of NiSO4.6H2O concentration on cathodic polarization of nickel electrodeposition
Addition of trisodium citrate to the bath changes the nature of the cathodic polarisation curves and such curves are characterized by an initial rapid potential shifted to more negative direction, followed by a gradual increase with rise of current density (Fig.9). Such a potential shift could be attributed to the formation of
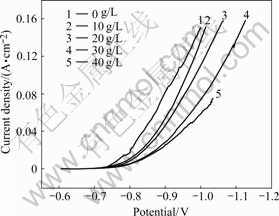
Fig.9 Effect of trisodium citrate concentration on cathodic polarization of nickel electrodeposition
different soluble complex species. Table 2 shows the dissociation constant of citric acid and the stability constant of the nickel-citrate complexes used for the calculation.
Table 2 Acid-dissociation constant of citric acid and stability constant of nickel-citrate complexes
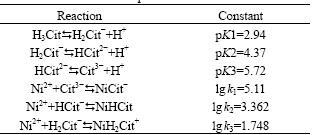
From these equilibrium constants, we can conclude that the NiCit- is the predominant complex species in the solution because it has a much higher equilibrium constant. In addition, because the concentration of the total citrate ion is lower than the metal ion concentration, there will also be significant amounts of free Ni2+ present in the solution at all values of pH. The following electroreduction reactions are possible, therefore, with hydrogen evolution from an independent side reaction:
Ni2++2e= Ni (1)
NiCit-+2e=Ni+Cit- (2)
The inhibition effect of citrate ion (i.e., the increase in cathodic polarization), especially at low current densities, could be assigned to the decrease in the concentration of the free Ni2+ as a result of complexation. In addition, citrate ion may be adsorbed on the cathode surface and block the active sites available for the Ni2+ discharge process. So the increase in cathodic polarisation with increasing the citrate concentration is observed.
The effect of bath pH on cathodic polarization is shown in Fig.10. It is clear that increasing the pH value of the bath, the cathodic polarization increases.
It is suggested that a decrease in pH increases the concentration of the uncompleted Ni2+ ions in the bath. It is probable that decreasing the pH value of the bath allows decreasing in the overpotential of the simultaneous hydrogen evolution reaction.
Fig.11 illustrates the effect of temperature on the cathodic polarization. The data reveal that a rise of bath temperature shifts the cathodic polarization curve to the less negative values. The behavior could relate to the decrease in the overpotential of both hydrogen evolution and nickel deposition reactions. Moreover, an increase of temperature enhances the concentration of the reducible species in the diffusion layer as a result of increasing
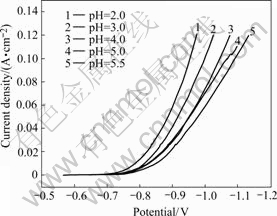
Fig.10 Effect of bath pH on cathodic polarization of nickel electrodeposition

Fig.11 Effect of bath temperature on cathodic polarization of nickel electrodeposition
their diffusion rates. In addition to the effect of tem- perature on the relative abundance of both the completed and free Ni2+ ions in the solutions was obtained.
3.5 Appearance and surface morphology of deposit
Black and rough nickel deposits were produced from bath containing nickel sulphate only. Addition of citrate ions into the bath, however, greatly improves the appearance and adherence of the deposits. Qualitative examination by simple procedures such as scrubbing, bending and heat/quench test does not result in separation of the Ni deposit from the base metal, indicating good adherence.
Surface examination of the nickel deposit on the lower carbon steel sheet from citrate baths was carried out by scanning electron microscopy (Fig.12). The nickel deposited from citrate baths is compact and composed of fine grains covering the entire surface with a growth resembling a “cauliflower” pattern. The surface morphologies of the deposits show an uneven surface as accumulation of fine spherical deposits. The grains of nickel deposits from the baths containing trisodium citrate are more compact than those obtained from Watts baths. We concluded that the presence of citrate complexes in the citrate bath affects the crystal structure of the deposits—producing fine deposits. Such characteristics cannot be obtained in the traditional nickel plating using boric acid.
