
J. Cent. South Univ. (2019) 26: 3295-3304
DOI: https://doi.org/10.1007/s11771-019-4253-x
Preparation of defect free ceramic/Ti composite membranes by surface modification and in situ oxidation
ZHANG Dong-qiang(张栋强), YANG Ping(杨平), WU Jian-yang(吴见洋),ZHAO Jing(赵静), CHEN Yan-an(陈彦安)
College of Petrochemical Technology, Lanzhou University of Technology, Lanzhou 730050, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Abstract:
Al2O3 ceramic powder was applied to modify the large pores defects on the surface of the porous metal Ti support, in situ oxidation method was a convenient method to prepare defect free ceramic/Ti composite membranes on this basis. In situ oxidation conditions experimental results show that the best condition for preparing the TiO2-Al2O3/Ti composite membrane is under 800 °C for 2 h, and the microstructure and pore sizes of the TiO2-Al2O3/Ti composite membranes are affected obviously. The thickness and composition of the TiO2/Ti composite membranes are determined by SEM and XRD completely. The pore size distribution of the composite membrane is measured by bubble pressure method, the most probable aperture is about 3.12 μm, while the average pore size of defect free TiO2-Al2O3/Ti is about 3.23 μm. After ultrasonic treatment, the slight weight change of membranes reveals no observable change, which indicates that TiO2-Al2O3/Ti composite membranes maintain a good stability.
Key words:
porous Ti; ceramic; TiO2 layer; in situ oxidation; composite membrane; surface modification;
Cite this article as:
ZHANG Dong-qiang, YANG Ping, WU Jian-yang, ZHAO Jing, CHEN Yan-an. Preparation of defect free ceramic/Ti composite membranes by surface modification and in situ oxidation [J]. Journal of Central South University, 2019, 26(12): 3295-3304.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-019-4253-x1 Introduction
The ceramic/metal composite membranes are composed of a porous ceramic layer and porous metal support, which is endowed with desirable properties of the two components [1-4]. Metal supports are considered to be an ideal porous materials applied to preparing ceramic membranes because of the excellent characteristics, such as high mechanical strength, high pressure resistance, rare anti-crack property and easily fixed by welding, crimping or brazing [5, 6]. Meanwhile, porous ceramic membrane layers with a relatively smooth surface containing rather uniform small pore systems can be prepared easily [7-10]. As is known to all, Ti plate has many advantages as an excellent metal material [11-13], porous plates made by Ti show lower apparent specific gravity, higher specific surface area, higher penetration performance [14-17]. In addition, TiO2 film is used to gas separation and water purification for its good separation performance [18-25].
Common methods for preparing ceramic membrane on metal support are listed as follows: electrophoretic deposition [26], wet powder spraying [27], screen printing [28] and other molding processes, unfortunately, all of the technologies have several obvious shortcomings such as complex preparation process and harsh conditions. Therefore, there is an urgent need to develop novel methods for improving the binding force between the ceramic layer and metal support. It is amazing that in situ oxidation can solve the problems by preparing an oxide layer on the surface of metal support. MEULENBER et al [29] manufactured TiO2 nanowire thin membranes by in situ oxidation, the Ti plate in a mixture solution of concentrated H2O2 and NaOH and the novel nanowire membrane was promising a practical aqueous purification. MA et al [6] fabricated an oxide layer on porous stainless steel supports by in situ oxidation method when the temperature is higher than 600 °C, the results indicated that the oxide layer is an effective diffusion layer between Pd membrane and metal support. In addition, ZHANG et al [30] prepared a ceramic/Ti-Al alloy composite membrane by in situ oxidation method, and the ceramic membranes with different thickness were fabricated at different temperatures ranging from 650 to 900 °C, however, the composite membrane had some transfer of large pores defects in the oxide layer.
In this work, defect free TiO2-Al2O3/Ti composite membranes with uniform pores were prepared by surface modification and in situ oxidation method. The effects of surface modification on macroporous defect transfer during in-situ oxidation procedure were studied. Furthermore, the thickness, surface aperture and the stability of the composite membranes were explored in detail.
2 Experimental
2.1 Experiment materials
Support materials are lab-made porous Ti disks made by Ti elemental powder, the particle diameter of which is 100-200 μm, the average diameter of porous Ti disks is about 30 mm, the maximum diameter of aperture is about 100 μm, the average pore size is about 40 μm, the thickness is about 3 mm.
Modified material is alumina ceramic powder, the average diameter of which is 400 nm.
2.2 Experiment method
The experiment will be divided into two processes, the first process is the test of in situ oxidation condition and another process is modified and in situ oxidation experiment.
First process. Ti disks was roasted for 2 h under the specified temperature, and then cooled down to room temperature. Suitable temperature was selected according to the characterization results of the prepared ceramic/Ti composite membranes.
