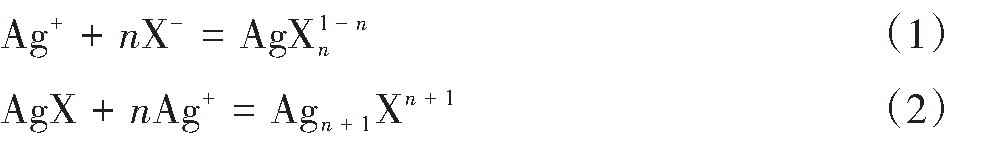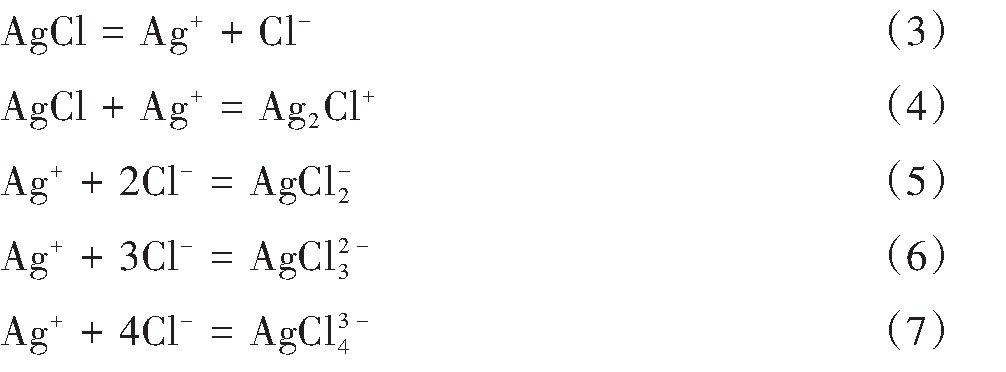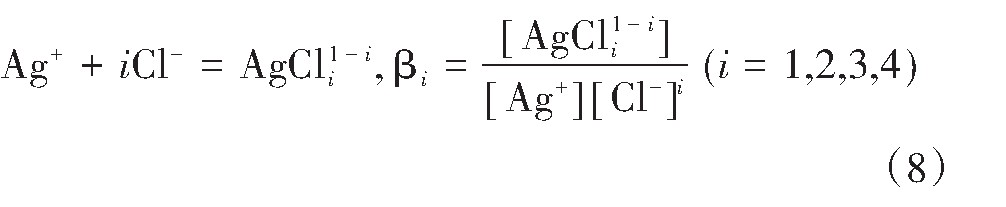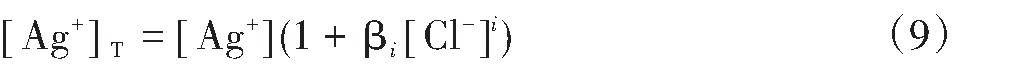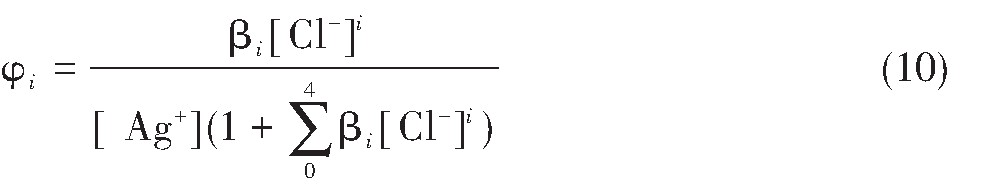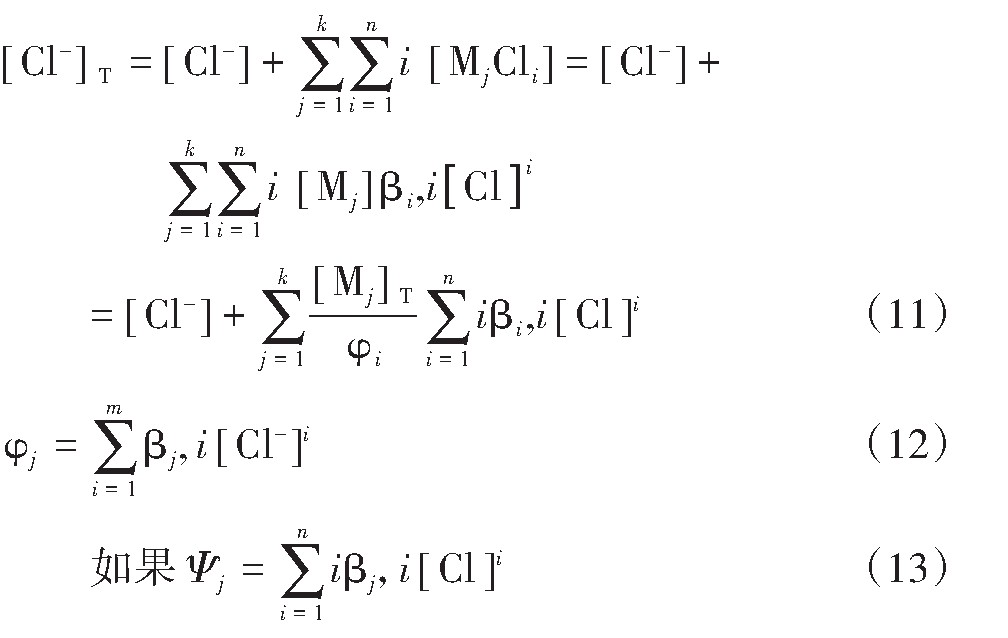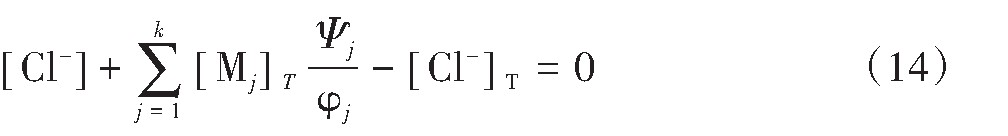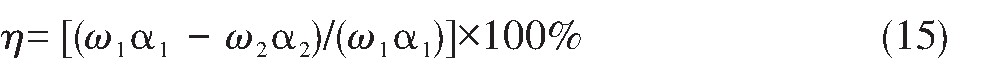网络首发时间: 2019-11-01 12:14
从钢铁厂烧结机头灰中选择性配位提取银
昆明理工大学冶金与能源工程学院
摘 要:
根据钢铁厂烧结机头灰富含银、氯、铁、碳等多种有价元素的特点,采用烧结机头灰自身含有的氯元素为配位剂,通过配位浸出的方式提取回收银。本实验研究溶液pH、反应温度、配位剂浓度、反应时间、液固比等反应条件对银浸出率的影响规律。采用X射线衍射仪(XRD)、扫描式电子显微-光微区(SEM-EDS)、粒度和化学成分(ICP-MS)分析手段分别对原料和浸出渣进行表征可知:烧结机头灰中的银主要以AgCl形式存在,通常被铁氧化合物、碳颗粒等物质包裹,铁主要以Fe2O3和Fe3O4物相存在;浸出渣的主要成分是铁氧化合物及元素碳等。实验结果表明:在弱酸性和中性溶液中,配位浸出过程中氯化银主要以AgCl43-络合离子形态被浸出,而铁、碳等对烧结有利的元素不被浸出进入渣中,浸出过程具有选择性。在溶液pH值为3.0、反应温度80℃、氯离子浓度6 mol·L-1、浸出时间2 h、液固比10:1 ml·g-1、搅拌速度400 r·min-1的最佳条件下,银的浸出率可达93.90%,铁的浸出率仅为1.28%,银的选择性分离提取效果好。
关键词:
中图分类号: X757
作者简介:世仙果(1995-),男,云南曲靖人,硕士研究生,研究方向:加压湿法冶金及材料制备,E-mail:1844234601@qq.com;;*李兴彬,副教授,电话:0871-65188819,E-mail:lixingbin2011@126.com;
收稿日期:2019-07-01
基金:国家重点研发计划固废资源化专项(2018YFC1900402);国家自然科学基金项目(51664029,51564030,51664030,51964029)资助;
Selective Coordination Extraction of Silver from Sintering Dust in Steel Plants
Shi Xianguo Li Xingbin Wei Chang Wang Chenyu Deng Zhigan Li Minting
Faculty of Metallurgy and Energy Engineering,Kunming University of Science and Technology
Abstract:
As one of the solid wastes produced in steel plant,sintering dust had a huge annual output and its chemical composition was complex,so it was not suitable to directly return to sintering ingredients to avoid damaging equipment,nor was it suitable to direct--y stockpile,so as to avoid the waste of resources caused by heavy metals seeping into the land and polluting the environment.Therefore,it was urgent to develop new recovery and treatment technologies.According to the fact that the insoluble compound silver chloride could be dissolved in hydrochloric acid,and could also form the coordination ion dissolved in a high concentration of halogen ion solution,combined with the high content of chlorine element in the sintering dust.Therefore,chlorine was used as coordination agent in the experiment to change the silver in the sintering dust into a soluble complex and increased its solubility into the solution,so as to realize the chlorination coordination leaching of silver.Firstly,quantitative sintering dust was weighed,and then quantitative leaching agent(ammonium chloride) and deionized water with predetermined concentration were added.Finally,the temperature in the flask was heated to the preset experimental temperature,and stirred continuously at a constant temperature for 3 h.During stirring,pulp pH was measured every half hour until the end.After leaching,liquid-solid separation was completed by pumping filtration,and the leaching solution and leaching residue were obtained.In this experiment,the effect of reaction conditions on silver leaching ratio was investigated,such as the pH of solution,reaction temperature,coordination agent concentration,reaction time,liquid-solid ratio and so on.With the increase of pH value,the leaching ratio of silver first increased and then decreased.When pH was 3.0,the maximum silver leaching ratio was 93.9%.As pH continued to increase,the concentration of hydrogen ions in the solution decreased,the dissolved silver chloride decreased,and coordination complexation weakened.With the increase of reaction temperature,the leaching ratio of silver showed an increasing trend.As the temperature rose,the reactivity of reactants was enhanced,and the diffusion ratio of reactants and products was accelerated,the dissolution ratio of silver was accelerated,and the reaction ratio was faster.When the temperature was 80℃,the silver leaching ratio reached the maximum of 93.9%.The leaching ratio of silver increased with the concentration of coordination agent increasing.When the concentration of Cl-was 2 to 6 mol·L-1,the leaching ratio of silver was from 32.6% to 93.9%.The higher the concentration of Cl-,the greater the probability of the formation of a