3.5 Structure of deposits
Fig.13 shows the XRD patterns of Ni deposited
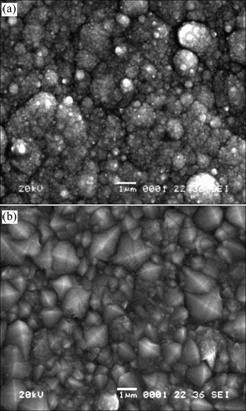
Fig.12 SEM micrographs of Ni deposited from electrolyte at I= 2 A/dm2, pH=4 and t=55 ℃: (a) Citrate bath; (b) Watts bath
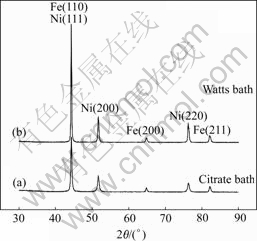
Fig.13 XRD patterns from Ni plated at 2 A/dm2, pH=4 and t=55℃: (a) Citrate bath; (b) Watts bath
from the citrate and Watts bath, separately. XRD data indicate that the deposit of Ni has face-centered cubic in the citrate bath, the nickel deposit exhibits a (111) growth orientation with assignation (200), (220) reflections as well. In the Watt bath, the deposit has also a (111) growth orientation with pronounced (100) and (110) Bragg lines.
4 Conclusions
1) Trisodium citrate bath exhibits excellent cathodic current efficiency and buffering capacity for nickel electrodeposition. It offers a practical, effective, and more environmentally friendly substitute for boric acid in a Watts bath.
2) The appearance of the plating surface, the cathodic current efficiency and crystal structure of the deposits obtained from the citrate bath, are dependent on the effect of electroplating variables. such as bath composition, current density, pH and temperature.
3) The nickel deposition obtained from citrate bath is compact, non-porous fine grains and with a growth resembling a ‘cauliflower’ pattern. X-ray analysis shows that the deposit of Ni has face-centered cubic structure.
References
[1] SUPICOVA M, ROZIK R, TRNKOVA L, ORINAKOVA R, GALOVA M. Influence of boric acid on the electrochemical deposition of Ni [J]. J Solid State Electrochem, 2006, 10: 61-68.
[2] GOLODNITKY D, GUDIN N V, VOLYANUK G A. Study of nickel-cobalt alloy electrodeposition from a sulfsmate electrolyte with different anion additives [J]. Journal of the Electrochemical Society, 2000, 147(11): 4156-4163.
[3] TILAK B V, GENDRON A S, MOSOIU M A. Borate buffer equilibria in nickel refining electrolytes [J]. J Appl Electrochem, 1977, 7: 495-500.
[4] DOI T, MIZUMOTO K. Notification of the direction general of the environment agency in Japan [R]. 1999-02-22.
[5] TAROZAITE˙R, GYLIENE O, STALNIONIS G. Adipate adsorption and its incorporation into NiP coatings from citrate electroless nickel plating solutions [J]. Surface & Coatings Technology, 2005, 200: 2208-2213.
[6] GHORBANI M, DOLATI A G, AFSHAR A. Electrodeposition of Ni-Fe alloys in the presence of complexing agents [J]. Russian Journal of Electrochemistry, 2004, 8(11): 1173-1177.
[7] YOUNES O, GILEADI E. Electroplating of Ni/W alloys (I): Ammoniacal citratebaths [J]. Journal of The Electrochemical Society, 2002, 149(2): C100-C111.
[8] YING R Y. Electrodeposition of copper-nickel alloys from citrate solutions on a rotating disk electrode [J]. J Electrochem Soc, 1988, 135(12): 2957-2964.
[9] DOI T, MIZUMOTO K. Bright nickel plating from nickel citrate electroplating baths [J]. Metal finishing, 2004(4): 26-35.
[10] IBRAHIM M A M, ABD EI REHIM S S, ASD EI WAHAAB S M, DANKERVA M M. Nickel electrodeplating on steel from acidic citrate bathd [J]. Plating and Surface Finishing, 1999, 86(4): 69-74.
[11] RODE S, HENNINOT C, MATLOSZ M. Complexation chemistry in nickel and copper-nickel alloy plating from citrate baths [J]. Journal of the Electrochemical Society, 2005, 152 (4): C248-C254.
[12] RASHWAN S M. Electrodeposition of Co-Ni alloys from citrate bath onto steel substrate [J]. Metall, 1999, 53(12): 686-691.
(Edited by LI Xiang-qun)
Foundation item: Project(2007CB613607) supported by the National Basic Research Program of China
Corresponding author: LI Xin-hai; Tel: +86-13873106090; E-mail: Lichaoqun65@163.com