Second process. At first, a stable and evenly dispersed suspension of ceramic particles was prepared by adding the alumina powders (400 nm) into the water and then dispersant (polyethylene, or polymethacrylate, or polyacrylamide, or a mixture of two or three of them) and thickener (methyl cellulose, or ethyl cellulose, or polyvinyl alcohol, or a mixture of two or three of them) were added to adjust the solution. Next, porous Ti supports were put into the suspension of ceramic particles for suction negative pressure dipping pulp adsorption that the channels of the pores on the surface of the support were filled with ceramic particles. And then, porous Ti supports were put into the suspension for suction negative pressure dipping pulp adsorption, furthermore, the channels of the pores on the surface of the support were filled with ceramic particles. And then the modified Ti disks were heated at a specified temperature for a period of time. The TiO2-Al2O3/Ti composite membranes were boiled in deionized water and cleaned in ultrasonic cleaner to remove the Al2O3 powders on the surface of membranes.
2.3 Characterization of Ti disks and ceramic/Ti composite membranes
The oxidation temperature in air of porous Ti support was analyzed by DTA-TG Analysis (DTA-TG: DT-40, SHIMADZU, Japan). X-ray was used to investigate the phase composition of the oxide layer (XRD: D/MAX-2400, Science, Japan). The surface and cross-sections morphology of composite membranes was studied by scanning electron microscope (SEM: JSM6700F, Japan Electronics Corporation, Japan), meanwhile, the size of surface pore can be determined by SEM. Electronic balance was applied to measure the weight gains and losses of the TiO2/Ti composite membranes (Electronic Balance: JJ-500, G & G Measurement Plant, China). The permeability of TiO2/Ti composite film was detected by gas permeation device under room temperature. The gas flow of N2 was measured by a mass flow measurer (MFC, Models D08-4D/ZM, Beijing Sevenstar Electronics Co., Ltd., Beijing, China). The surface pore size distribution of the TiO2-Al2O3/Ti composite membranes was investigated by gas bubble pressure method [31].
3 Results and discussion
3.1 Characterization of porous Ti disk
Porous Ti disks were tested by the thermogravimetric-differential scanning calorimetry (TG-DSC) after heating treatment at 1000 °C in air atmosphere. As shown in Figure 1, there is no change in mass to from room temperature to 750 °C, which indicates that the porous Ti support is very stable when the temperature is less than 750 °C. It is worth noting that when the oxidation temperature is higher than 800 °C, Ti support begins to oxidize, and the oxidation degree is severe at 900 °C. Based on the results of TG analysis, 750, 800, 850 and 900 °C were selected as the in situ oxidation temperature of porous Ti support.
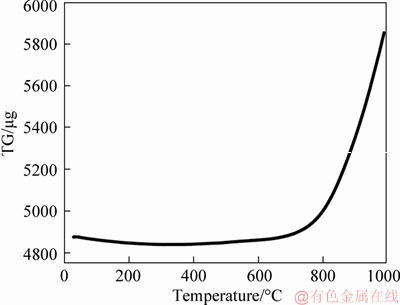
Figure 1 TG curve of porous Ti disks
Figure 2(a) shows the SEM images of porous Ti disks. It is seen obviously that the pore size distribution is uniform and the average pore diameter is 30-50 μm, moreover, the defect diameter is about 120 μm.
3.2 Characterization of porous ceramic/Ti composite membranes
3.2.1 Surface SEM images of TiO2 /Ti composite membranes
In situ oxidation method was applied to preparing TiO2/Ti composite membranes at 750, 800, 850 and 900 °C, respectively. The SEM images of the composite membranes are shown in Figure 2. It can be seen that the morphology and pore sizes distribution of the samples are changed due to the oxidation reaction of Ti element on the surface of Ti disks. The microstructure of TiO2/Ti composite membrane is shown in Figure 2(b), the results indicate that there is a slight change of morphology after oxidation at 750 °C for 2 h. However, the pore size has little change. Although the in situ oxidation has reacted on the surface of the support at 750 °C, the degree of the reaction is too slight to influence the distribution of surface pore size.
The changes of Ti particles could find obviously in the SEM images (Figure 2(c)) of TiO2/Ti composite membrane. In addition, the surface of Ti disks has changed obviously, these changes have no impact on the pore sizes and distributions. In the surface area of Ti disks, Ti particles are so small that the permeability of TiO2/Ti membrane is hardly affected by Ti particles.
The morphology of TiO2/Ti composite membrane oxidized at 850 °C is shown in Figure 2(d). The results prove that the degree of adhesion among Ti particles is serious. Moreover, the morphology of Ti particles has changed completely, and a large number of crystals are grown significantly on the surface of Ti support, the pore size is decreased due to the adhesion among Ti particles becomes severer. Hence, the in situ oxidation at 850 °C not only forms a continuous oxide layer, but also modifies the pore structure.
