
Thermodynamic investigation of ZrO2-BaO system
GONG Wei-ping(龚伟平), CHEN Teng-fei(陈腾飞), JIN Zhan-peng(金展鹏)
State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China
Received 28 June 2006; accepted 11 January 2007
Abstract:
Thermodynamic description of ZrO2-BaO system was developed using the available experimental information. Special attention was paid to the modelling of the perovskite phase BaZrO3 by a temperature-dependent polynomial to fit the experimental thermodynamic properties. The liquid phases, CSS (cubic ZrO2 solid solution) and TSS (tetragonal ZrO2 solid solution) were modelled with Redlich-Kister formula. The compounds Ba2ZrO4 and Ba3Zr2O7 were treated as stoichiometric phases and the BaOSS and MSS (halite BaO and monoclinic ZrO2 solid solutions) were treated as pure compounds. Comparisons between the calculated and the measured phase diagram as well as the thermodynamic quantities indicate that the most reliable experimental information is satisfactorily accounted for by the present thermodynamic calculation.
Key words:
ZrO2-BaO system; thermodynamic description; perovskite phase; Redlich-Kister formula;
1 Introduction
Doped with certain amount of MgO, CaO, SrO, BaO, Y2O3 or CeO2, the disruptive martensitic phase transformation of pure ZrO2 from monoclinic to tetragonal can be suppressed, thus the mechanical properties are greatly improved[1]. While the mechanical properties of partially stabilized zirconia(PSZ) or tetragonal zirconia polycrystals(TZP) with a smaller amount than needed to produce fully stabilized zirconia, are quite different from each other[2-4]. Obviously, the knowledge of the phase diagram and thermodynamic properties is of fundamental importance to define the processing conditions for making these materials and subsequent treatments to obtain optimal engineering properties. Associated with the sluggish kinetics at low temperatures, the formation of meta-stable phases, and the difficulties in conducting experiments at high temperatures, etc, the phase equilibria in ZrO2-included systems have been proved to be difficult to determine with certainty. Since only calorimetry, the electromotive force and mass spectrometry are practicable, measurements of the thermodynamic properties in ZrO2-included systems are also not easy from the experimental point of view. When the present investigation was initiated, the literature experimentally dealing with the ZrO2-BaO system gave an incomplete and confusing picture[5-8]. In this work, the Calphad technique was employed to determine the phase relations and thermodynamic properties in ZrO2-BaO system with the aim to assess the thermodynamic properties of the various phases in the ZrO2-BaO system and to provide the optimized thermodynamic parameters, which can reproduce the reliable experimental phase diagram, thermodynamic data and structural information satisfactorily.
2 Evaluation of experimental data in literature
2.1 Phase diagram information
PASCHOAL et al[6] and SHEVCHENKO et al[7] were responsible for the major contributions to understanding the ZrO2-BaO liquidus phase diagram. By means of optical microscopy, X-ray diffraction(XRD) and differential thermal analysis(DTA) techniques, PASCHOAL et al[6] measured the liquidus in the BaO-rich side. Using the DTA method, SHEVCHENKO et al[7] constructed a series of the phase diagrams of ZrO2/HfO2-based systems, and the attention was paid to the ZrO2-rich side. The ZrO2-BaO liquidus equilibria reported by SHEVCHENKO et al[7] were in good agreement with those measured by WARTENBERG et al[8]. Based on the available experimental information, the ZrO2-BaO system was characterized by BaZrO3 and Ba2ZrO4 compounds, in which BaZrO3 melted congruently at (2 978±10) K, and Ba2ZrO4 decomposed peritectically at about 2 600 K. The present work was mainly based on these experimental data to set up the liquidus equilibria, on the grounds that the high-purity specimens were employed, and experimental procedures were well described.
Both PASCHOAL et al[6] and SHEVCHENKO et al[7] did not report the compound Ba3Zr2O7, while the available information on {(1-x)(BaO)+x(ZrO2)} indicated the existence of Ba3Zr2O7 in the ZrO2-BaO system[8-9] and the phase diagram calculated by DASH et al[10] for {(1-x1-x2)(Ba)+x1(Zr)+x2(O)} indicated that Ba3Zr2O7 co-existed with BaZrO3 at 1 287 K. And more, Ba3Zr2O7 was really prepared by the reaction of BaCO3(s) and ZrO2(s) in the mole ratio of 3/2 in an alumina boat at 1 300 K for 400 h in dry air[10] and was found at 1 650 K[11]. Consequently, special attention was paid to the compound Ba3Zr2O7 in this work with the aim to understand the difference existing in Refs.[6-11].