complex coordinated with free Ag+ and the greater the solubility of silver chloride,the higher the concentration of silver in the leaching solution and the higher the leaching ratio of silver.With the increase of leaching reaction time,the leaching of silver first increased and then decreased.When the leaching time was from2 to 4 h,the leaching ratio was from 94.8% to 84.5%.With the extension of leaching reaction time,the volatilization loss of leaching agent would increase,the coordination effect of Ag-Cl would be weakened gradually,and the silver leaching ratio would decrease.The silver leaching ratio increased with the increase of the liquid-solid ratio.When the liquid-solid ratio was from 10 to 12 ml·g-1,the leaching ratio was from 93.9% to 95%.From the use of experimental raw materials and reagents,the larger the liquid-solid ratio was,the greater the consumption of raw materials and reagents would be,resulting in higher cost and complicated subsequent treatment of the solution.The compositions and phase results of raw material and leaching residue were characterized by X-ray diffraction(XRD),scanning electron microscope-energy dispersive spectrometer(SEM-EDS),particle size analysis and chemical composition analysis(ICP-MS).According to the characterization results,the silver content in the sintering dust reached 610.4 g·t-1 and mainly existed in the form of AgCl,which was usually wrapped by ferric oxides,carbon particles and other substances.The chlorine content was 1.5%;the iron content in the sintering dust was 28.5% and mainly existed in the form of Fe2 O3 and Fe304.The average diameters of raw materials and leaching residue were 9.63 and 3.59 μm respectively,and the average diameters of specific surface areas were 3.74 and 0.78 μm respectively.Compared with the size distribution of the raw material,the size distribution of the leaching residue was smaller,which proved that the soluble material in the raw material could get a more adequate reaction.The leaching residue was iron oxide in phase,and its chemical composition was as follows:Ag content decreased to 78.2 g·t-1,chlorine content was only 0.096%,iron content increased to 52.6%,and element carbon content was 4.5%.The metal elements which were harmful to sintering process were almost all leached out,while the iron leach ratio was low and remained in the leaching residue.The leaching ratio of chlorine element was the highest,indicating that in the process of leaching of sintering dust,chlorine ion and silver element were selectively coordinated to form soluble compounds into the solution.High content of iron and carbon in the leaching residue,heavy metals such as zinc and alkali metals such as potassium were dissolved into the solution,the efficient leaching of zinc and alkali metals could avoid the cycle enrich?ment of zinc and alkali metals in the recycling process of leaching residue,and reduced the harm of zinc and alkali metals to sintering,blast furnace and other ironmaking processes.The experiment results showed that in weak acidic and neutral solution,silver chloride was leached mainly in the form of AgCl43- complex ions during the process of coordinate leaching,while iron and carbon were not leached into the residue,which meant that the leaching process was selective.Under the optimal conditions of the solution pH of 3.0,the reaction temperature of 80℃,chloride ion concentration of 6 mol·L-1,the reaction time of 2 h,liquid-solid ratio of 10-1 ml·g-1and stirring velocity of 400 r·min-1,the leaching ratio of silver was up to 93.90%,and the leaching ratio of iron was only 1.28%,the selective separation and extraction of silver was effective.Compared with other methods,chlorine self-coordination method was effective in extracting silver from sintered ash,and it was a new method to treat sintered dust.The extraction of silver from silver-containing solution was also a worthy research direction.Meanwhile,other ionic applications,such as potassium ion could be used to produce potash fertilizer,could also be studied.