Finally, as shown in Figure 2(e), great changes take place on the surface of ceramic/Ti composite membrane at 900 °C. The pore size has completely blocked due to a big mass of oxides produced on the surface of Ti support.
3.2.2 Cross-section SEM images of TiO2/Ti composite membranes
Figure 3 shows the cross-section SEM images of Ti support and TiO2/Ti composite membranes, the pores size changes seriously with the increasing oxidation temperature, and the change near the surface of the support is more obvious than in the channel in Figure 3(a). There is a slight change of channel in the SEM images of Figures 3(b) and 3(c) about TiO2/Ti composite membranes. However, the oxidation layer can be found obviously in Figures 3(d) and (e). As shown in Figure 3(d), a large adhesion area is formed on the surface of the support and affects the permeability and porosity of the composite membranes. A continue oxidation layer is formed on the top of composite membrane (Figure 3(e)), indicating that the oxide layer of porous support has completely changed.
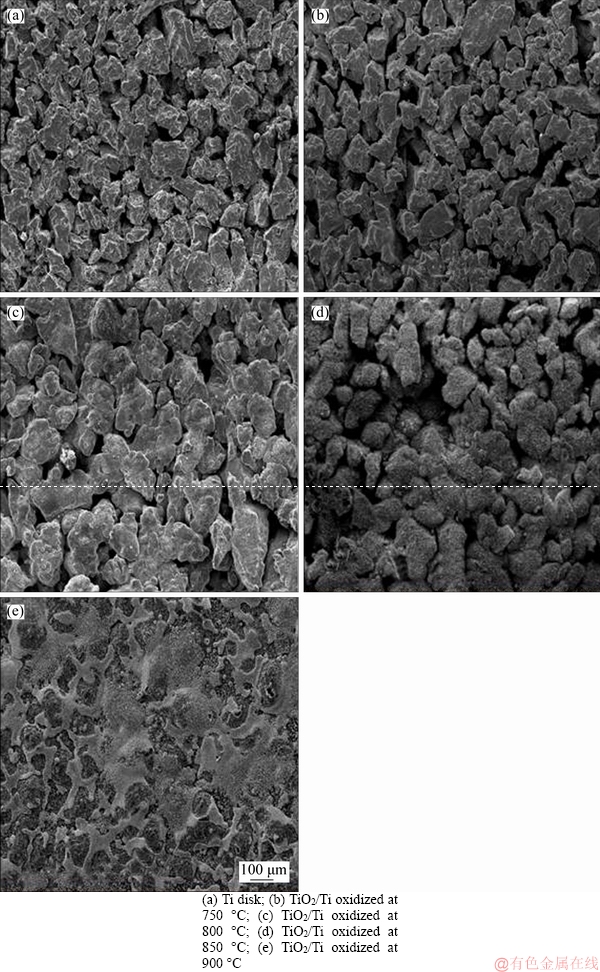
Figure 2 Surface microstructure of Ti disk and TiO2/Ti after oxidation at different temperatures:
It is unsuitable to use the TiO2/Ti composite membranes as support for preparation of ceramic membranes under 850 °C and 900 °C, because the permeability of the membrane is affected or even lost. The pore structures of porous supports are little affected after in situ oxidation process at 750 °C and 800 °C for 2 h.
3.2.3 XRD analysis of TiO2/Ti composite membranes
XRD analysis of porous Ti support and TiO2/Ti composite membranes made by in situ oxidation at different temperatures are shown Figure 4. The results prove that the main components of Ti disk are Ti peaks (PDF: 05-0682), meanwhile, the peaks of Ti become receded and the rutile phase peak of TiO2 appears after heating at 750 °C for 2 h. It is indicated that the ceramic layer formed on the surface of porous Ti disks is uncompleted. The peak of rutile (PDF: 21-1276) can be found only in the XRD patterns of TiO2 which was roasted at 800 °C and 850 °C, respectively. Therefore, a continuous oxide layer formed on the top of Ti disk is confirmed. The peaks of rutile (PDF: 21-1276), brookite (PDF: 29-1360) and anatase (PDF: 21-1272) can be discovered in the XRD patterns of TiO2/Ti sample.
