
J. Cent. South Univ. (2020) 27: 3278-3289
DOI: https://doi.org/10.1007/s11771-020-4546-0
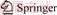
Cations stress on low-grade nickel sulfide ore oxidation leaching
KANG Jin-xing(康金星), WANG Xin(王鑫), WANG Ya-yun(王亚运), LIU Zhao-bo(刘召波),
HAN Guo-qiang(韩国强), LIU Zhi-guo(刘志国), WANG Chuan-long(王传龙)
ENFI Research Institute, China ENFI Engineering Co. , Beijing 100038, China
Abstract:
The effects of cations stress of magnesium ion and sodium ion on the low-grade nickel sulfide ore oxidative leaching in simulated sulfuric acid solutions were investigated. This study was performed in two courses, including the effect of the cations on the valuable metals leaching efficiencies of the nickel ore and its influences on the electrochemical oxidation behavior of the nickel ore. The leaching results present that parts of magnesium-containing gangues and ferrous sulfide are preferentially dissolved into lixivium, and the leaching efficiencies of Ni and Cu decreased much related to the leached concentrations of Mg2+ increased. The results of electrochemical measurements show that the oxidation leaching of the low-grade nickel sulfide ore is controlled by the intermediates oxidative diffusion. Mg2+, as well as Na+, affects the transformations of the Fe3+/Fe2+ couple and sulfur-containing species, and those cations are apt to be attracted by the anions and directionally adhere to the negative active site of the metal sulfide surface, causing an increase in the electrochemical activities, which facilitates the electron transfer between the ore and leaching mediums. By comparative study of the role of Mg2+ and Na+, it is found that Mg2+ negatively affects the oxidative diffusion of the intermediates through promoting the generation of a compact film, which lowers the metals leached efficiencies, and the unfavorable effect of Na+ tends to be the coupled effect of the leached Mg2+ and Fe3+.
Key words:
magnesium ion; sodium ion; intermediates diffusion; directional adsorption; passivation film;
Cite this article as:
KANG Jin-xing, WANG Xin, WANG Ya-yun, LIU Zhao-bo, HAN Guo-qiang, LIU Zhi-guo, WANG Chuan-long. Cations stress on low-grade nickel sulfide ore oxidation leaching [J]. Journal of Central South University, 2020, 27(11): 3278-3289.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-020-4546-01 Introduction
Nickel is one of the important strategic raw materials and widely used in electronic engineering, machinery realm, military industry, chemical engineering, and other fields [1]. At present, the extraction of nickel from nickel sulfide ores constitutes the majority, especially in China mainly occurring nickel sulfide deposits [2, 3]. For a long time, the outstanding contradiction between the consumption needs and the independent production of nickel increased in China [4]. Low-grade nickel sulfide ore, as important nickel resources, is one of the hot spots in nickel metallurgy attracting more and more attention [5-7].
Most of the low-grade nickel sulfide ores are polymetallic sulfide ores, and often associated with copper, cobalt, and iron-bearing sulfide minerals. The gangue minerals of the ores, commonly magnesium-containing silicate minerals, constituted the basic matrix [8, 9]. The desired minerals or elements separations from those ores are usually adopted during the beneficiation-pyrogenic process and wet extraction process, of which sulfide flotation-fire melting is in the majority [10-12]. However, because of the appreciable impact of magnesium-bearing silicates, mineral separation efficiency of the turbulent flotation process is poor [13]. The disposition of the raw ores or flotation concentrates using the fire method causes high environmental stress and smelting cost [14]. The wet extraction of the valuable metals from the low-grade nickel sulfide ore is an environmental and efficient method in relative terms.
Aiming at the wet process for low-grade nickel sulfide ore, researchers have reported many biological and abiotic metallurgic methods [15-17]. The nickel hydrometallurgy commonly uses sulfuric acid as a leaching medium. In sulfuric acid oxidative leaching systems, a great majority of chemical extractions pay close attention to metals recoveries and leaching cost, and the vast majority of microbes leaching is focused on the metals recoveries and biological tolerance [18, 19]. It has shown that during the sulfuric acidic oxidation leaching, as well as weakly acidic bioleaching, part of cations such as magnesium, sodium, and iron ions, could be preferentially leached into lixivium and disturb the whole leaching process [20]. However, studies of the oxidation leaching behavior of low-grade nickel sulfide ore under the intimidation of prioritized cations are reported less.
Nickel minerals presented in the low-grade nickel ore are bound up with iron-containing sulfide in ore properties resulting in the extraction of nickel strongly linked with ferrous-bearing sulfide oxidation dissolution. Based on previous studies, it can be drawn that magnesium ion (Mg2+) and iron ion (Fe3+) are the main underlying positive ions during valuable metals extraction in nickel sulfide ore oxidation leaching. Sodium-ion (Na+), as a positive ion and flexible ion, is usually used as a comparison of the influences of the basic rock cations on metals sulfide oxidative leaching.
This work attempted to evaluate the effect of preferentially leached cations on the oxidation behavior of sulfide minerals by using low-grade nickel sulfide ore as raw material. The oxidation leaching efficiencies of Ni, Cu, Fe, and Mg in the nickel ore were measured by using different oxidative systems, including Mg2+ or Na+ added in Fe3+-free solutions and Fe3+-containing oxidative solutions. Correspondingly, the electrochemical behaviors of the nickel ore in the imitated leaching mediums were investigated. The roles of Mg2+ and Na+ in the ore leaching were discussed of those cations in a sulfuric acid system.