The maximum solubility of BaO in c-ZrO2 (cubic ZrO2) and t-ZrO2 (tetragonal ZrO2) is 0.025 mol [7]. This small solubility of BaO in ZrO2 benefits the mechanical properties of zirconia very much and the measured data [7] were used to evaluate the thermodynamic properties of CSS (cubic ZrO2 solid solution) and TSS (tetragonal ZrO2 solid solution). The very limited solubility of ZrO2 in halite BaO (about 0.005 mol)[6-8] was ignored. No experimental data were reported for the solubility of BaO in m-ZrO2 (monoclinic ZrO2).
2.2 Structural and thermodynamic information
The structural behavior and the thermodynamic properties of the perovskite phase BaZrO3 have been the subjects of many investigations associated with the technological application[12-19]. Using high- temperature X-ray diffractometry, MATHEWS et al[12] measured the cell parameters, unit-cell volumes and the coefficients of volume thermal expansion of the zirconia CaZrO3, SrZrO3 and BaZrO3 in the temperature range from 298 to 1 675 K. The linear variation of the unit-cell volume and the cell parameters without any discontinuity indicated that the compound BaZrO3 did not take place any phase transition, and the structure of BaZrO3 was cubic. Using electromotive force, JOCAB et al[13] reported the enthalpies of formation of both SrZrO3 and BaZrO3 from the component oxides in the temperature range from 960 to 1 210 K. Based on the slope of electromotive force, SrZrO3 was detected to undergo a structural transformation at about 1 123 K, while no structural transformation was detected for the compound BaZrO3[13]. Because the experimental procedures were well controlled and the experimental results were generally consistent with each other[12-13], the structure of BaZrO3 was treated as cubic in the whole temperature range.
Several groups[13-19] investigated the thermo- dynamic properties of BaZrO3. Using high-temperature differential calorimeter, NAGARAJAN et al[14] reported the enthalpy increments H(T)-H(298) for BaZrO3 from 1 000 to 1 700 K. Drop calorimetry was used by LEVITSKII[15], for H(T)-H(298) measurements on BaZrO3, respectively. BANERJEE et al[16] determined H(T)-H(298) of BaZrO3 by a precise high-temperature Calvet micro- calorimeter in the temperature range from 365.7 to 981 K. CORDFUNKE et al[17] also reported the enthalpy values for BaZrO3 in their compilations. GOSPODINOV et al[18] reported enthalpy data for BaZrO3 in the temperature range between 298 and 500 K by differential scanning calorimeter(DSC). These experimental data[14-16] except those of Ref.[18] were in good agreement with each other and join smoothly with the adiabatic measurements carried out by KING et al[19]. They were used to evaluate the Gibbs energy of BaZrO3. Data from GOSPODINOV et al[18] were too high to have any physical meaning and were excluded in this work. The estimated data from CORDFUNKE et al[17] were not adopted, since they were directly connected with the thermodynamic parameters.
The estimated thermodynamic properties at 298 K, i.e. the enthalpy of formation of BaZrO3 ?fH(298)= (-1 768.58±2) kJ/mol[13,15], the molar heat capacity Cp(298)=(101.71±0.31) J/(mol?K)[19] and the standard entropy SΘ(298)=(124.7±2) J/(mol?K)[19] were adopted so that the evaluation can be carried out practically, but a low weight factor was applied to them.
The Gibbs energies of formation of BaZrO3 relative to the pure oxides in the temperature range from 960 to 1 210 K, and from 1 180 to 1 320 K were determined by JOCAB et al[13] and LEVITSKII[15] using electro- motive force, respectively. JOCAB et al[13] also reported the enthalpies of formation of BaZrO3 from the component oxides in the temperature range from 960 to 1 210 K, which were in good agreement with the value at 1 060 K reported by MUROMACHI et al[20]. These data were not used in the optimization. However, they were compared with the calculated results in order to check the final modelling.
