Trans. Nonferrous Met. Soc. China 25(2015) 4201-4206
Effect of milling time on carbothermic reduction of ilmenite
Min CHEN1, Ai-tao TANG2, Xuan XIAO1
1. College of Materials Engineering, Panzhihua University, Panzhihua 617000, China;
2. College of Materials Science and Engineering, Chongqing University, Chongqing 400044, China
Received 10 February 2015; accepted 28 September 2015
Abstract:
A systematic investigation on the effect of milling time on carbothermic reduction of ilmenite was carried out by a combination of thermal analysis, X-ray diffraction (XRD), scanning electron microscopy (SEM) and particle size analysis. It was shown that the graphite crystalline structure was easily destroyed along the (002) crystal plane during milling process. The particle size of ilmenite/graphite mixture was largely decreased and a fully mixed composite structure was obtained after milling for 4 h at high milling intensity. Milling exerts positive effect on the decreasing temperatures of ilmenite reduction and gradual deoxidizing of titanium oxides was completed.
Key words:
milling time; carbothermic reduction; ilmenite;
1 Introduction
As the sources of high-grade titanium minerals are decreasing worldwide, the processes involving application of low-grade minerals have attracting more attention. Ilmenite is cheap and very rich in China. Different methods have been used for application of ilmenite [1]. Among these methods, carbothermic reduction is a potential and economical method [2,3]. However, high temperatures are required for deep reduction. It is found that unstable titanium oxides still exist at high temperature in reduction process of ilmenite. In order to promote the efficiency of reduction process, different pretreatments and additives were tried, such as catalysts, preoxidation and ball milling [4,5]. Different types of raw materials (such as different carbon sources) were also tested [6]. TRIPATHY et al [6] investigated the reduction of an ilmenite ore with different carbonaceous reducing agents and found that graphite was more effective at higher temperature. GUPTA et al [7] found that the addition of ferric chloride promoted the nucleation of iron which increased the rate of reduction. TAIWIL et al [4] carried out reduction tests of ilmenite in the presence of sodium carbonate and found that additions of sodium carbonate at levels of up to 30% in the ore enhanced the reduction efficiency. However, the investigations on deep reduction of ilmenite with high efficiency were rarely reported, especially for the stable reduced products of TiCN at high temperatures. It is known that TiCN is a useful hard phase with excellent characteristics in cutting and wear- resistant materials.
It is generally accepted that mechanical activation process is an effective way to increase the rate of carbothermic reduction reactions and it plays an important role in mineral processing. During mechanical milling, repeated welding and fracturing of powders increase contacting areas among the mixtures, which produced more contacting surfaces [8,9]. This allows the reaction to occur at lower temperature, which exerts significant effect on decreasing equipment requirements and improving reduction efficiency as well. WELHAM et al [10,11] and CHEN [12] found that ball milling can accelerate the reduction process of ilmenite by carbon after long time milling. It was found that the reduction was a complex process. Simultaneous reactions occurred and overlapped with each other in reductions. Many factors have an impact on the reactions, including mineralogical composition of the ore, particle size, milling parameters, etc. GUPTA et al [7] observed that different types of ore also had significant influence on the reduction degree of ilmenite. However, the effect of ball milling parameters on the reduction process had rarely been reported. Therefore, the aim of the present work is to investigate the effect of milling time on ilmenite reduction.
2 Experimental
In this work, ilmenite was supplied by Titanium Company of Panzhihua Steel & Iron (Group) Corporation (Sichuan Province, China). The chemical composition is shown in Table 1. Mixtures of ilmenite with graphite were ball milled in a vertical planetary ball mill (QM-3SP4, China) under Ar condition. A ball-to- powder mass ratio of 20:1 was used with stainless steel balls of two types (6 mm and 10 mm in diameter). Three relative levels of milling intensity were tested: 1) milling speed lower than 250 r/min; 2) middle speed between 350 r/min and 400 r/min; 3) milling speed higher than 450 r/min. In this work, 250, 350 and 450 r/min were used to represent levels 1, 2 and 3, respectively. Carbothermal nitridation reduction tests of ilmenite were carried out in nitrogen atmosphere.
After milling, the particle size distribution was measured by the laser diffractometer MASTERSIZER 2000 with the range from 0.02 μm to 2000 μm. Mean particle diameter was obtained by d(0.5) from the particle size distribution data.
Comprehensive thermal analysis was carried out in a multifunctional thermal analyzer (STA449C). Samples were heated at a rate of 20 K/min to 1400 °C under flowing nitrogen.