Keyword:
sintering filtrated dust; coordinate leaching; comprehensive utilization of resources;
Received: 2019-07-01
烧结机头灰是在铁矿石烧结过程中,通过电除尘器收集的烟尘,且烟尘产量一般占烧结矿产量的2%~4%
自然界中银的存在形式多种多样,主要以硫化银的形式伴生于黑色金属矿石中
本文以云南某钢铁厂的烧结机头灰为原料,在分析其物化特性的基础上,采用烧结机头灰自身含有的氯元素为配位剂,通过配位浸出的方式提取回收银,为烧结机头灰中银的综合回收提供一种高效环保的提取方法。
1 实验
1.1 烧结机头灰化学成分和X射线衍射(XRD)分析
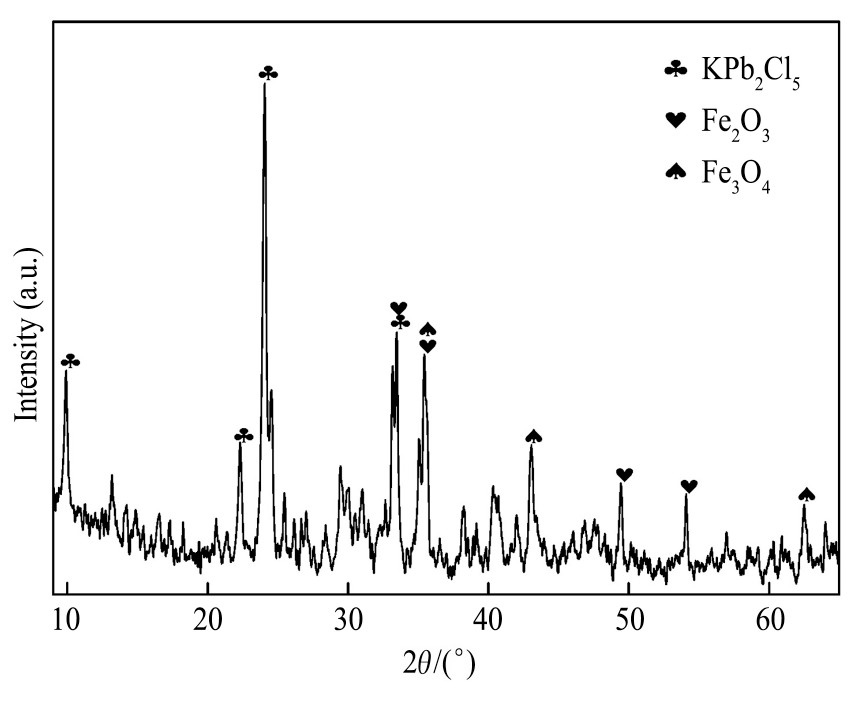
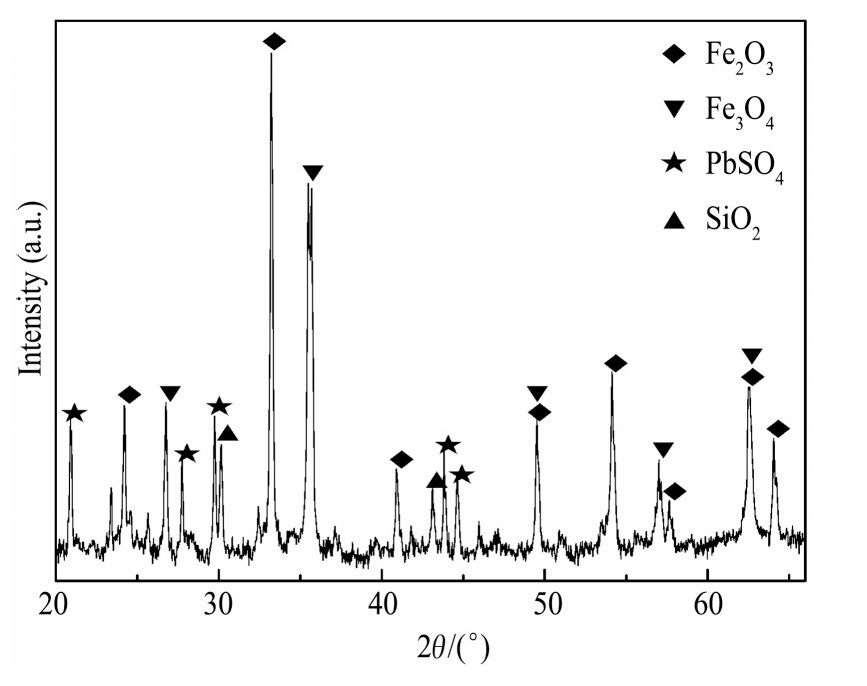
实验所用烧结机头灰源于云南某钢铁厂,外观呈灰褐色细小颗粒,原料主要的化学成分见表1,X射线衍射(XRD)分析如图1所示。
由表1可知,烧结机头灰中银含量达到610.4g·t-1,回收价值高。由图1可知,烧结机头灰中铁、铅、氯的主要物相分别为KPb2Cl5,Fe2O3和Fe3O4。烧结机头灰中的银主要以银、氯化银、氧化银、硫化银的形式存在,且被烧结机头灰中的铁的化合物(磁铁矿和赤铁矿)、铅化合物等包裹存在于烧结机头灰中
表1 烧结机头灰的化学成分分析 下载原图
Table 1 Chemical composition of analysis of sintering dust(%,mass fraction)
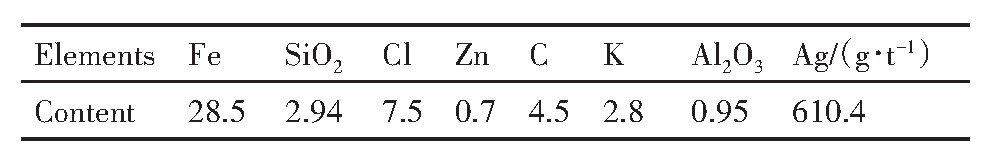
图1 烧结机头灰的XRD图谱
Fig.1 XRD pattern of sintering dust
1.2 实验原理
根据难溶化合物氯化银可以溶解于盐酸中,亦能形成配离子溶解在高浓度的卤离子溶液中
由于最稳定的一价银的配合物是线型结构,能产生多核配离子
计算表明,在处于热力学平衡的Ag-Cl--H2O系的水溶液中Ag2Cl+很少,可以忽略不计
Ag+与Cl-在水溶液中反应形成Ag Cli1-i(i=1,2,3,4)。总反应式如下
水溶液中Ag+总浓度[Ag+]T为:
对于水溶液中的Ag+,Ag Cl,Ag Cl2-,Ag Cl32-以及Ag Cl43-的[Ag+]T可以用φi(i=0,1,2,3,4)来代替,则有