For the compounds Ba2ZrO4 and Ba3Zr2O7, the enthalpies of formation from the component oxides at 298 K were determined[11, 21]. These data were adopted to optimize the parameters of the compounds.
All temperatures quoted in this work were converted into the International Temperature Scale of 1990[22]. The thermodynamic functions were calculated at the normal constant pressure.
3 Thermodynamic models
The Gibbs energy function ![]() =
=![]() -
- ![]() for the component i (i=ZrO2, BaO) in the phase F is expressed by
for the component i (i=ZrO2, BaO) in the phase F is expressed by
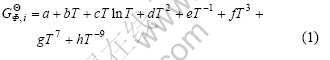
where ![]() is the molar enthalpy of the component i at 298.15 K and 101 325 Pa in its standard element reference state, and T is the absolute temperature. The last two terms in Eqn.(1) are used only outside the ranges of stability, the term gT7 is relative to the liquid below the melting point and hT –9 to the solid phases above the melting point.
is the molar enthalpy of the component i at 298.15 K and 101 325 Pa in its standard element reference state, and T is the absolute temperature. The last two terms in Eqn.(1) are used only outside the ranges of stability, the term gT7 is relative to the liquid below the melting point and hT –9 to the solid phases above the melting point.
In the present work, the Gibbs energy functions of pure ZrO2, ![]() and
and ![]() were taken from the assessments of DU et al[23]. The Gibbs energy functions of BaO,
were taken from the assessments of DU et al[23]. The Gibbs energy functions of BaO, ![]() and
and ![]() were consistent with those used by LU et al[24], which were little different from those of SGTE substance database since some modification was made. Here, l denotes liquid, c, t and m stand for cubic, tetragonal and monoclinic ZrO2, respectively, h represents halite BaO. The Gibbs energy functions employed in this work are expressed as
were consistent with those used by LU et al[24], which were little different from those of SGTE substance database since some modification was made. Here, l denotes liquid, c, t and m stand for cubic, tetragonal and monoclinic ZrO2, respectively, h represents halite BaO. The Gibbs energy functions employed in this work are expressed as


Due to the lack of experimental information, the Gibbs energies of the hypothetical meta-stable ZrO2 and BaO were expressed relative to those of their stable structures:
![]()
where the coefficients Ai and Bi (i=1, 2) are to be assessed by checking their influence on the calculated phase equilibria.
The liquid phase and solid solutions CSS and TSS were described by Redlich-Kister polynomials. The Gibbs energy for the liquid was expressed by
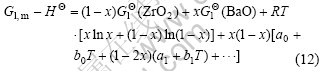
where HΘ is the abbreviation of ![]()
![]() R is the gas constant, and X is the molar fraction of BaO. The interactive parameters a0, b0, a1 and b1 are to be optimized. The CSS and TSS were described by an analogous equation. Due to the very limited solubility of ZrO2 in halite BaO and BaO in m-ZrO2, the BaOSS (halite BaO solid solution) and MSS (monoclinic ZrO2 solid solution) were treated as pure compounds in this work.
R is the gas constant, and X is the molar fraction of BaO. The interactive parameters a0, b0, a1 and b1 are to be optimized. The CSS and TSS were described by an analogous equation. Due to the very limited solubility of ZrO2 in halite BaO and BaO in m-ZrO2, the BaOSS (halite BaO solid solution) and MSS (monoclinic ZrO2 solid solution) were treated as pure compounds in this work.
The compounds Ba2ZrO4 and Ba3Zr2O7 were modelled as stoichiometric phases due to the very limited information. The Gibbs energy of Ba2ZrO4 was given by
![]()
where A3 and B3 are connected with the enthalpy and entropy of formation from pure oxides in solid state, which will be evaluated in the course of optimization. The analogous equation, Eqn.(14) can be written for the Gibbs energy of Ba3Zr2O7:
![]()
Since there were experimental thermodynamic data for BaZrO3 in a wide temperature range[12-19], it was preferable to express its Gibbs energies relative to the SER state, and the following equation was used:
![]()
where the coefficients c1, d1 and e1 can be evaluated mainly from the measured enthalpy increments[14-16].
4 Optimization procedure
The Thermo-calc software package was used to optimize the thermodynamic parameters. The critically selected experimental data were employed with some certain weights, and the weights can be changed systematically until most of the experimental data were accounted for within the claimed uncertainty limits.
