Trans. Nonferrous Met. Soc. China 25(2015) 490-496
Arginine functionalized hydroxyapatite nanoparticles and its bioactivity for gene delivery
Guo-hui WANG1, Yan-zhong ZHAO1, Juan TAN1, Shai-hong ZHU1, Ke-chao ZHOU2
1. The Third Xiangya Hospital, Central South University, Changsha 410013, China;
2. State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China
Received 13 December 2014; accepted 20 January 2014
Abstract:
In order to further improve the transfection efficiency of hydroxyapatite nanoparticle (HAp), arginine functionalized hydroxyapatite (HAp/Arg) was synthesized by hydrothermal synthesis. The morphology, crystallite size and zeta potential of the HAp/Arg were characterized by transmission electron microscopy (TEM), atomic force microscopy (AFM) and zeta potential analyzer. The loading and protecting properties of HAp/Arg to DNA were tested by electrophoresis. Its cytotoxicity was also measured in Hela cells and HAEC cells by MTT and LDH, and its transfection efficiency was examined by fluorescence microscope and flow cytometry. The results reveal that HAp/Arg is short rod-like and nano single crystal, the mean diameter is 50-90 nm and zeta potential is 35.8 mV at pH 7.4. HAp/Arg to DNA can be condensed by electrostatic effect and protect DNA against degradation in DNase I, and shows high transfection efficiency without cytotoxicity. These results suggest that HAp/Arg can be a promising alternative as a novel gene delivery system.
Key words:
hydroxyapatite nanoparticles; arginine; functionalization; hydrothermal synthesis; gene delivery;
1 Introduction
Hydroxyapatite [Ca10(OH)2(PO4)6] is an inorganic compound with composition similar to that of the mammalian bone and dentin mineral compartment. In recent years, hydroxyapatite nanoparticles (HAp) have attracted considerable attention as new candidates of nonviral vectors for gene therapy due to its excellent properties: 1) biodegradability and biocompatibility, 2) protection of DNA from degradation, 3) ease of synthesis and surface modification to strong interaction with their payload, 4) capability of targeted delivery to specific organs or cells and 5) other bioactivity such as inhibition to some cancer cells growth [1-4]. In our previous studies, HAp can transfer a therapeutic gene and a reporter gene in vitro and in vivo [5-7]. However, the relatively low efficiency of gene delivery is one of major concerns for popularizing the HAp in clinic trials.
Recently, researches have revealed that arginine with guanidyl group can facilitate the cellular uptake of covalently conjugated particles, even though the uptake mechanism is still controversial [8,9]. And our previous studies also indicated that the hydrophilic arginine with guanidyl group —(CH2)3NHC(NH2)+ modified HAp could enhance gene delivery efficiency [10,11]. Under the experimental conditions, provided that the pH value of the reaction fluid is consistently lower than the isoelectric point 0.76 of arginine, the arginine will have positive zeta potentials during the whole process. Positively charged HAp nanoparticles allow higher extent of internalization, apparently as a result of the ionic interactions established between positively charged particles and negatively charged cell membranes [12,13]. Moreover, positively charged nanoparticles were reported to be able to escape from lysosomes after being internalized and exhibit perinuclear localization, whereas the negatively and neutrally charged nanoparticles prefer to colocalize with lysosomes [14-17].
In this study, the preparation, physicochemical characterization, and the cytotoxicity of arginine functionalized HAp prepared via hydrothermal treatment were introduced. It demonstrated that positively charged HAp/Arg nanoparticles can improve transfection efficiency for non-viral vectors by simply mixing with the plasmid DNA pEGFP-N1 via electrostatic self-assembly. The transfection efficiency of the HAp/Arg nanoparticles was evaluated as follows: 1) to determine if the positively charged HAp can improve the negatively charged HAp transfection; 2) to compare the transfection effects of HAp/Arg with lipofectamine 2000 in Hela cell lines.
2 Experimental
2.1 Hydrothermal synthesis
Pure HAp and arginine functionalized HAp were synthesized according to an established method [18,19]. Typically, a calcium phosphate precipitate was produced at pH 9.5 and room temperature by the addition of aqueous ammonium phosphate to a solution containing calcium nitrate and an amino acid at a Ca2+:PO43-: amino acid (arginine) molar ratio of 3:1:6. After the calcium, phosphate and terbium solution was stirred evenly, the solution was transferred into an autoclave. Then the reaction was continued under the set solution temperature until completion. At the end of the experiment, the solids were collected by centrifugation (10000 r/min) and filtration and then were washed thoroughly using ethanol and deionized water. The product was dried overnight at vacuum condition.