X-ray diffraction (XRD) was carried out using a Rigaku D/MAX-2500PC diffractometer with Cu Kα radiation at 40 kV and 30 mA and a scan rate of 0.02 (°)/s in a 2θ range of 10°-90°. The microstructure was observed by scanning electron microscopy (TESCAN VEGAⅡLMU) and Carl Zeiss Auriga dual column focused ion beam-scanning electron microscopy (FIB-SEM).
Table 1 Chemical composition of ilmenite (mass fraction, %)

3 Results and discussion
3.1 Effect of ball milling parameters on granulometrical and morphological changes of powders
Figure 1 shows the particle size of milled samples under different conditions. The mean particle size decreased rapidly during the initial milling stage and then leveled off or increased slightly with milling time. The particle size decreased gradually and tended to be stable after milling for 4 h at the low milling intensity. The particle size decreased to the same level after milling for 4 h in both levels 1 and 2 condition. At the high milling intensity, the particle size decreased fast in the first hour and then changed to the minimum value within 2 h. However, a slight increase was observed with extending milling time. Samples with different milling time under level 3 were studied.
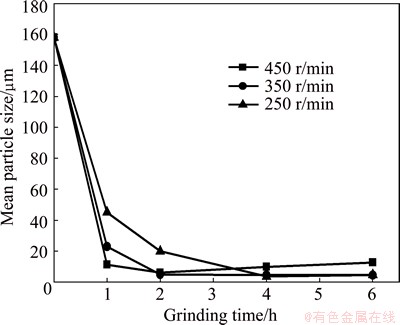
Fig. 1 Mean particle size of samples as function of milling time under different milling intensities
Microstructural evolution of samples during ball milling is shown in Fig. 2. It can be seen that the initial ilmenite-graphite mixture consists of distinct angular particles (Fig. 2(a)). After ball milling for 2 h, the size of ilmenite and graphite particles decreased significantly and the graphite particles were embedded in ilmenite particles. When the milling time reached 4 h, fine ilmenite and graphite particles uniformly combined and a fully composite structure was achieved. However, as the milling time increased to 6 h, no significant particle size decrease can be further observed in the structure. High magnification graphs of milled samples between 4 h and 6 h are shown in Fig. 3 and part of particles tend to agglomerate in local regions after milling for 6 h.
3.2 Effect of milling time on phases analysis
To study the structural change, both unmilled and milled samples under level 3 were subjected to XRD examinations and the results are shown in Fig. 4. No new diffraction peaks were observed, suggesting that no significant chemical reactions took place, and differences in peak intensities can be found in the main graphite peak (002), which diminished more than 95% after milling for 2 h and disappeared after 4 h. When samples were milled for 2 h, the intensity of graphite peak (002) decreased from 51192 counts per second to 1385 counts per second. However, the intensity of ilmenite peak (104) decreased from 3328 counts per second to 739 counts per second for the samples milled for 6 h. This implies that the graphite crystalline structure is easily deformed along (002) crystal plane and changes into an amorphous state. However, the main peak (104) intensity of ilmenite experienced a slower decrease. The phenomenon of amorphous graphite state was reported by POURGHAHRAMANI and FORSSBERG [13] and CHEN et al [14]. Changes of diffraction peaks in the milling process are consistent with the average size reduction detailed above. When the particle size of raw material is finer, the contact area enlarges and carbothermal nitridation reduction of solid-solid and gas-solid reactions is enhanced. At the same time, the reaction activity is increased by lattice distortion caused in the milling process. Thus, the high rate of reduction in subsequent process was expected.