Optimization was started with the perovksite phase BaZrO3. By using the measured thermodynamic data [14-16] selected in the above section, the numerical values of the coefficients c1, d1 and e1 in Eqn.(15) describing the Gibbs energy function of BaZrO3 could be critically evaluated. Once c1, d1 and e1 have been estimated, the coefficients a1 and b1 in Eqn.(15) could be estimated from the reported heat capacity, entropy, and enthalpy of formation of BaZrO3 at 298 K[13, 19]. The obtained coefficients were subjected to further optimization, the calculated thermodynamic properties of BaZrO3 at 298 K were compared with the literature available data[13,15, 19-20] in Table 1.
Table 1 Estimated thermodynamic properties of perovskite phase BaZrO3 at 298 K compared with measured values

Since the liquidus has been measured over a wide composition and temperature region[6-8], the optimization for its thermodynamic parameters is very important to the successful description of the ZrO2-BaO system. And since there are not any independent data for the liquid phase, such as experimental enthalpy of the mixture, the CSS, TSS and Ba2ZrO4 should be modelled to reproduce the liquidus throughout the system. In this case, a suitable number of the thermodynamic parameters for each phase was really important to the optimization. It was found that the parameters a0, b0, a1 and b1 in Eqn.(12) should be introduced to describe the properties of the liquid satisfactorily. For the CSS and TSS phases, the experimental data were few, so the regular substitutional parameters were introduced to describe the relative properties. The coefficients A3 and B3 in Eqn.(13), were used to describe the compounds Ba2ZrO4. With most of experimental data[5-8] reproduced quite well, special attention was paid to the compound Ba3Zr2O7 to check the possibility of its existence. Because there were not any phase diagram data about the compound Ba3Zr2O7, the estimated enthalpy of formation of Ba3Zr2O7 from the component oxides at 298 K[11] was used to optimize the coefficients A4 and B4 in Eqn.(14).
All the thermodynamic parameters were finally evaluated together and slight adjustments were made to give the best description of the system.
5 Results and discussion
The present evaluated thermodynamic parameters of the ZrO2-BaO binary system are as follows:
The interaction parameters of liquid:
a0=-357 817.66, b0=73.4
a1=-282 549.78, b1 = 93.9
The interaction parameters of the CSS phase:
a0=-104 672.96, b0=-8.4
The interaction parameters of the TSS phase:

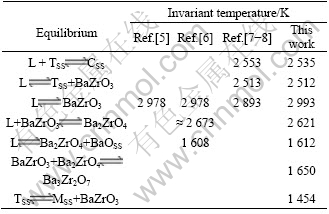
A comparison between the calculated ZrO2-BaO phase diagram and that measured by ADAMSON et al [5-8] is shown in Fig.1. The calculated invariant reaction temperatures are compared with the experimental ones[5-8] in Table 2. It can be seen from Table 2 that the present modelling can account for the measured invariant temperatures[5-8] quite well. The eutectoid reactions TSS![]() MSS+BaZrO3 at 1 454 K and the peritectiod reaction Ba3Zr2O7
MSS+BaZrO3 at 1 454 K and the peritectiod reaction Ba3Zr2O7![]() Ba2ZrO4+BaZrO3 at 1 650 K are the results of calculations. These reactions are not easy to be experimentally detected because of the following reasons: 1) it is not easy to detect Ba3Zr2O7 from Ba2ZrO4 or BaZrO3 due to the near composition; 2) it is very difficult for oxides to achieve equilibrium at a low temperature such as 1 454 K. Better knowledge on the phase equilibria at low temperatures is useful for further refinement of the ZrO2-BaO system.
Ba2ZrO4+BaZrO3 at 1 650 K are the results of calculations. These reactions are not easy to be experimentally detected because of the following reasons: 1) it is not easy to detect Ba3Zr2O7 from Ba2ZrO4 or BaZrO3 due to the near composition; 2) it is very difficult for oxides to achieve equilibrium at a low temperature such as 1 454 K. Better knowledge on the phase equilibria at low temperatures is useful for further refinement of the ZrO2-BaO system.