2.2 Physicochemical characterization
HAp/Arg nanoparticle samples were characterized by a transmission electron microscope (TEM, JEOL, Japan) to analyze the nanoparticle crystalline appearance and particle size. The zeta potential of HAp nanoparticles both with and without arginine functionalization was measured with a Zetasizer 3000HSa (lasetHeeNe (633 nm)) from Malvern Instruments. Complexes between HAp/Arg and DNA were prepared in distilled water by gently mixing pEGFP-N1 (0.1 μg) with HAp solution at different N/P ratios. The DNA condensation ability of the HAp/Arg copolymer was confirmed by electrophoresis. The protection and release of DNA in complexes were measured by electrophoresis, too. The morphology and microstructure of HAp/Arg and HAp/Arg-DNA complexes were observed by atomic force microscopy (AFM, PICOPLUS, USA). Prior to the experiments, the solution was diluted in an aqueous solution containing 0.01 mol/L NaCl and adjusted to the desired pH.
2.3 Cell line and cell culture
During this study, the Hela cells (Human cervical carcinoma cell line) and HAEC cells (Human Aortic Endothelial Cells) were cultured in Dulbecco’s Modified Eagle medium (DMEM) with the addition of 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin. The cells were maintained in a humidified incubator set to 37 °C under 5% CO2 atmosphere. Upon 80%-90% confluency, the cells were passaged every 3 or 4 d. To passage cells, the cell culture flasks were washed with phosphate-buffered saline three times and trypsin was added to detach cells from the bottom of the flasks.
2.4 MTT assay
The effect of concentration of HAp/Arg on cytotoxicity evaluation in Hela cells and HUVEC cells was studied using MTT colorimetric assay. In brief, both cell lines (Hela and HAEC) at a density of 105 cell/well were seeded in 96-well plates and were cultured for 24 h at 37 °C in a 5% CO2 incubator. Four different concentrations of HAp/Arg nanoparticles in the medium (0, 20, 100, 200 μg/mL) were added to the wells. After the cells were exposed to HAp/Arg nanoparticles for 4, 24, 48, or 72 h, respectively, 10 μL of MTT reagent was added to each well and was further incubated for 4 h. Formazan crystals formed after 4 h in each well were dissolved in 150 μL of detergent and the plates were read immediately in a microplate reader (BIO-RAD microplate Reader-550) at 570 nm. The protocol of this assay followed manufacturer’s instructions. Untreated Hela and HUVEC cells were taken as controls with 100% viability. The cell viability percent was calculated by using the following relation: Cellular viability=OD treated cells/OD control cells ×100.
2.5 LDH assay
Quantitative cytotoxicity/cytolysis was assayed using a cytoplasm enzyme lactate dehydrogenase (LDH) cytotoxicity detection kit (Sigma Aldrich). This assay was based on the measurement of the release of LDH from damaged cells into the supernatant. LDH activity was determined in an enzymatic test as kit Manual. Cells (Hela and HAEC) cultured in a flat-bottomed 96-well polystyrene coated plate were treated with increasing concentrations of HAp/Arg nanoparticles (0, 20, 100, 200 μg/mL). After the cells were exposed to HAp/Arg nanoparticles for 4, 24, 48, or 72 h, respectively, culture supernatant was collected and incubated with reaction mixture. The LDH catalyzed conversion results in the reduction of the tetrazolium salt to formazan, which can be read at about 500 nm absorbance. These data were measured in LDH activity as a percentage of the control. Any significant increase in LDH levels would indicate cellular disruption or death due to the treatment.