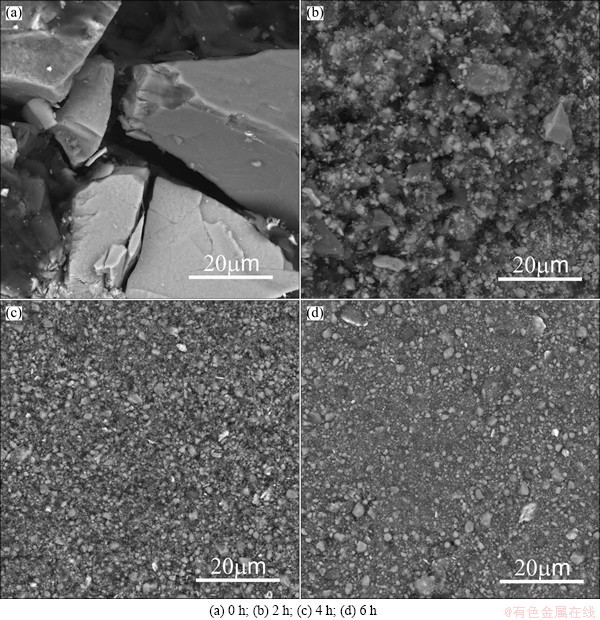
Fig. 2 BSE micrographs of samples milled for different time
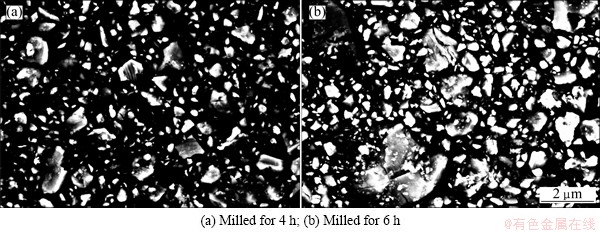
Fig. 3 BSE micrographs of milled samples at high magnification
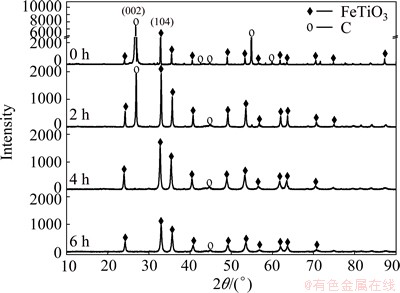
Fig. 4 XRD patterns of unmilled and milled samples
3.3 Effect of milling on reduction behavior of powders
As shown in Fig. 5, TG and DSC techniques were used to evaluate the reduction behavior of ilmenite, all samples before and after milling were heated to 1400 °C at a rate of 20 °C/min. The total mass loss of unmilled sample did not reach a steady level with increasing temperature to 1400 °C. The TG curve exhibited a three- step sequential mass loss and reached reaction platform after 1360 °C as the milling time is longer than 4 h. In the first stage, with temperature lower than 1000 °C, the mass decreased at a slow rate and the total mass loss was less than 5% with milling time shorter than 2 h. When milling time was more than 4 h, the mass loss was higher than 10%. This could be associated with solid state reaction which occurred by direct contacts between carbon and ilmenite particles. When milling time was more than 4 h, increased contact areas accelerated solid state reduction of iron and the main reaction equation is: FeTiO3+C=Fe+TiO2+CO. During this stage, the thermal effect of solid-solid reaction was not obvious. Probably, the thermal effect was weaker and subsequently masked or overlapped by the much stronger endothermic peaks for the two later gaseous reaction steps. The mass of unmilled sample had little decrease until 1080 °C. For the sample after milling for 2 h, the mass started to fall from 940 °C and the mass loss curve was much steeper than that of unmilled one, showing that milling advanced the reduction process of ilmenite. As the milling time extended to 4 h, the onset reaction temperature advanced to 870 °C. However, the milling time has no positive effect in advancing the onset reaction temperature any more after milling for more than 4 h. In addition, the mass of samples milled for more than 4 h showed a gradually decrease before the onset temperatures, which was attributed to desorption of gas [15,16]. The effect of mechanical activation in the first stage has been suggested for two reasons. Firstly, particle size was reduced and the contact areas of graphite and ilmenite were increased in the milling process. Secondly, the activation energy of solid state reaction can be reduced in milling process [17].
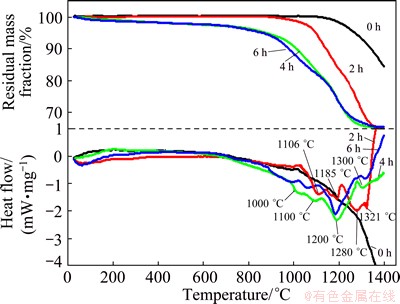
Fig. 5 TG and DSC curves for unmilled and milled samples as function of temperature
As the temperature exceeded 900 °C, the gaseous reactions were the main type and the curve decreased quickly. For the sample milled for 6 h, the rate of mass loss was a little faster than that milled for 4 h in the second stage, while the mass experienced a slower rate of mass loss in the third stage. This suggests that longer milling time delayed the reduction at higher temperature, which was attributed to the formation of agglomerates during extended dry milling [18]. Compared with the unmilled mixture, separated endothermic peaks were observed in the milled samples, which revealed that the mechanical activation discriminates different steps of reduction. The endothermic peaks evidently moved to lower temperatures with extending milling time to 4 h and then changed little. The temperatures of endothermic peaks for 2 h milled sample were 1106, 1185, 1280, 1321 °C and the temperatures advanced to 1000, 1100, 1200, 1300 °C for 4 h milled sample. The endothermic peaks stand for gradual deoxidizing of titanium oxides. The first two small endothermic peaks correspond to the formation of intermediate titanium oxides. Ti4O7 and Ti3O5 were usually detected and titanium oxides TinO2n-1 with n higher than 5 were difficult to be detected due to fast transformation. The equations are as follows:
TiO2+1/4C=1/4Ti4O7+1/4CO (1)
Ti4O7+1/3C=4/3Ti3O5+1/3CO (2)
In nitrogen atmosphere, the transformation of Ti3O5 to TiN was easier and then changed to Ti(C,N). The main equations corresponding to the third big endothermic peak and the fourth small endothermic peak were as follows:
Ti3O5+5C+3/2N2→3TiN+5CO (3)
TiN+C→Ti(C,N)+N2 (4)
In order to study the reaction process, XRD patterns of 4 h milled sample after heating (20 °C/min) to 1000, 1100, 1200, 1300 and 1400 °C which related to various stages of the endothermic peaks are shown in Fig. 6. Most of Fe has been reduced from ilmenite, since little ilmenite and TiO2 can be detected after heating to 1000 °C and Fe was the main phase. As the samples were heated to 1100 °C, intermediate titanium suboxide Ti4O7 was detected. At 1200 °C, Ti4O7 disappeared and the main phases were Ti3O5 and TiN. Ti3O5 is known as the most stable phase of titanium oxides under nitrogen atmosphere [19]. It is also proved by BERGER et al [20] that Ti3O5 is the precursor for formation of TiN. Then the peak intensity of TiN strengthened at the expense of Ti3O5, but the incorporation of carbon is a very slow process [21]. When the sample was heated to 1300 °C, small amounts of Ti3O5 were remained and main products were Fe and Ti(C,N). When the temperature rose to 1400 °C, the gradual deoxidizing completed and the detected products were Fe and Ti(C,N).

Fig. 6 XRD patterns for 4 h milled sample heated to 1000, 1100, 1200, 1300 and 1400 °C
4 Conclusions
1) During the milling process, the graphite crystalline structure was easily deformed along the (002) crystal plane and the graphite peaks disappeared after 4 h. However, the intensity of ilmenite peak (104) experienced a slower decrease. Changes of diffraction peaks are consistent with the average size reduction.
2) Milling exerts positive effect on the decreasing temperatures of ilmenite reduction. The sample milled for more than 4 h could reach reaction platform after 1360 °C and temperatures for gradual deoxidizing advanced.
3) Mechanical activation discriminated gradual deoxidizing of titanium oxides and separated endothermic peaks were observed corresponding to the solid-gas reactions. The phase evolution sequences of titanium oxides were: TiO2→Ti4O7→Ti3O5→TiN→ TiCN.
References
[1] ZHANG Wen-sheng, ZHU Zhao-wu, CHENG Chu-yong. A literature review of titanium metallurgical processes [J]. Hydrometallurgy, 2011, 108: 177-188.
[2] WANG Yu-ming, YUAN Zhang-fu, GUO Zhan-cheng, TAN Qiang-qiang, LI Zhao-yi, JIANG Wei-zhong. Reduction mechanism of natural ilmenite with graphite [J]. Transactions of Nonferrous Metals Society of China, 2008, 18(2): 962-968.
[3] LIU Chen-hui, ZHANG Li-bo, PENG Jin-hui, LIU Bing-guo, XIA Hong-ying, GU Xiao-chun, SHI Yi-feng. Effect of temperature on dielectric property and microwave heating behavior of low grade Panzhihua ilmenite ore [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(11): 3462-3469.
[4] TAWIL S Z, MORSI I M, YEHIA A, FRANCIS A A. Alkali reductive roasting of ilmenite ore [J]. Canadian Metallurgical Quarterly, 1996, 35(1): 31-37.
[5] CHEN Y, HWANG T, WILLIAMS J S. Ball milling induced low-temperature carbothermic reduction of ilmenite [J]. Materials Letters, 1996, 28: 55-58.
[6] TRIPATHY M, RANGANATHAN S, MEHROTHA S P. Investigations on reduction of ilmenite ore with different sources of carbon [J]. Mineral Processing and Extractive Metallurgy, 2012, 121(3): 147-155.
[7] GUPTA S K, RAJAKUMAR V, GRIEVESON P. The influence of weathering on the reduction of ilmenite with carbon [J]. Metallurgical Transactions B, 1989, 20B: 735-745.
[8] SASIKUMAR C, SRIKANTH S, MUKHOPADHYAY N K, MEHROTRA S P. Energetics of mechanical activation—Application to ilmenite [J]. Minerals Engineering, 2009, 22: 572-574.