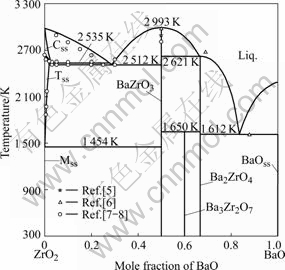
Fig.1 Comparison between calculated ZrO2-BaO phase diagram and experimental data in Refs.[5-8]
Table 2 Comparison between calculated and measured coordinates of invariant temperatures in ZrO2-BaO system
In Fig.2, the calculated H(T)-H(298) for BaZrO3 is compared with the experimental values[14-16]. The agreement between the calculated and measured values of H(T)-H(298) is acceptable within the experimentaluncertainties.
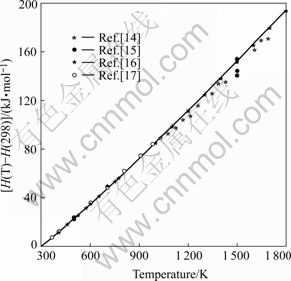
Fig.2 Comparison of calculated H(T)-H(298) of BaZrO3 with measured data [14-16]
The calculated Gibbs energy and enthalpy of formation for BaZrO3 from the pure oxides are compared with the experimental data[13, 15, 19-20] in Tables 3 and 4, respectively. The calculation can describe these experimental data reasonably although they are not used in the present optimization.
Table 3 Gibbs energy of formation of BaZrO3 from component oxides compared with measured values in Refs.[13, 15]

Table 4 Enthalpy of formation of BaZrO3 from component oxides compared with measured values in Refs.[13, 20]

The present calculated enthalpy of formation for the compounds Ba2ZrO4 and Ba3Zr2O7 from the component oxides at 298 K are compared with the literature data in Table 5, a good agreement is obtained.
Table 5 Comparison of enthalpy of formation for Ba2ZrO4 and Ba3Zr2O7 from component oxides at 298 K

6 Conclusions
1) The thermodynamic function of the perovskite phase BaZrO3 is critically evaluated with all reliable experimental data reproduced within the estimated experimental uncertainty. A set of reasonable and self-consistent thermodynamic parameters for the ZrO2-BaO binary system is obtained. The calculated phase diagram and thermodynamic properties data are in good correspondence with the experimental information.
2) The compound Ba3Zr2O7 decomposes to Ba2ZrO4 and BaZrO3 at 1 650 K. Thermodynamic and phase diagram measurements on Ba3Zr2O7 are useful for further refinement of the compound and the ZrO2-BaO system.
References
[1] RUFF O, EBERT F. Refractory ceramics (I): The form of zirconium dioxide [J]. Z Anorg Allg Chem, 1929, 180: 19-41.
[2] GARVIE R C, HANNINK R H, PASCOE R T. Ceramic steel [J]. Nature, 1975, 258: 703-704.
[3] PORTER D L, HEUER A H. Mechanisms of toughening partially stabilized zirconia (PSZ) [J]. J Am Ceram Soc, 1977, 60: 183-184.
[4] GUPTA T K, LANGE FF, BECHTOLD J H. Effect of stress-induced phase transformation on the properties of polycrystalline zirconia containing metastable tetragonal phase [J]. J Mater Sci, 1978, 13: 1464-1470.
[5] ADAMSON M G, AITKEN E A, CAPUTI R W. Experimental and thermodynamic evaluation of the melting behavior of irraniated oxide fuels [J]. J Nuclear Materials, 1985, 130: 349-365.
[6] PASCHOAL J O A, KLEYKAMP H, TH?MMLER F. Phase equilibria in the pseudo-quaternary BaO-UO2-ZrO2-MoO2 system [J]. J Nuclear Materials, 1987, 151: 10-21.
[7] SHEVCHENKO A V, LOPATO LM, GERASIMYUK G I, ZAITSEVA Z A. Reactions in the systems hafnium dioxide-stronium oxides, hafnium dioxide-barium oxide and zirconium dioxide-barium oxide in the regions with a high content of a hafnium dioxide (zirconium dioxide) [J]. Izv Akad Nauk SSSR Neorg Mater, 1987, 23(9): 1495.
[8] WARTENBERG H V, GURR W. A compilation of phase-rule diagrams of interest to the ceramist and silicate technologist [J]. Z Anorg Allg Chem, 1931, 196: 374-383.