2.6 In vitro transfection assays
The transfection efficiency of the DNA plasmid pEGFP-N1 was evaluated in Hela cells. According to the optimum binding mass ratio of DNA to nanoparticles as above, HAp/Arg-DNA complexes were prepared. In short, the DNA plasmid pEGFP-N1 was diluted in serum-free DMEM medium to get a final concentration of 1 μg/mL. 200 μL diluted DNA solution was added to 1 μL HAp/Arg solution (1 μg/μL) and was mixed immediately by vigorous pipetting, and then was incubated for 30 min at room temperature to form HAp/Arg-DNA complexes. 8 h prior to transfection, Hela cells were seeded at a density of 2×105 cell/well in 12-well plates. The cells were grown as adherent cultures in a humidified atmosphere at 37 °C and 5% CO2 in DMEM medium containing 10% fetal bovine serum, and grown to 70%-80% confluence. And then the cells were washed twice with phosphate-buffered saline and the medium was replaced with fresh serum-free medium. The freshly prepared 200 μL/well HAp/Arg-DNA complex solutions were added to the 12-well cell culture plate. After mixing, the cells were incubated for a further 6 h at 37 °C and 5% CO2, and then the medium was removed and replaced with fresh serum-containing medium. Meanwhile, the DNA pEGFP-N1 plasmid liposomes were prepared and to transfect Hela cells as a control. After 48 h post-transfection, the cells were rinsed with phosphate-buffered saline, and examined by the fluorescence microscope and flow cytometry to determine the green fluorescence protein (GPF) expression.
2.7 Statistical analysis
All of the measurements were performed in triplicate. Data were presented as the mean ± standard deviation. A one-way analysis of variance (ANOVA) (SPSS13.0) test was performed to determine significant differences among treatment groups. P<0.05 was considered to be statistically significant.
3 Results and discussion
3.1 Characterization of HAp/Arg
HAp/Arg prepared by hydrothermal synthesis has hexagonal and monoclinic structures, and has the preferential growth habit with rod-like and needle-like morphology during the synthesis process. A representative TEM image of HAp with superficial modification with arginine is shown in Fig. 1(a). HAp/Arg appears to be rod-like or needle-like shape with one-dimensional (1D) size of 50-100 nm with an average value (mean±SD) of 73.09±27.32 nm and with excellent dispersivity even over two weeks at 4 °C. Figure 1(b) shows electron diffraction pattern of HAp/Arg corresponding to the selected area in Fig. 1(a). The diffraction pattern of rectangular area in TEM image of HAp/Arg also showed that the single hydroxyapatite particle synthesized was short rod-like and nano single crystal.
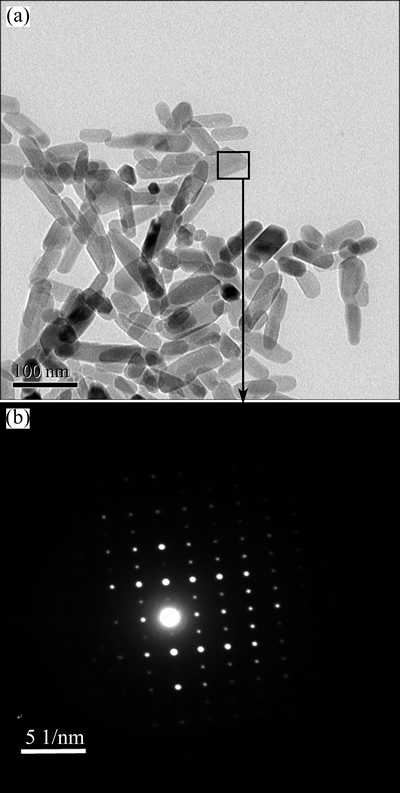
Fig. 1 TEM image (a) and SAED pattern (b) of HAp/Arg
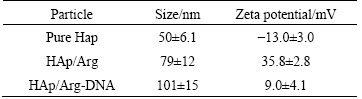
Zeta potential measurement (Table 1) showed that the unmodified HAps were shorter than the modified ones in average sizes. And it showed that the unmodified HAp was negatively charged at pH 7.4, whereas all of arginine modified HAps were positively charged at this pH value. Such results illustrated that cationic aminated functional groups of arginine could increase the zeta potential value of HAp, as a result of absorption of amino acid residue on the HAp surface. To confirm this opinion, arginine was extracted from HAP aqueous solution and titrated to further analyze how hydrothermal crystalline behavior of HAp is affected by arginine as well as the underlying mechanism of surface electronic charge status of HAp. The data are consistent with a binding model in which the amino acid a-carboxylate is preferentially bound to the HAp crystal such that the positively charged amino groups exposed at the crystal/solvent interface dominate the surface charge unless compensated by an additional side chain carboxylate [20,21].