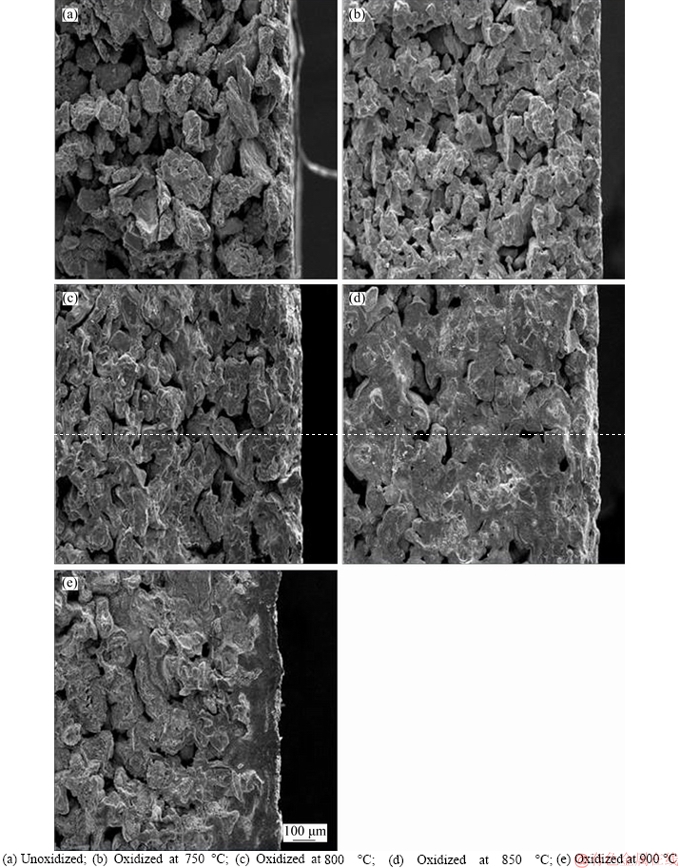
Figure 3 SEM images of cross-sections of Ti and TiO2/Ti composite:
3.3 Permeability of TiO2/Ti composite membranes
The permeability of the porous Ti disks and TiO2/Ti composite membranes were measured by permeation apparatus. Equations (1) and (2) are the general formula for calculating the gas flux through a membrane.
J=F·(P1-P2) (1)
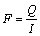 (2)
(2)
where J is the N2 flux of the diffusing, P1 is the N2 partial pressure on the feed side of the membrane and P2 is conversely the N2 partial pressure on the permeate side, F is the permeability coefficient, Q is permeability and l is the membrane thickness.
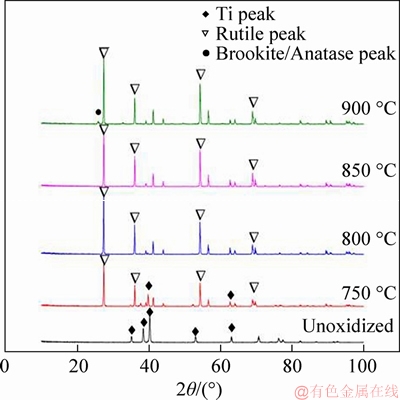
Figure 4 XRD patterns of porous Ti disc and ceramic/Ti composite membranes
The changes of the permeability of each sample are shown in Figure 5, it is indicated that the N2 permeation flux of the TiO2/Ti composite membranes is decreased by 13.4%, 24.5%, 74.3% and 100%, respectively. Permeation flux can be calculated according to the following formula:
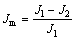 (3)
(3)
where Jm is the N2 flux change of the membrane after in situ oxidation, J1 is N2 flux before the oxidation and J2 is the after. The effect of temperature (700 and 800 °C) on the permeability of the porous disk is negligible. After the temperature is up to 850 °C, it exerts a tremendous influence on the permeability of porous disks, furthermore, the permeability of porous disks dismisses, while the temperature is up to 900 °C. The result is consistent with the conclusions of SEM image of Figures 2(e) and 3(e).
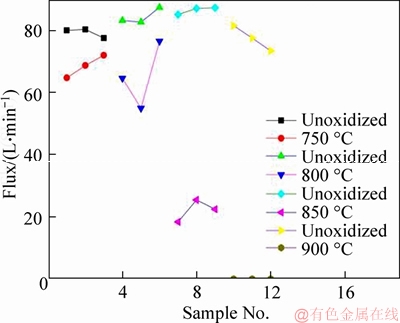
Figure 5 Permeability curves of disks
3.4 TiO2-Al2O3/Ti composite membranes
3.4.1 Microstructures of TiO2-Al2O3/Ti composite membrane
According to the above conclusions, Ti support was modified by Al2O3 powder. And then, the in situ oxidation method was applied to preparing TiO2-Al2O3/Ti composite membranes at 800 °C for 2 h. The SEM images of surface and cross-section are shown in Figures 6 and 7.
Figure 6 shows that the surface of porous disks become smooth, and the various pores are filled with dry Al2O3 powder. According to Figures 6(c) and (d), smaller apertures generate among Al2O3 particles after surface modification and in situ oxidation process, which indicates that the porous surfaces of Ti disks are not blocked completely.
Figure 7 shows that the Al2O3 particles only exist in the surface, however, the short channels structure and permeability of support have not been destroyed significantly.