[9] APPENDINO P, RAMONDA G. New compounds in the barium oxide-zirconium oxide system [J]. Ann Chim, 1971, 61(1): 61-65.
[10] DASH S, SINGH Z, PRASAD R, SOOD D D. The standard molar Gibbs free energy of formation of Ba3Zr2O7 (s) [J]. J Chem Therm, 1994, 26: 737-744.
[11] MARUSHKIN K N, ALIKHANYAN A S, GRINBERG Y K, KURTASOV O V, GORGORAKI V I. Vapor composition in the barium oxide-zirconium dioxide system and thermodynamic properties of barium zirconates [J]. Zh Nerg Khim, 1989, 34(6): 1592-1597.
[12] MATHEWS M D, MIRZA E B, MOMIN A C. High-temperature X-ray diffractometric of CaZrO3, SrZrO3 and BaZrO3 [J]. J Mater Sci Lett, 1991, 10: 305-306.
[13] JOCAB K T, WASEDA Y. Potentiometric determination of the Gibbs energies of formation of SrZrO3 and BaZrO3 [J]. Metall Mater Trans, 1995, 26B: 775-781.
[14] NAGARAJAN K, SAHA R, BABU R, MATHEWS C K. Thermodynamic functions of barium and strontium zirconates from calorimetric measurements [J]. Therm Acta, 1985, 90: 297-304.
[15] LEVITSKII V A. Thermodynamics of double oxides (I): Some aspects of the use of CaF2-type electrolyte for thermodynamic study of compounds based on oxides of alkaline earth metals [J]. J Solid State Chem, 1978, 25: 9-22.
[16] BANERJEE A, DASH S, PRASAD R, SOOD D D. Enthalpy increments of strontium and barium zirconates [J]. Therm Acta, 1997, 298: 59-64.
[17] CORDFUNKE E H P, KONINGS R J M. Enthalpy increments of barium zirconate, BaZrO3, and an assessment of its thermochemical properties [J]. Therm Acta, 1989, 156: 45-51
[18] GOSPODINOV G G, MARCHEV V M. The temperature relations of the thermodynamic quantities of Ca, Sr, Ba, and Pb zirconates [J]. Therm Acta, 1993, 222: 137-141.
[19] KING E G, WELLER W W. Thermochemistry and Thermodynamic Properties of Substances [R]. US Bur Mines Rept 5571 Invest, 1960.
[20] MUROMACHI E T, NAVROTSKY A. Energetics of compounds (A2+B4+O3) with the perovskite structure [J]. J Solid State Chem, 1988, 72: 244-256.
[21] DASH S, SINGH Z, PRASAD R, SOOD D D. The determination of the standard molar Gibbs free energy of formation of Ba2ZrO4 [J]. J Chem Therm, 1994, 26: 745-750.
[22] THOMAS H P. The international temperature scale of 1990 (ITS-90) [J]. Metrologia, 1990, 27: 3-10.
[23] DU Y, JIN Z P. Optimization and calculation of the ZrO2-MgO system [J]. Calphad, 1991, 15(1): 63-73.
[24] LU X G, JIN Z P. Thermodynamic assessment of the BaO-TiO2 quasibinary system [J]. Calphad, 2000, 24(3): 319-338.
Foundation item: Project(33354) supported by the Natural Science Foundation of Guangdong Province, China
Corresponding author: CHEN Teng-fei; Tel: +86-731-8877242; E-mail: tengfei@mail.csu.edu.cn
Abstract: Thermodynamic description of ZrO2-BaO system was developed using the available experimental information. Special attention was paid to the modelling of the perovskite phase BaZrO3 by a temperature-dependent polynomial to fit the experimental thermodynamic properties. The liquid phases, CSS (cubic ZrO2 solid solution) and TSS (tetragonal ZrO2 solid solution) were modelled with Redlich-Kister formula. The compounds Ba2ZrO4 and Ba3Zr2O7 were treated as stoichiometric phases and the BaOSS and MSS (halite BaO and monoclinic ZrO2 solid solutions) were treated as pure compounds. Comparisons between the calculated and the measured phase diagram as well as the thermodynamic quantities indicate that the most reliable experimental information is satisfactorily accounted for by the present thermodynamic calculation.