Table 1 Particle sizes and zeta potential of nanoparticles tested at pH=7.4 (n=5)
It’s known that hydrophilic and negatively charged DNA molecules prevent them from entering the cell membrane. Therefore, to conceal the net negative charge of DNA, condensation of DNA molecules with cationic HAp/Arg is prerequisite for HAp/Arg-DNA complexes binding to and transpassing the cell membrane. The capability of complex formation between HAp/Arg and DNA was observed by the retardation of the DNA in gel electrophoresis, as shown in Fig. 2. In addition, HAp/Arg protected DNA against degradation by forming HAp/Arg-DNA complex, whereas the naked DNA was completely degraded by the enzyme DNase I (Fig. 3). As shown in Fig. 4, AFM analysis further showed that the naked DNA was irregular and incompact shape (size of about 800 nm), but the complexes were spherical and compact shape (size of about 100 nm). Such results indicated that the size of HAp/Arg-DNA complexes decreased because of the condensation between negative DNA and cationic HAp/Arg.
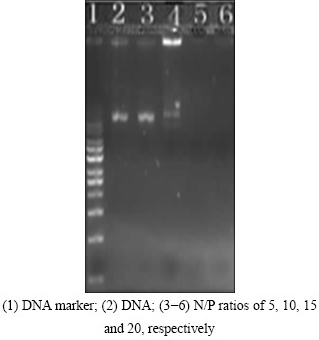
Fig. 2 Representative agarose gel electrophoresis result of HAp/Arg-DNA complexes (pEGFP-N1 control) at different N/P ratios
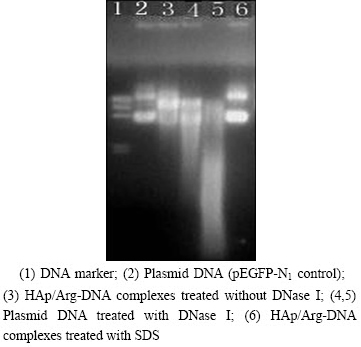
Fig. 3 Protection and release assay of DNA (DNA was released by adding 1% SDS to HAp/Arg-DNA complexes at N/P ratio of 20)
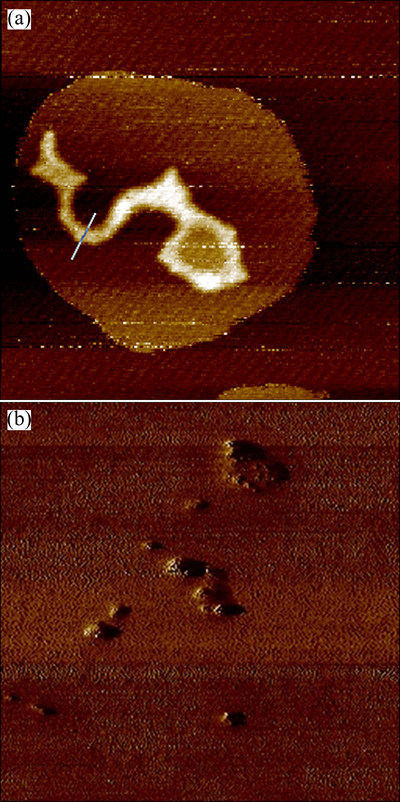
Fig. 4 AFM images of naked DNA at PBS buffer (0.2 μm× 0.2 μm) (a) and HAp/Arg-DNA complexes with N/P ratio of 20 (2 μm×2 μm) (b)
3.2 Effect of HAp/Arg on cell viability and cytotoxicity
Cell viability and cytotoxicity are also important parameters for evaluating biocompability of gene carrier that can ensure biosafety during clinical trials, therefore the MTT assay and the LDH leakage assay were performed. The MTT assay is a cell viability assay often used to determine cytotoxicity following exposure to toxic substances. MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) is a water soluble tetrazolium salt, which is converted to an insoluble purple formazan by cleavage of the tetrazolium ring by succinate dehydrogenase within the mitochondria. The formazan product is impermeable to the cell membranes and therefore it accumulates in healthy cells.