Therefore, the porous Ti disks modified by Al2O3 powder not only reduce the size of surface pores, but also change nothing of the internal pore structure and internal permeability of the support.
3.4.2 Surface pore size distribution of TiO2-Al2O3/ Ti composite membrane
Surface pore size distribution test of TiO2-Al2O3/Ti composite membrane is conducted by gas bubble pressure and the results are shown in Figure 8. The most probable aperture (D) of the TiO2-Al2O3/Ti composite membrane is 3.12 μm while the average pore size of TiO2-Al2O3/Ti composite membrane is 3.23 μm, indicating that the pore size of porous disk has reduced to 10%, and the TiO2-Al2O3/Ti composite membrane has a uniform pore size distribution. It is proved that the TiO2-Al2O3/Ti composite membrane has a uniform pore size distribution. It is proved that surface modification and in situ oxidation method plays an important role in improving the structure performance of support surface.
3.4.3 Stability of TiO2-Al2O3/Ti composite membrane
The stability of TiO2-Al2O3/Ti composite membranes treated in boiling deionized water and ultrasonic cleaning was evaluated by testing changes of mass and permeability.
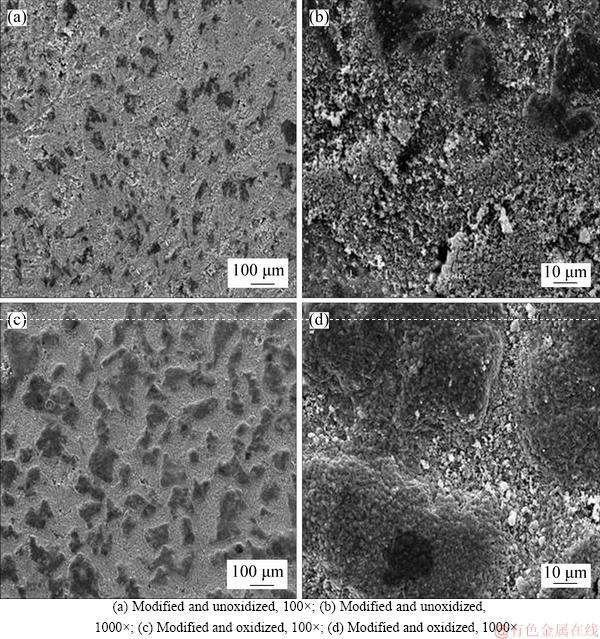
Figure 6 SEM images of surfaces of TiO2-Al2O3/Ti:
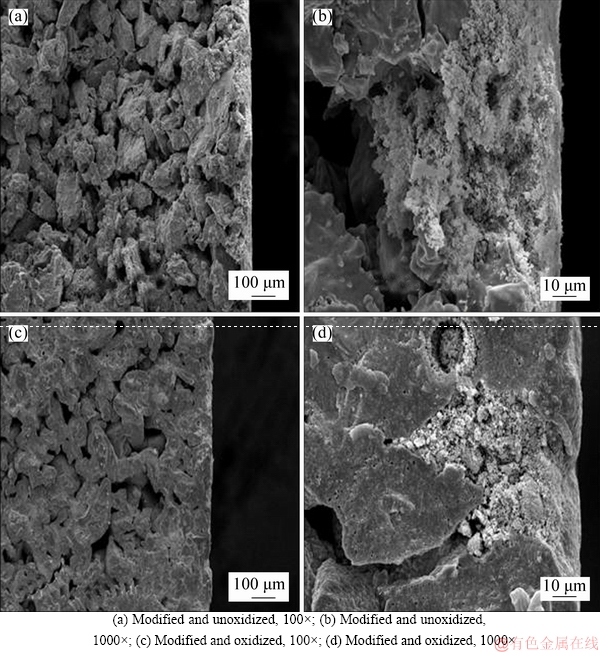
Figure 7 SEM images of surfaces of TiO2-Al2O3/Ti:

Figure 8 Pore size distribution of TiO2-Al2O3/Ti composite membrane
The sample was weighted to obtained m0 and permeability test was taken to get Q0. Then, the sample was treated as described above and dried at 120 °C for 12 h followed by weighting and testing to get m1 and Q1, and the treatment circles were taken to get m2, m3, Q2 and Q3. The permeation experiment was carried out at room temperature and pressure difference of 0.025 MPa, the effective area of sample is a circular area with the diameter of 20 mm. The results are listed in Table 1.
Table 1 Mass and permeability changes testing
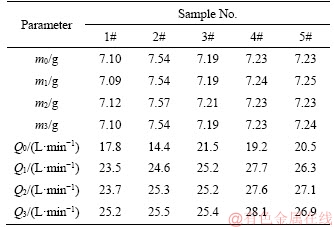
According to Table 1, the change of permeability is not obvious after first treatment procedure, which indicates that TiO2-Al2O3/Ti composite membranes have an excellent stability.