假设溶液中的金属离子Mj(j=0,1,2,…k)能和氯离子结合生成氯配合物Mj Cli(i=0,1,2…n),则水溶液中的Cl-的总浓度[Cl-]T为
那么式(11)可以转化为式(14):
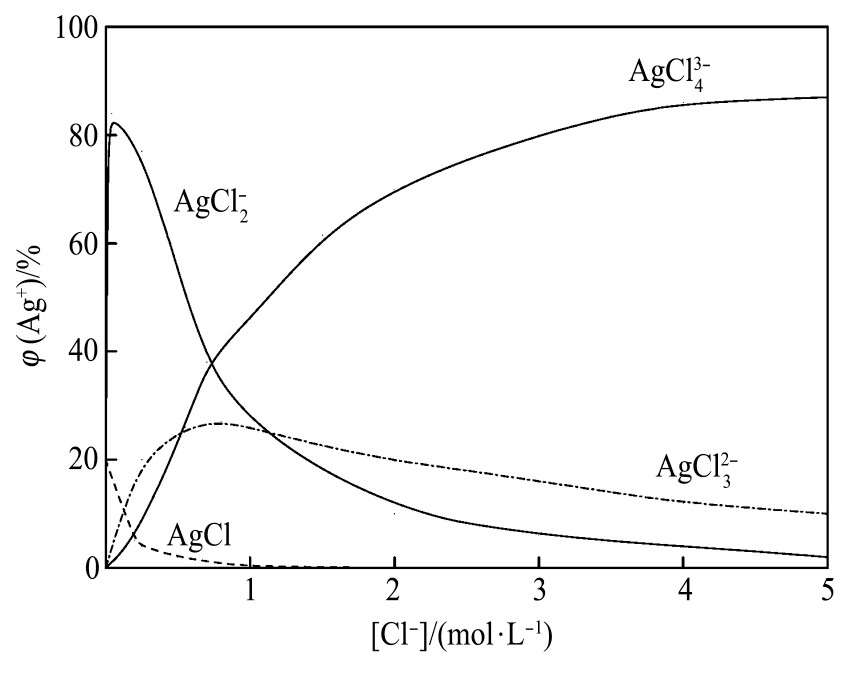
图2是Ag-Cl-H2O系中银氯的配合物随着溶液中[Cl-]变化的曲线图
由图2可见,溶液中Ag+和Cl-形成的配合物随着[Cl-]的变化而变化。当[Cl-]的浓度逐渐增大至3.0 mol·L-1时,溶液中80%以上都是Ag Cl43-。
1.3 实验设备与方法
采用数显恒温水浴锅(金坛市大地自动化仪器厂HH-S24S)控制浸出温度,使用三口烧瓶(云南科仪化玻有限公司)为浸出反应容器。利用电子天平(JE1001,上海浦春计量仪器有限公司)称量一定质量的烧结机头灰,同时量取适量氯化铵溶液,混匀后加入三口烧瓶中,调节搅拌转速为400 r·min-1,利用盐酸调节矿浆的p H值,控制反应温度的变化范围±0.5℃。
采用p H计(PHSJ-5,上海沪粤明科学仪器有限公司)测量溶液的p H值,反应结束后,停止搅拌,矿浆采用旋片真空泵(2XZ-2型,浙江台州求精真空泵有限公司)抽滤;滤渣经等体积蒸馏水洗涤抽滤后,在电热鼓风干燥箱内(DZF-6090,上海一恒科学仪器有限公司)恒温干燥(温度75℃)12 h后,制样送化验分析。
图2 银氯的配合物分布
Fig.2 Distribution of silver chloride complex
银的浸出率(η)计算公式为:
式中,η为银的浸出率;ω1为物料的质量;ω2为浸出渣的质量;α1为原料中银含量;α2为浸出渣中银含量。
1.4 烧结机头灰扫描电镜(SEM)图和粒度分析
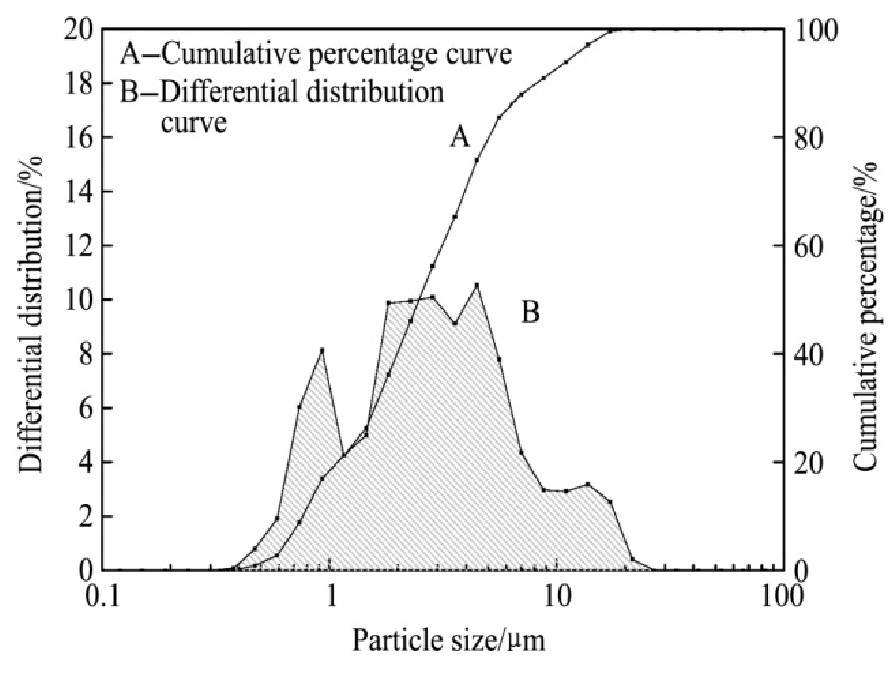
对烧结机头灰进行扫面电镜(SEM)和粒度分析,结果如图3和4所示。
图3 不同放大倍数的烧结机头灰的SEM图
Fig.3 SEM images of sintering dust with different magnifica-tion(a-d)
图4 烧结机头灰粒度分布图
Fig.4 Particle size distribution of sintering dust
从图3可以看出,烧结机头灰的粒度小,大部分颗粒表面较致密,界面明显,四周无粘附物,少部分颗粒表面有絮状物粘附,且疏松多孔。由图4粒度分析结果可知:烧结机头灰的粒径范围是0.38~66.2μm,粒径分布较集中,体积平均直径为9.63μm,比表面积平均直径为3.74μm,90%的粒子最大粒径为21.00μm,其余的颗粒均以小颗粒形式存在。