The effects of varying concentration of HAp/Arg and exposure time on cell viability were evaluated using a model cancer cell line (Hela cells) and a model normal cell line (HAEC cells). The results indicated that HAp/Arg did not affect cell viability or exert detectable cytotoxicity in the context of changes of concentration and exposure time. The cells exposed to nanoparticles survived well similar to the controls (Fig. 5), even at the highest 200 μg/mL concentration, suggesting that biocompatible HAp/Arg is a remarkably safe gene transporter system.
The MTT assay is mainly based on the enzymatic conversion of MTT in the mitochondria whereas the LDH leakage assay is based on the release of the enzyme into the culture medium after cell membrane damage. Lactate dehydrogenase is of medical significance and is found extensively in body tissues, such as blood cells and heart muscle. The loss of intracellular LDH and its release into the culture medium are an indicator of irreversible cell death due to cell membrane damage. Reliability, speed and simple evaluation are some of the characteristics of this assay [22].
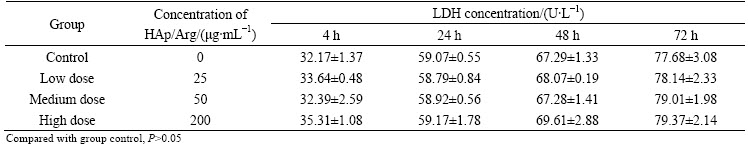
In this work, we evaluated the level of extracellular LDH released from damaged cells for evaluating the cytotoxicity of HAp/Arg. Tables 2 and 3 show the LDH released effect of different concentrations of HAp/Arg nanoparticles in the Hela and HAEC cells during the exposure time of 4-72 h. The results show that there is no significant difference for LDH concentration between each test group and control. As shown, in Hela and HAEC cells no any cytotoxicity following exposure to HAp/Arg is observed when the LDH leakage assay is employed.

Fig. 5 Effects of concentration of HAp/Arg nanoparticles and exposure time on Hela (a) and HAEC (b) cell viability
3.3 Transfection efficiency of HAp/Arg in vitro
To a certain extent, surface modification can dramatically change some properties of nanoparticles,such as hydrophilicity or hydrophobicity, surface charge. What’s more, it can realize other new functions including targeting, membrane permeability, long- circulation in vivo, and so on. In order to enable the direct use of nanoparticles in biomedical applications, the nanoparticles have been further functionalized by conjugating them with functional groups. These groups such as endothelial growth factors (EGF), Arg-Gly-Asp (RGD), folic acid, transferring, permit specific recognition of cell types and target the nanoparticles to a specific tissue or cell type by binding to a cell surface receptor. Although some above mentioned functionalities have been utilized in the gene carrier research, the transfection efficiency of nanoparticles is still unsatisfied [23,24].
Table 2 Effect of HAp/Arg nanoparticles on LDH concentration (U/L) in Hela cells (n=6)
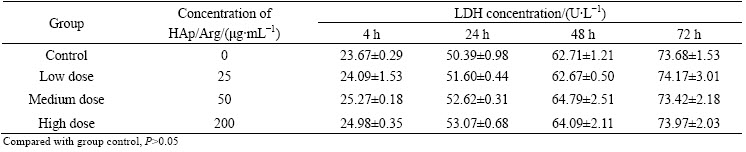
Table 3 Effect of HAp/Arg nanoparticles on LDH concentration (U/L) in HAEC cells (n=6)
In this study, arginine functionalized HAp can bind plasmid pEGFP-N1 efficiently and form compact HAp/Arg-DNA complex. To test the transfection efficiency of the HAp/Arg nanoparticles, the Hela cells were treated with the plasmid pEGFP-N1 and nanoparticles for 48 h and then analyzed by microscope to determine the transfection efficiency. Meanwhile, the plasmid pEGFP-N1 transfection was carried out using lipofectamine 2000 as positive control according to the manufacturer’s protocol. Non-transfected cells were used as negative control of fluorescence expression. After 48 h incubation, high transfection efficiencies were achieved with both the nanoparticles (approximately 45% of cells expressed GFP fluorescence, Fig. 6(b)) and lipofectamine 2000 approaches (approximately 50%, Fig. 6(a)), indicating that the HAp/Arg nanoparticle is just as efficient as a carrier for DNA delivery as lipofectamine 2000. In addition, in order to increase the transfection efficiency, multiple conditions were modified, including DNA plasmid concentration, HAp/Arg solution volume, cellular density, and incubation time; however, neither of the transfection conditions increased the transfection percentage beyond 50% (data not shown). However, little fluorescence (below 10%) could not be observed in the Hela cells transfected by pure HAp/DNA complex (Fig. 6(c)).