4 Conclusions
Al2O3 ceramic powder is used to modify the large pores defects on the surface of the porous metal Ti support, and then the in situ oxidation method is applied to preparing defect free TiO2-Al2O3/Ti composite membranes.
Ti support is immersed into the prepared suspension of ceramic particles by impregnation method, and Al2O3 ceramic powder can modify effectively the large pores defects on the surface of the porous Ti support. The best condition to prepare the TiO2-Al2O3/Ti composite membranes is at temperature of 800 °C for 2 h, moreover, the surface of the composite membrane is intact. Furthermore, the most probable aperture is about 3.12 μm, while the average pore size of defect free ceramic/Ti is about 3.23 μm. Finally, the result of the stability tests shows that TiO2-Al2O3/Ti composite membranes have an excellent stability. Therefore, the composite membranes will be applied extensively in the field of water treatment.
References
[1] CHEN Fang-lin, FANG Shu-min, BRINKMAN K S. Chemically stable ceramic-metal composite membrane for hydrogen separation: USA, US 9687775 B2 [P]. 2017-06-27.
[2] NOVIKOV V I, SHARAPAEV A I, PETUNIN A B, MURADOVA A G. An increase in abrasive resistance of composite metal ceramic membranes with selective layers based on oxide ceramics [J]. Theoretical Foundations of Chemical Engineering, 2016, 50(5): 827-830. DOI: 10.1134/S0040579516050195.
[3] NOVIKOV V I, SHARAPAEV A I, KOROSTYLEY D A, KUZ’MIN A V. Preparation of metal-ceramic membranes based on the powder of titanium and titanium dioxide [J]. Theoretical Foundations of Chemical Engineering, 2016, 50(5): 822-826. DOI: 10.1134/S0040579516050183.
[4] SUKANTA C, md RUSHDIE I I, AMIT S, RAMACHANDRA L S, REID S R. A computational framework for modeling impact induced damage in ceramic and ceramic-metal composite structures [J]. Composite Structures, 2017, 164: 263-276. DOI: 10.1016/j.compstruct. 2016.12.064.
[5] BOWKER M, JAMES D, STONE P, PERKINS N, MILLARD L, GREAVES J, DICKINONS A. Catalysis at the metal-support interface: Exemplified by the photocatalytic reforming of methanol on Pd/TiO2 [J]. Journal of Catalysis, 2003, 217(2): 427-433. DOI: 10.1016/ s0021-9517(03)00074-5.
[6] MA Y H, AKIS B C, AYTHURK M E, GUAZZONE F. Characterization of intermetallic diffusion barrier and alloy formation for Pd/Cu and Pd/Ag porous stainless steel composite membranes [J]. Industrial & Engineering Chemistry Research, 2004, 43(12): 2936-2945. DOI: 10.1021/ie034002e.
[7] POLFUS J M, XING W, FONTAINE M L, DENONVILLE C, HENRIKSEN P P, BREDESEN R. Hydrogen separation membranes based on dense ceramic composites [J]. Journal of Membrane Science, 2015, 479: 39-45. DOI: 10.1016/ j.memsci.2015.12.054.
[8] MONTALEONE D, MERCADELLI E, GONDOLINI A, PINASCO P, SANSON A. On the compatibility of dual phase BaCe0.65 Zr0.2 Y0.15 O3-based membrane for hydrogen separation application [J]. Ceramics International, 2017: S0272884217308416. DOI: 10.1016/j.ceramint.2017. 05.039.
[9] LANGE R S A D, HEKKINK J H A, KEIZER K, BURGGRAAF A A. Formation and characterization of supported microporous ceramic membranes prepared by sol-gel modification techniques [J]. Journal of Membrane Science, 2017, 99(1): 57-75. DOI: 10.1016/0376-7388(94) 00206-E.
[10] ELGAMOUZ A, TIJANI N. From a naturally occurring material (clay mineral) to the production of porous ceramic membranes [J]. Microporous & Mesoporous Materials, 2018, 271: 52-58. DOI: 10.1016/j.micromeso.2018.05.030.
[11] NGUYEN H Q, DEPORTER D A, PILLIAR R M, VALIQUETTE N, YAKUBOVICH R. The effect of sol–gel formed calcium phosphate coatings on bone ingrowth and osteoconductivity of porous-surfaced Ti alloy implants [J]. Biomaterials, 2004, 25(5): 865-876. DOI: 10.1016/s0142- 9612(03)00607-0.
[12] LI Jun, YU Hui, SHI Qing-nan, LIU Li-gang, REN Wan-bo. Hot deformation behavior of pure titanium and its application in hot sheet finish rolling [J]. Journal of Central South University: Science and Technology, 2016, 47(6): 1889-1895. DOI: 10.11817/j.issn.1672-7207.2016.06.010. (in Chinese)
[13] WEN Yu-hui, ZHU Guo-ming, DAI Si-yu, KANG Yong-lin. Effect of Ti on microstructure and strengthening behavior in press hardening steels [J]. Journal of Central South University: 2017, 24(10): 2215-2221.