2 结果与讨论
2.1 溶液p H对银浸出率的影响
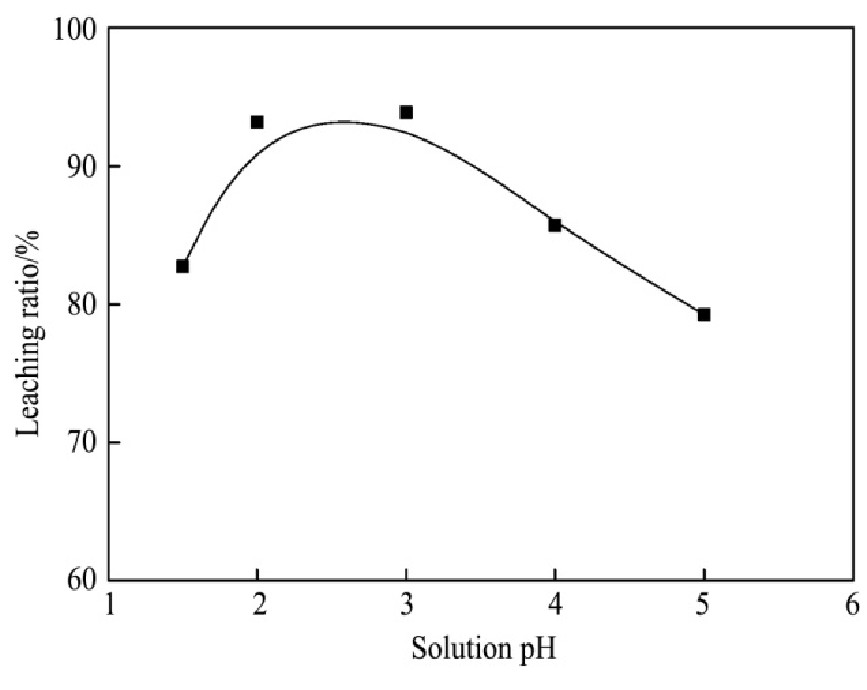
实验条件:反应温度80℃,氯离子浓度6 mol·L-1,浸出3 h,液固比10∶1 ml·g-1,改变溶液p H值,考察不同p H对银浸出率的影响。实验结果如图5所示。
由图5可见,随着溶液p H值的增大,银的浸出率呈现先上升后下降的趋势。当p H=1.5时,银浸出率为82.7%;当p H=2.0时,银浸出率为93.2%;当p H=3.0时,银浸出率达到最大,为93.9%。随着反应的进行,溶液p H逐渐上升,溶液中的H+浓度逐渐减小,Cl-与Ag+的配位作用逐渐减弱,氯化银的溶解度减小
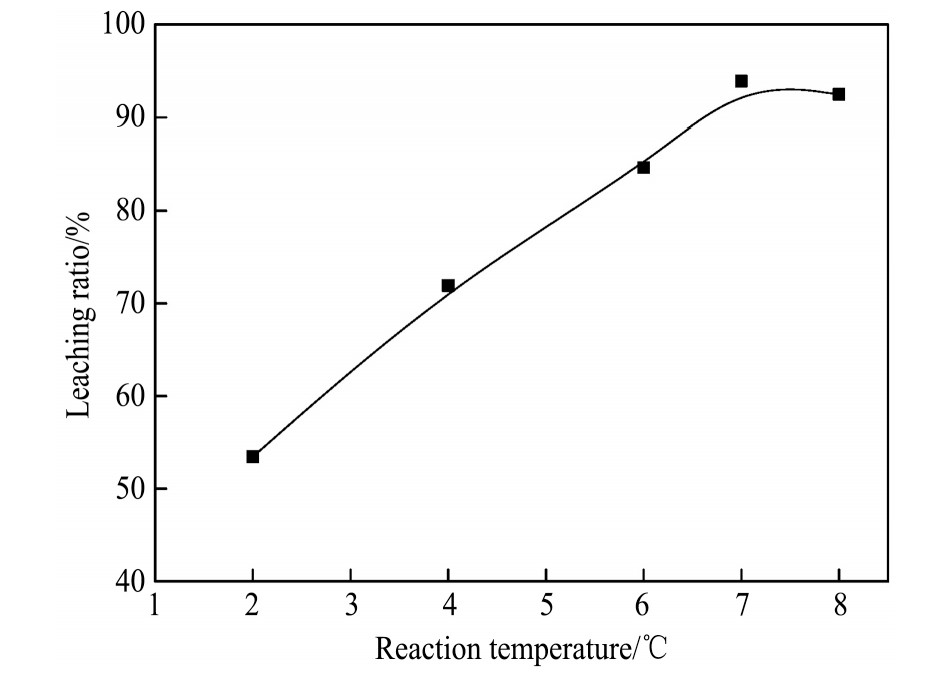
2.2 反应温度对银浸出率的影响
实验条件:溶液p H=3.0,氯离子浓度6 mol·L-1,浸出3 h,液固比10∶1 ml·g-1,改变浸出反应温度,考察温度对银浸出率的影响。实验结果如图6所示。
由图6可见,升高反应温度,银的浸出率呈现上升趋势。温度升高,反应物的反应活性增强,反应物和产物扩散速度加快
图5 溶液p H对银浸出率的影响
Fig.5 Effect of solution p H on leaching ratio of silver
图6 反应温度对银浸出率的影响
Fig.6 Effect of reaction temperature on leaching ratio of silver
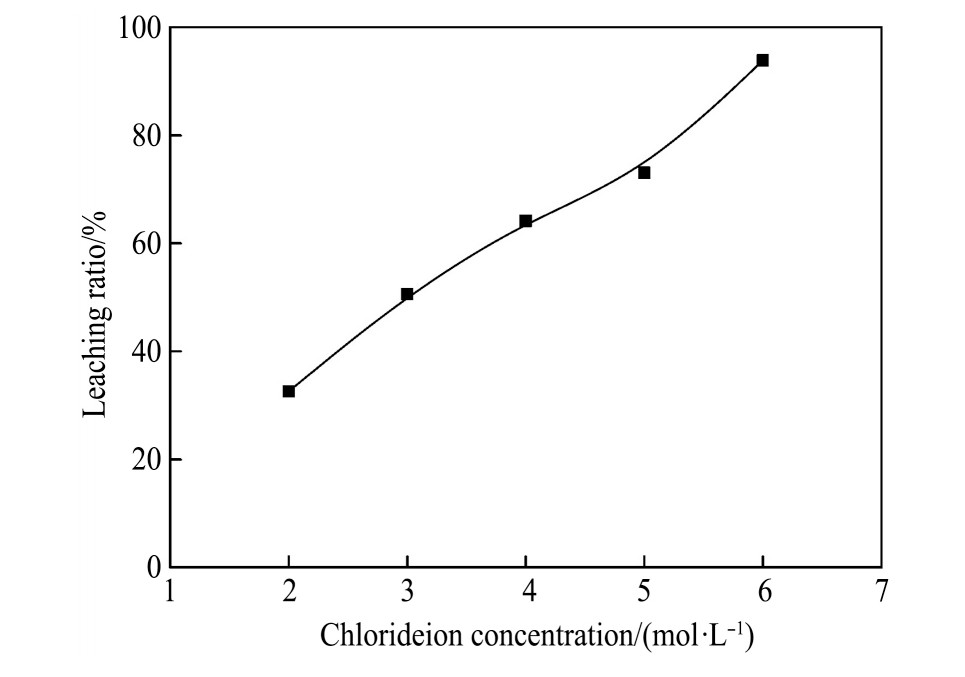
2.3 配位剂浓度对银浸出率的影响
实验条件如下:溶液p H=3.0,反应温度80℃,浸出3 h,液固比10∶1 ml·g-1,改变加入氯离子的浓度,考察在不同浓度下银浸出率的影响。实验结果如图7所示。
由图7可见,在一定范围内,随着配位剂浓度的增加,银的浸出率呈上升趋势。当Cl-浓度为2mol·L-1时,银浸出率为32.6%;随着浓度的升高,当Cl-的浓度为6 mol·L-1时,银的浸出率最大,为93.9%。Cl-浓度越高,与游离Ag+配位生成络合物的几率就越大,氯化银的溶解度也越大
图7 氯离子浓度对银浸出率的影响