Actually, in recent years much more interest has been concentrated on arginine functionalized non-viral delivery system, such as lipidosome, chitosan [25-27]. The studies found that its transfection efficiency was increased drastically after being arginine modified, and at the same time, its cytotoxicity of the gene carrier itself can be reduced, too. Therefore, this study shows that arginine functionalizing HAp nanoparticles can effectively increase the HAp nanoparticles mediated transfection efficiency of plasmid DNA, which is consistent with the literature reported arginine role.
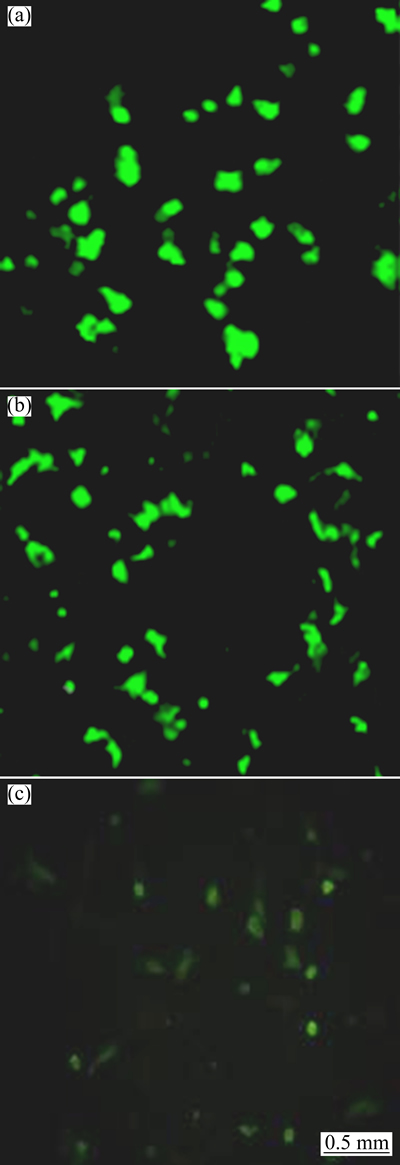
Fig. 6 Fluorescence characteristic in Hela cells transfected by cationic liposome (a), HAp/Arg-DNA complex (b) and pure HAp/DNA complex (c)
4 Conclusions
1) Arginine functionalized HAp samples were successfully synthesized via hydrothermal method. All test samples were found to be short rod and nano single crystal particles. The average sizes of HAp/Arg powders are in the range of 50-90 nm, and their zeta potential is about 35.8 mV at pH 7.4.
2) The synthesized HAp/Arg had excellent physicochemical properties. HAp/Arg-DNA complex can be formed by electrostatic self-assembly. The formed complex is compact with size of approximately 101 nm and slight positive surface charge (around +9.0 mV). MTT and LDH study revealed that HAp/Arg had promising biocompatibility.
3) In vitro transfection assay results showed that HAp/Arg can protect DNA against degradation in DNase I and have high transfection efficiency in Hela cells. It suggests that the HAp/Arg nanoparticles can be a promising alternative as a novel gene delivery system.
References
[1] AOKI H. Science and medical applications of hydroxyapatite [M]. Tokyo: Takayama Press System Center Co. Inc., 1991: 163-170.
[2] TOMMY N, ANNIKA S, SUSANN E. Generation of a new protein purification matrix by loading ceramic hydroxyapatite with metal ions-demonstration with poly-histidine tagged green fluorescent protein [J]. Biotechnology, 1999, 69(2): 123-133.
[3] DESCAMPS M, HORNEZ J C, LERICHE A. Manufacture of hydroxyapatite beads for medical applications [J]. Journal of the European Ceramic Society, 2009, 29(3): 369-375.