[14] LI Liang-hao, HUANG Zhuang-peng, FAN Xiao-xiao, ZHANG Zhen, DOU Rong-ni, WEN Shu-long, CHEN Yuan, CHEN Yuan-cai, HU Yong-you. Preparation and characterization of a Pd modified Ti/SnO2-Sb anode and its electrochemical degradation of Ni-EDTA [J]. Electrochimica Acta, 2017, 231: 354-362. DOI: 10.1016/j.electacta.2017. 02.072.
[15] METIKOS-HUKOVIC M, TKALCEC E, KWOKAL A, PILJAC J. An in vitro study of Ti and Ti-alloys coated with sol–gel derived hydroxyapatite coatings [J]. Surface & Coatings Technology, 2003, 165(1): 40-50. DOI: 10.1016/ s0257-8972(02)00732-6.
[16] LV Dong-sheng, XU Jiu-hua, DING Wen-feng, FU Yu-can, YANG Chang-yong, SU Hong-hua. Tool wear in milling Ti40 burn-resistant titanium alloy using pneumatic mist jet impinging cooling [J]. Journal of Materials Processing Tech, 2016, 229: 641-650. DOI: 10.1016/j.jmatprotec.2015. 10.020.
[17] JIANG Dian-lu, ZHANG Shan-qing, ZHAO Hui-jun. Photocatalytic degradation characteristics of different organic compounds at TiO2 nanoporous film electrodes with mixed anatase/rutile phases [J]. Environmental Science & Technology, 2007, 41(1): 303-308. DOI: 10.1021/ es061509i.
[18] BAGHERI S, HIR Z A M, YOUSEFI A T, HAMID S B A. Progress on mesoporous titanium dioxide: synthesis, modification and applications [J]. Microporous & Mesoporous Materials, 2015, 218: 206-222. DOI: 10.1016/ j.micromeso.2015.05.028.
[19] YONG Zhao, ZHANG Xin-tong, ZHAI Jin, HE Jin-ling, JIANG Lei, LIU Zhao-yue, NISHIMOTO S, MURAKARMI T, FUJISHIMA A, ZHU Dao-ben. Enhanced photocatalytic activity of hierarchically micro/nano-porous TiO2 films [J]. Applied Catalysis B-Environmental, 2008, 83(1): 24-29. DOI: 10.1016/j.apcatb.2008.01.035.
[20] CHOI H, SOFRANKO A C, DIONYSIOU D D. Nanocrystalline TiO2 photocatalytic membranes with a hierarchical mesoporous multilayer structure: Synthesis, characterization, and multifunction [J]. Advanced Functional Materials, 2010, 16(8): 1067-1074. DOI: 10.1002/adfm. 200500658.
[21] PARK J H, KIM S, BARD A J. Novel carbon-doped TiO2 nanotube arrays with high aspect ratios for efficient solarwater splitting [J]. Nano Lett, 2006, 6(1): 24-28. DOI: 10.1021/nl051807y.
[22] DU Zhi-ming, HAN Zhi-yue, YAO Qian, ZHANG Ying-hao. Research progress of titanium dioxide anode in dye-sensitized solar cells [J]. Transactions of Beijing Institute of Technology, 2015, 35(2): 111-117. DOI: 10.15918/j.tbit1001-0645.2015.02.001.
[23] CHEN Yong-sheng, CRITTENDEN, J C, HACKNEY, S, SUTTER, L, HAND D W. Preparation of a novel TiO2-based p–n junction nanotube photocatalyst [J]. Environmental Science & Technology, 2005, 39(5): 1201-1208. DOI: 10.1021/es049252g.
[24] GONG Qing, YIN Li-song, GUO Zhi-bo, YANG Su-yu, AN Ke-yun. Titanium oxide nanotube arrays prepared by anodic oxidation method and photocatalytic degradation of chloramine phosphorus [J]. Journal of Central South University: Science and Technology, 2011, 42(11): 3270-3276. (in Chinese)
[25] ZHU Jin, FAN Yi-qun, XU Nan-ping. Modified dip-coating method for preparation of pinhole-free ceramic membrane [J]. Journal of Membrane Science, 2011, 367(1, 2): 14-20. DOI: 10.1016/j.memsci.2010.10.024.
[26] UCHIKOSHI T, KREETHAWATE L, MATSUNAGA C. Fabrication of ceramic membranes on porous ceramic supports by electrophoretic deposition [J]. Advances in Applied Ceramics, 2014, 113(1): 3-7. DOI: 10.1179/ 1743676113Y.0000000111.