[9] MOHAMMAD N, HOSSEIN Y, MASOUD A, NAOAKI K, JUNICHI K, JUNYA K, FUMIO S, HIDEKAZU K, HIROYUKI F. Effect of mechanical milling on carbothermic reduction of magnesia [J]. ISIJ International, 2010, 50(5): 668-762.
[10] KERR A, WELHAM N J, WILLIS P E. Low temperature mechanochemical formation of titanium carbonitride [J]. Nano Structured Materials, 1999, 11(2): 233-239.
[11] WELHAM N J. Enhanced gas-solid reaction after extended milling [J]. Journal of Materials Science Letters, 2001, 20: 1849-1851.
[12] CHEN Y. Mechanically enhanced carbothermic synthesis of iron-TiN composite [J]. Journal of Materials Science Letters, 1997, 16: 37-39.
[13] POURGHAHRAMANI P, FORSSBERG E. Effects of mechanical activation on the reduction behavior of hematite concentrate [J]. International Journal of Mineral Processing, 2007, 82: 96-105.
[14] CHEN Y, TWANG T, MARSH M, WILLIAMS J S. Mechanically activated carbothermic reduction of ilmenite [J]. Metallurgical and Materials Transactions A, 1997, 28: 1115-1121.
[15] WELHAM N J, BERBENNI V, CHAPMAN P G. Effect of extended ball milling on graphite [J]. Journal of Alloys and Compounds, 2003, 349(1-2): 255.
[16] KARBASI M, SAIDI A, TAHMASEBI M H. Carbothermic reduction of mechanically activated hematite–graphite–copper mixture [J]. Ironmaking & Steelmaking, 2009, 36(2): 82-86.
[17] SETOUDEH N, SAIDI A, WELHAM N J. Carbothermic reduction of anatase and rutile [J]. Journal of Alloys and Compounds, 2005, 390: 138-143.
[18] PAN Fu-sheng, LI Kui, TANG Ai-tao, WANG Yu, ZHANG Jing, GUO Z-xiao. Influence of high energy ball milling on the carbothermic reduction of ilmenite [J]. Materials Science Forum, 2003, 437-438: 105-108.
[19] WHITE G V, MACKENZIE K J D, BROWN I W M, BOWDEN M E. Carbothermal synthesis of titanium nitride [J]. Journal of Materials Science, 1992, 27: 4294-4299.
[20] BERGER L M, ETTMAYER P, SCHULTRICH B. Influencing factors on the carbothermal reduction of titanium dioxide without and with simultaneous nitridation [J]. International Journal of Refractory Metals & Hard Materials, 1993-1994, 12: 161-172.
[21] BERGER L M, GRUNER W. Investigation of the effect of a nitrogen-containing atmosphere on the carbothermal reduction of titanium dioxide [J]. International Journal of Refractory Metals & Hard Materials, 2002, 20: 235-251.
球磨时间对钛精矿碳热还原的影响
陈 敏1,汤爱涛2,肖 玄1
1. 攀枝花学院 材料工程学院,攀枝花 617000;
2. 重庆大学 材料科学与工程学院,重庆 400044
摘 要:结合热分析、XRD、SEM和粒度分析系统研究球磨时间对钛精矿碳热还原的影响。结果表明:球磨过程中石墨晶体结构容易沿(002)晶面被破坏。高转速下球磨4 h后钛精矿/石墨混合粉料的粒度大幅度降低,获得了均匀的复合组织结构。球磨有效降低了钛精矿不同阶段的还原反应温度,钛氧化物逐级还原反应完成。
关键词:球磨时间;碳热还原;钛精矿
(Edited by Yun-bin HE)
Foundation item: Project (2014JY0132) supported by the Program for Application Basic Research of Sichuan Province, China; Projects (2013CY-G-7, 2014CY-G-26-1, 2015CY-G-18) supported by the Technology Research Program of Panzhihua City, China; Project (2015TX-11) supported by the Innovative Project of Panzhihua City, China
Corresponding author: Min CHEN; Tel: +86-13118300185; E-mail: cmrre@163.com
DOI: 10.1016/S1003-6326(15)64070-5
Abstract: A systematic investigation on the effect of milling time on carbothermic reduction of ilmenite was carried out by a combination of thermal analysis, X-ray diffraction (XRD), scanning electron microscopy (SEM) and particle size analysis. It was shown that the graphite crystalline structure was easily destroyed along the (002) crystal plane during milling process. The particle size of ilmenite/graphite mixture was largely decreased and a fully mixed composite structure was obtained after milling for 4 h at high milling intensity. Milling exerts positive effect on the decreasing temperatures of ilmenite reduction and gradual deoxidizing of titanium oxides was completed.