[4] LIU Z S, TANG S L, AI Z L. Effects of hydroxyapatite nanoparticles on proliferation and apoptosis of human hepatoma BEL-7402 cells [J]. World Journal of Gastroenterology, 2003, 9(9): 1968-1971.
[5] ZHU S H, HUANG B Y, ZHOU K C, HUANG S P, LIU F, LI Y, XUE Z G, LONG Z G. Hydroxyapatite nanoparticles as a novel gene carrier [J]. J Nanopart Res, 2004, 6: 307-311.
[6] SUN H, JIANG M, ZHU S H. In vitro and in vivo studies on hydroxyapatite nanoparticles as a novel vector for inner ear gene therapy [J]. Chin J Otorhinolaryngol Head Neck Surg, 2008, 43(1): 51-57. (in Chinese)
[7] ZHAO Y Z, ZHU S H, TAN J, HUANG Y Y, LI Z Y, ZHOU K C. Arginine modification and gene binding of hydroxyapatite nanoparticles [J]. The Chinese Journal of Nonferrous Metals, 2010, 20(6): 1203-1208. (in Chinese)
[8] KIM T H, JIANG H L, JERE D, PARK I K. Chemical modification of chitosan as a gene carrier [J]. Prog Polym Sci, 2007, 32(738): 726-753.
[9] CHANG M, CHOU J C, LEE H J. Cellular internalization of fluorescent proteins via arginine-rich intracellular delivery peptide in plant cells [J]. Plant and Cell Physiology, 2005, 46: 482-488.
[10] SCHOLL F A, OLIVER S F, WENDER P A. Gene transfer via reversible plasmid condensation with cysteine-flanked, internally spaced arginine-rich peptides [J]. Human Gene Therapy, 2003, 14: 1225-1233.
[11] FUTAKI S, OHASHI W, SUZUKI T. Stearylated ariginine-rich peptides: A new class of transfection systems [J]. Bioconjugate Chem, 2001, 12: 1005-1011.
[12] GRATTON S E, ROPP P A, POHLHAUS P D. The effect of particle design on cellular internalization pathways [J]. Proc Natl Acad Sci USA, 2008, 105: 11613-11618.
[13] HARUSH-FRENKEL O, ROZENTUR E, BENITA S, ALTSCHULER Y. Surface charge of nanoparticles determines their endocytic and transcytotic pathway in polarized MDCK cells [J]. Biomacromolecules, 2008, 9: 435-443.
[14] RABINOVICH-GUILATT L, COUVREUR P, LAMBERT G, DUBERNET C. Cationic vectors in ocular drug delivery [J]. J Drug Target, 2004, 12: 623-633.
[15] VOLKER M, KATHARINA L. Interaction of nanoparticles with cells [J]. Biomacromolecules, 2009, 10: 2379-2400.
[16] FOGED C, BRODIN B, FROKJAER S, SUNDBLAD A. Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model [J]. Int J Pharm, 2005, 298: 315-322.
[17] VASIR J K, LABHASETWAR V. Quantification of the force of nanoparticle–cell membrane interactions and its influence on intracellular trafficking of nanoparticles [J]. Biomaterials, 2008, 29: 4244-4252.
[18] ZHAO Y Z, ZHU J, ZHU S H, HUANG Y Y, LI Z Y, ZHOU K C. Synthesis and characterization of arginine-modified and europium- doped hydroxyapatite nanoparticle and its cell viability [J]. Transactions of Nonferrous Metals Society of China, 2011, 21: 1173-1778.
[19] ZHANG H B, ZHOU K C, LI Z Y, ZHAO Y Z. Morphologies of hydroxyapatite nanoparticles adjusted by organic additives in hydrothermal synthesis [J]. J Cent South Univ Technol, 2009, 16: 871-875.
[20] PALAZZO B, WALSH D, IAFISCO M. Amino acid synergetic effect on structure, morphology and surface properties of biomimetic apatite nanocrystals [J]. Acta Biomaterialia, 2009, 5: 1241-1252.
[21] JACK K S, VIZCARRA T G, TRAU M. Characterization and surface properties of amino acid-modified, carbonate-containing hydroxyapatite particles [J]. Langmuir, 2007, 23: 12233-12242.
[22] DECKER T, LOHMANN-MATTHES M L. A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity [J]. J Immunol Meth, 1988, 115: 61-69.