[27] ZHANG Xiao-yu, ZHANG Bing, WU Yong-hong, WANG Tong-hua, QIU Jie-shan. Preparation and characterization of a diatomite hybrid microfiltration carbon membrane for oily wastewater treatment [J]. Journal of the Taiwan Institute of Chemical Engineers, 2018: S187610701830258X. DOI: 10.1016/j.jtice.2018.04.035.
[28] GESTEL V T, SEBOLD D, MEULENBERG W A, BRAM M, BUCHKREME H P. Manufacturing of new nano-structured ceramic metallic composite mieroporous membranes consisting of ZrO2, Al2O3, TiO2 and stainless steel [J]. Solid State Ionics, 2008, 179: 1360-1366. DOI: 10.1016/j.ssi.2008.02.046.
[29] MEULENBER W A, MERTENS J, BRAM M, BUCHKREME H P, STOVER D. Graded porous titania membranes for microfiltration [J]. Journal of the European Ceramic Society, 2006, 26: 449-454. DOI: 10.1016/ j.jeurceramsoc.2005.06.035.
[30] ZHANG Dong-qiang, WU Jian-yang, LI Bo, FAN Yi-qun. Preparation of ceramic membranes on Ti-Al alloy supports by an in-situ oxidation method [J]. Journal of Membrane Science, 2015, 476: 554-560. DOI: 10.1016/j.memsci.2014. 10.053.
[31] WU Ya-hui, LONG M, CAI Wei-min, DAI Si-di, CHEN Chao, WU De-yong, BAI Jing. Preparation of photocatalytic anatase nanowire films by in situ oxidation of titanium plate [J]. Nanotechnology, 2009, 20(18): 185703. DOI: 10.1088/ 0957-4484/20/18/185703.
[32] ZHANG Dong-qiang, ZHOU Shou-yong, FAN Yi-qun, XU Nan-ping, HE Yue-hui. Preparation of dense Pd composite membranes on porous Ti–Al alloy supports by electroless plating [J]. Journal of Membrane Science, 2012, 387(1): 24-29. DOI: 10.1016/j.memsci.2011.10.004.
[33] YU Jian, HU Xiao-juan, HUANG Yan. A modification of the bubble-point method to determine the pore-mouth size distribution of porous materials [J]. Separation & Purification Technology, 2010, 70(3): 314-319. DOI: 10.1016/j.seppur.2009.10.013.
(Edited by FANG Jing-hua)
中文导读
表面改性和原位氧化法制备无缺陷陶瓷/Ti复合膜
摘要:本文采用Al2O3陶瓷粉末对多孔金属Ti载体表面的大孔缺陷进行修饰,在此基础上采用原位氧化法制备了无缺陷陶瓷/Ti复合膜。结果表明,制备的陶瓷/Ti复合膜的最佳工艺条件为氧化温度800 °C、氧化时间2 h,随着氧化温度升高和氧化时间的延长,陶瓷/Ti复合膜的表面结构和孔径发生明显变化。采用SEM和XRD测定了所制备陶瓷/Ti复合膜的表面形貌、膜厚度和组成变化。通过气体泡压法测量了复合膜的孔径分布,所制备的陶瓷/Ti复合膜的最可几孔径约为3.12 μm,平均孔径约为 3.23 μm。经超声处理后,复合膜的质量无明显变化,表明所制备的陶瓷/Ti复合膜具有良好的稳定性。
关键词:多孔Ti支撑体;陶瓷;TiO2膜层;原位氧化;复合膜;表面修饰
Foundation item: Projects(212006065, 21666018) supported by the National Natural Science Foundation of China
Received date: 2018-09-30; Accepted date: 2019-04-04
Corresponding author: ZHANG Dong-qiang, PhD, Associate Professor; Tel: +86-931-7823115; E-mail: zhangdq@lut.cn; ORCID: 0000-0002-0430-0030
Abstract: Al2O3 ceramic powder was applied to modify the large pores defects on the surface of the porous metal Ti support, in situ oxidation method was a convenient method to prepare defect free ceramic/Ti composite membranes on this basis. In situ oxidation conditions experimental results show that the best condition for preparing the TiO2-Al2O3/Ti composite membrane is under 800 °C for 2 h, and the microstructure and pore sizes of the TiO2-Al2O3/Ti composite membranes are affected obviously. The thickness and composition of the TiO2/Ti composite membranes are determined by SEM and XRD completely. The pore size distribution of the composite membrane is measured by bubble pressure method, the most probable aperture is about 3.12 μm, while the average pore size of defect free TiO2-Al2O3/Ti is about 3.23 μm. After ultrasonic treatment, the slight weight change of membranes reveals no observable change, which indicates that TiO2-Al2O3/Ti composite membranes maintain a good stability.