Fig.7 Effect of chlorideion concentration on leaching ratio of silver
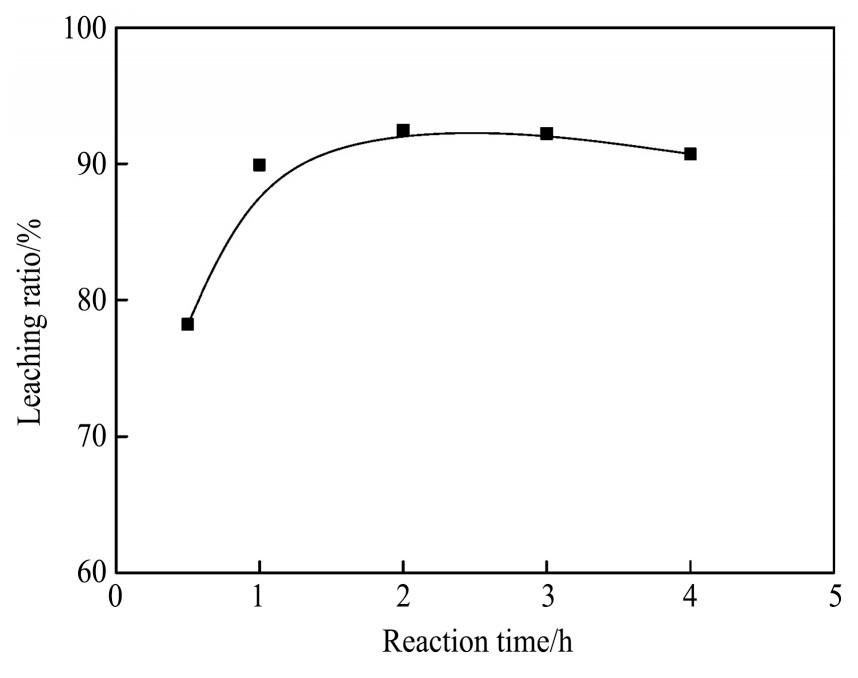
2.4 反应时间对银浸出率的影响
实验条件:溶液p H=3.0,反应温度80℃,氯离子浓度6 mol·L-1,液固比10∶1 ml·g-1,改变反应时间,考察时间对银浸出率的影响,实验结果如图8所示。
由图8可见,随着浸出反应时间的增加,银的浸出率先变大后降低。当浸出时间为0.5 h时,银浸出率为79.48%;当浸出时间为1 h时,银浸出率为89.4%;当浸出时间为2 h时,浸出率达到最大,为94.8%。随着浸出反应时间的延长,当浸出时间达到4 h时,浸出率只有84.5%。由于浸出时间越长,浸出剂的挥发损失将增多,银氯的配位作用逐渐减弱,银浸出率降低,同时也不利于后续工艺。因此浸出时间选择2 h为宜。
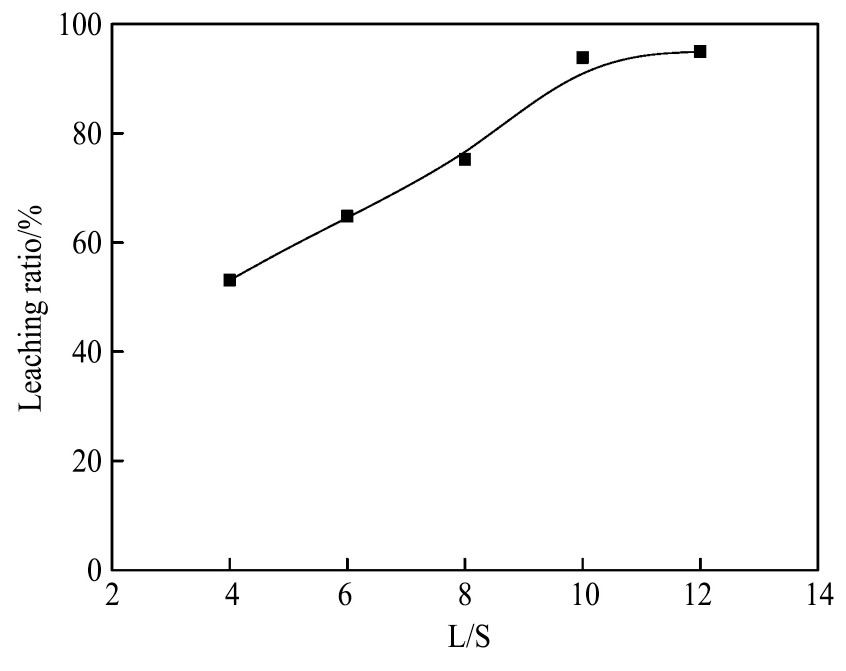
2.5 反应液固比(L/S)对银浸出率的影响
实验条件:溶液p H=3.0,反应温度80℃,氯离子浓度6 mol·L-1,浸出2 h,改变反应液固比,考察液固比(L/S)对银浸出率的影响,实验结果如图9所示。
由图9可见,液固比对银浸出率影响较大,随着反应液固比的增大,银浸出率逐渐上升。当L/S=4 ml·g-1时,银浸出率为53.1%;当L/S=8 ml·g-1时,银浸出率已为75.3%。随着反应液固比增大,银浸出率显著升高。当L/S=10 ml·g-1时,银的浸出率达到93.9%。此后,液固比继续增大,浸出率达到最大值95%,上升了1.1%,上升的幅度较小。而且从实验原料和实验试剂的使用情况来看,液固比越大,原料和试剂消耗越大,造成成本升高,溶液后续处理复杂,故选择液固比10:1 ml·g-1最合适。
图8 反应时间对银浸出率的影响
Fig.8 Effect of reaction time on leaching ratio of silver
图9 反应液固比对银浸出率的影响
Fig.9 Effect of reaction L/S on leaching ratio of silver
3 浸出渣表征
为了更清楚地了解烧结机头灰在浸出前后矿物发生的形态变化,在上述研究基础上选用最佳工艺条件:溶液p H=3.0,反应温度80℃,氯离子浓度6 mol·L-1,液固比10:1 ml·g-1,浸出时间2 h开展综合验证实验。对综合验证实验得到的浸出渣分别进行化学成分分析、XRD分析及SEM分析。