[23] ILLUM L, JABBAL-GILL I, HINCHCLIFFE M, FISHER A N, DAVIS S S. Chitosan as a novel nasal delivery system for vaccines [J]. Adv Drug Deliv Rev, 2001, 51(1-3): 81-96.
[24] BALTHASAR S, MICHAELIS K, DINAUER N. Preparation and characterization of antibody modified gelatin nanoparticles as drug carrier system for uptake inlymphocytes [J]. Biomaterials, 2005, 26(15): 2723-2733.
[25] GAO Y, XU Z, CHEN S. Arginine-chitosan/DNA self-assemble nanoparticles for gene delivery: In vitro characteristics and transfection efficiency [J]. Int J Pharm, 2008, 359(1-2): 241-246.
[26] FENG B, TOMIZAWA K, MICHIUE H. Development of a bifunctional immunoliposome system for combined drug delivery and imaging in vivo [J]. Biomaterials, 2010, 31(14): 4139-4145.
[27] XIANG B, DONG D W, SHI N Q. PSA-responsive and PSMA-mediated multifunctional liposomes for targeted therapy of prostate cancer [J]. Biomaterials, 2013, 34: 6976-6991.
纳米羟基磷灰石的精氨酸修饰及其基因转染活性
王国慧1,赵颜忠1,谭 娟1,朱晒红1,周科朝2
1. 中南大学 湘雅三医院,长沙 410013;2. 中南大学 粉末冶金国家重点实验室,长沙 410083
摘 要:为了提高羟基磷灰石(HAp)纳米颗粒的基因转染效能,采用水热合成法制备精氨酸表面修饰的羟基磷灰石(HAp/Arg)纳米颗粒。利用透射电镜、原子力显微镜、Zeta电位分析仪对HAp/Arg纳米颗粒及其与DNA结合的复合物的形貌、晶粒尺寸和zeta 电位进行表征;采用凝胶电泳实验研究HAp/Arg对DNA的负载与保护性能;采用MTT和LDH法,选取人正常血管内皮细胞和人肿瘤细胞Hela细胞,考察HAp/Arg纳米颗粒的细胞毒性,及其负载基因的转染活性。结果表明:制备的HAp/Arg 粒径较均匀,呈短棒状,尺寸为50~90 nm;在pH=7.4 时,HAp/Arg的表面净电荷均值为35.8 mV,可负载DNA静电效应浓缩形成HAp/Arg-DNA复合物,同时对结合的DNA 有明显的保护作用和转染活性,并无细胞毒性。经精氨酸表面修饰的HAp可成为一种有效的基因结合载体。
关键词:纳米羟基磷灰石;精氨酸;修饰;水热合成;基因转染
(Edited by Sai-qian YUAN)
Foundation item: Project (2013SK2024) supported by the Key Projects in Social Development Pillar Program of Hunan Province, China; Project (20130162120094) supported by Specialized Research Fund for the Doctoral Program of Higher Education (SRFDP), Ministry of Education, China; Projects (81071869, 51305464) supported by the National Natural Science Foundation of China
Corresponding author: Yan-zhong ZHAO; Tel: +86-731-88618669; E-mail: yanzhongzhao@163.com
DOI: 10.1016/S1003-6326(15)63629-9
Abstract: In order to further improve the transfection efficiency of hydroxyapatite nanoparticle (HAp), arginine functionalized hydroxyapatite (HAp/Arg) was synthesized by hydrothermal synthesis. The morphology, crystallite size and zeta potential of the HAp/Arg were characterized by transmission electron microscopy (TEM), atomic force microscopy (AFM) and zeta potential analyzer. The loading and protecting properties of HAp/Arg to DNA were tested by electrophoresis. Its cytotoxicity was also measured in Hela cells and HAEC cells by MTT and LDH, and its transfection efficiency was examined by fluorescence microscope and flow cytometry. The results reveal that HAp/Arg is short rod-like and nano single crystal, the mean diameter is 50-90 nm and zeta potential is 35.8 mV at pH 7.4. HAp/Arg to DNA can be condensed by electrostatic effect and protect DNA against degradation in DNase I, and shows high transfection efficiency without cytotoxicity. These results suggest that HAp/Arg can be a promising alternative as a novel gene delivery system.