3.1 浸出渣成分分析
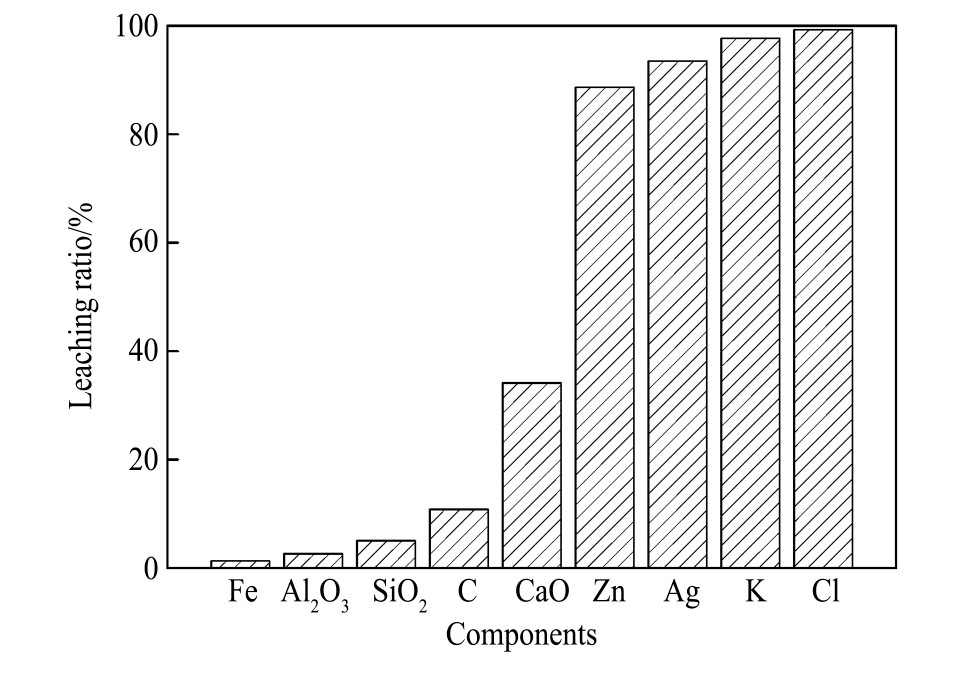
对浸出渣的化学成分进行检测,结果如表2所示,各组分的浸出率如图10所示。
由表2可见,浸出配位浸出反应后,浸出渣中的银、氯等元素含量较原料成分明显降低,结合图10可知,银、锌、钾、氯的浸出率分别为88.7%,93.5%,97.7%和99.1%;铁、铝、硅、碳的浸出率分别是1.3%,2.6%,5.01%和10.8%。对烧结过程有害的金属元素几乎全部浸出,而铁的浸出率低,残留在浸出渣中。氯元素的浸出率是最高的,表明在烧结机头灰浸出过程中氯离子与银的元素进行选择性配位浸出,形成可溶解的化合物进入溶液中。浸出渣中铁和碳元素含量高,分别为52.6%和4.5%,锌等重金属和钾等碱金属被溶解进入溶液,锌及碱金属的高效浸出可以避免浸出渣在循环利用过程中造成锌和碱金属的循环富集,降低锌和碱金属等对烧结、高炉等炼铁过程的危害。
表2 浸出渣的主要成分 下载原图
Table 2 Main components of leaching residue(%,mass fraction)
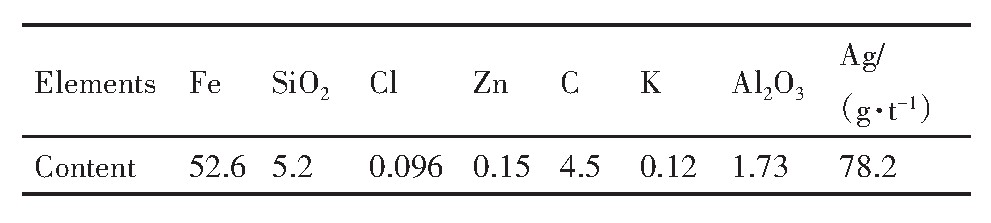
图1 0 浸出渣中几种主要组分浸出率
Fig.10 Leaching ratio of several main components in leaching residue
3.2 浸出渣XRD分析和粒度分析
为了进一步分析浸出渣的物相组成及粒度分布情况,对浸出渣进行XRD和粒度分析检测,结果如图11和12所示。
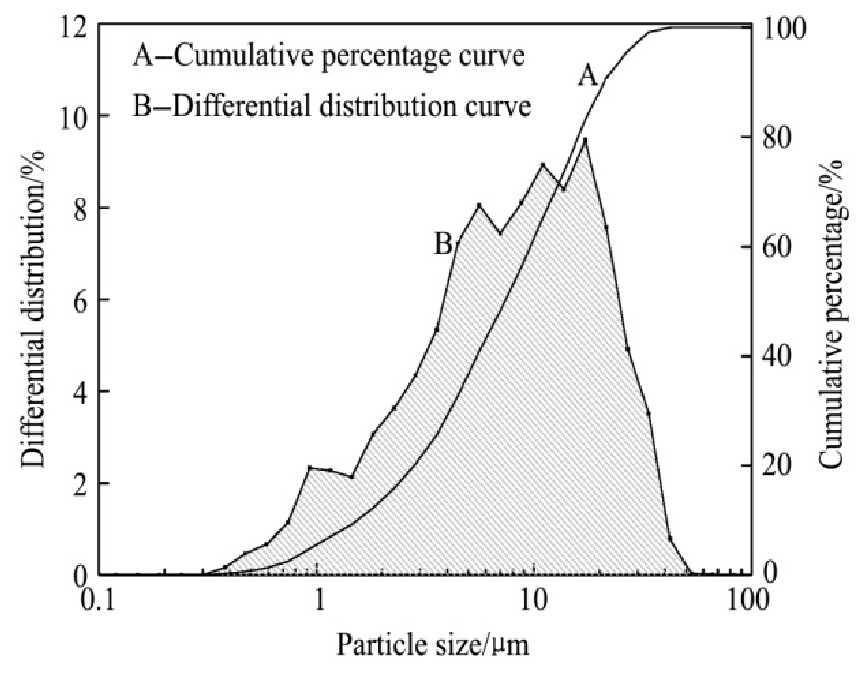
从图11的XRD分析结果可以知道,浸出渣中的物相组成简单,所含的有价元素主要是铁、硅、铅等。其中,铁主要以Fe2O3和Fe3O4两种形态存在,且可以看出渣中大部分为Fe2O3。由图12的粒度检测结果可知:浸出渣的粒径范围是0.38~21.53μm,粒径分布比较集中,浸出渣的体积平均直径为3.59μm,比表面积平均直径是0.78μm,90%粒子的最大粒径是8.20μm,粒度小。相比原料的粒径分布而言,浸出渣的粒径分布变小,证明原料中的可溶解物质得到较充分的反应,同时结果表明几乎所有较大的烧结机头灰颗粒在浸出过程中破碎成小颗粒物质,且反应完成后部分细小的颗粒团聚在较大颗粒周围,形成团聚体附着在部分烧结矿颗粒表面。
图1 1 浸出渣的XRD图谱
Fig.11 XRD pattern of leaching residue
图1 2 浸出渣粒度分布图
Fig.12 Particle size distribution of leaching residue
3.3 浸出渣能谱(SEM-EDS)分析
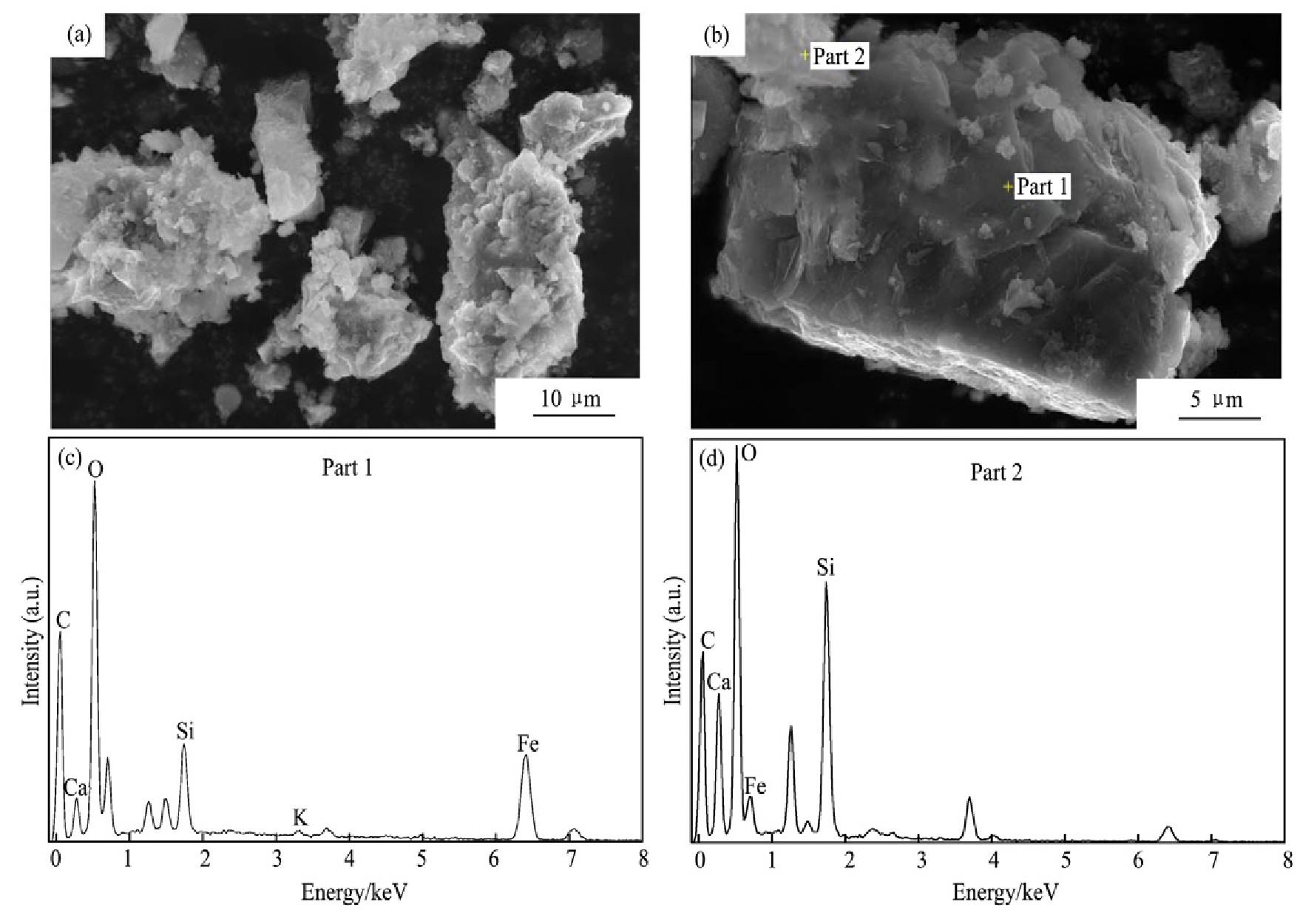
为了进一步分析浸出渣的微观形貌和化学成分,对浸出渣进行能谱(SEM-EDS)检测,结果如图13所示。
由图13的浸出渣SEM图和EDS谱图可见:烧结机头灰浸出渣颗粒较为规整,周围并无絮状物粘附,且根据粒径检测知颗粒的粒径不大,在颗粒表面多是反应完之后重新凝聚在一起的团聚体,有的颗粒表面较光滑致密,有的颗粒表面粗糙疏松。渣中主要是Fe,Ca,C,O 4种元素,且渣中铁元素含量达到52.6%,主要形态为铁的化合物Fe2O3和Fe3O4,表明烧结机头灰中铁氧化物在浸出时几乎不参与反应,浸出结束后在浸出渣中得到富集。
4 结论
本文采用氯化配位浸出的方法,研究了烧结机头灰中银的配位浸出行为,获得的主要结论如下:
1.经XRD,FESEM-EDS等分析知:烧结机头灰中Ag含量达到610.4 g?t-1,主要以Ag Cl的形态存在;原料中的氯含量达到7.52%,主要以氯化物形态存在;烧结机头灰中的铁主要以Fe3O4和Fe2O3两种形态存在。
图1 3 浸出渣的SEM图片和EDS谱
Fig.13 SEM images(a,b)and EDS spectra of selected positions(c,d)
2.根据烧结机头灰中的氯和银含量高的特点,采用配位浸出提取烧结机头灰中的银,使银转变为Ag Cl43-络合离子形态选择性溶解进入溶液。通过单因素实验得到最佳条件:溶液p H=3.0,反应温度80℃,氯离子浓度为6 mol·L-1,反应时间2 h,液固比10:1 ml·g-1,搅拌速度400 r·min-1。在此条件下,银的浸出率可达93.90%,铁的浸出率仅为1.28%,实现了银的选择性配位浸出。浸出渣中银含量很少,浸出渣中主要为对钢铁冶炼有用的铁和碳等元素,对炼铁有害的锌和碱金属均被高效溶出,实现与铁和碳的分离。因而,脱除有害杂质元素的浸出渣可以返回炼铁工艺流程循环利用,实现多元素的综合回收。
3.采用烧结机头灰中本身富含的氯配位离子为配位剂,直接配位浸出银的技术路线,具有操作简单、浸出率高、环境污染小、成本低等优点,为该类烧结机头灰中银的浸出提供了一条新的技术途径。
参考文献