2 Experimental
2.1 Materials
The low-grade nickel sulfide sample was obtained from Jinchuan, Gansu, China. The nickel ore sample was ground to 74 μm with a specific surface area of 3.2 m2/g before being used. The chemical compositions of nickel ore sample are shown in Table 1. Figure 1 shows the XRD pattern of the nickel ore. The main metallic minerals in the ore include pentlandite, violarite, pyrrhotite, and chalcopyrite, and the main gangue minerals are amesite, serpentine, and pyroxene. The other reagents used in this work were of analytical grade.
Table 1 Chemical compositions of low-grade nickel sulfide ore (mass fraction, %)
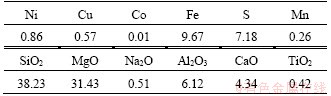
Figure 1 XRD pattern of low-grade nickel sulfide sample ore
2.2 Oxidative leaching performance tests
Oxidation dissolution tests of the nickel ore were performed in simulated solutions, including air environment and selected concentration ferric iron dilute sulfuric acidic oxidative solution with or without Mg2+or Na+. The imitated leaching media were prepared by dilute sulfuric acid dissolved ion-containing sulfate, incorporating MgSO4, Na2SO4, and Fe2(SO4)3. 100 mL configured solution for each test was added into a 250-mL conical flash, which was placed in a thermostatic water bath with a magnetic agitator. When the leaching solution was heated to a temperature of 90 °C, a mass of 1.0 g nickel ore sample was added for the oxidation leaching test. Each agitation leach was tested four times in parallel at the mixing speed of 400 r/min. After a selected time, the slurry was filtered, and the filtrate, as well as residue, was taken for the element content analysis. The oxidation leaching test was measured in a constant acidity of pH 0.8 (corresponding to H2SO4 concentration of 0.1 mol/L) to reduce the effects of pH fluctuation and jarosite formation. Leaching efficiency was calculated based on the increased concentration of leached metal in the lixivium with respect to input quantity. The leached Ni, Cu, Mg, and Fe concentrations in the pregnant solutions were determined by ICP-AAS analysis.
2.3 Electrode and electrochemical measurements
The electrochemical behavior of low-grade nickel sulfide was performed in simulated bath solutions using a typical three-electrode system connected to a CHI1140C microcomputer-based electrochemical work station. The working electrodes were plated a thin layer of mineral grain ink which was mixed by the nickel ore powder (90 wt.%) and polytetrafluroethylene (10 wt.%) in N-methyl-2-porrilione over a Pt disk (0.1 cm2) [21]. The reference electrode used a saturated calomel electrode (SCE) (0.24 V vs. standard hydrogen electrode (SHE)) and the auxiliary electrode employed a Pt plate electrode (1.0 cm2). Simulated dilute sulfuric acid bath solutions were prepared in an established concentration of Mg2+ or Na+ in the presence or absence of Fe3+ corresponding to Section 2.2. The three electrodes were immersed in the simulated mediums for 20 min and the scan was started. The cyclic voltammetry measurements of nickel sulfide ore were performed by scanning from 1.2 V to -0.8 V (negative-going potential scan), and back to the started potential with a scanning rate of 25 mV/s. The potentiodynamic polarization curves were obtained by sweeping from 1.0 V to -0.2 V with a scanning ratio of 5 mV/s. The impedance spectra were generated at the open circuit potential (OCP) by employing a sine wave voltage amplitude of 5 mV in the frequency range of 0.01-105 Hz. The data were displayed as Nyquist plots and analyzed using Zview2 software. The electrochemical tests were performed in the atmosphere at 25 °C by connecting a homeothermic thermotank. All potentials shown in this work referred to SCE.
3 Results and discussion
3.1 Mg2+ and Na+ stress on Ni, Cu, Fe, Mg leaching efficiencies
The oxidation acidic leaching of low-grade nickel sulfide ore is primarily concerned with the sulfide dissolutions and alkalescent matrixes in an acidic system. So, the experiment below focused on the leaching ratios of Ni, Cu, Fe, and Mg to discuss the influences of magnesium ion (Mg2+) and sodium ion (Na+) in simulated leaching media. Figure 2 shows the leaching ratios of Ni, Cu, Fe, and Mg over time in various initial leaching solutions, suggesting that the dissolution of Mg2+ is preferential relative to metal sulfides, and the initial use of Mg2+, as well as Na+, has adverse effects on the oxidation leaching of sulfide minerals but lightly affects the acid dissolution of the basic gangue minerals under test conditions.
For each group, the leached magnesium is around 60% referred to the raw nickel ore. It is relatively independent of the oxidation systems in the presence or absence of the cations, illustrating that parts of magnesium-containing alkaline minerals are decomposed forwardly in sulfuric acid media [22]. The addition of Fe3+ accelerates sulfide mineral oxidation significantly [23]. The higher leaching efficiency of Fe than Ni and Cu in the absence of Fe3+ solutions, suggests that the oxidation dissolution of the iron-bearing sulfide minerals could be prepositive, which generates an active species of Fe3+ and then affects the oxidation leaching of sulfide minerals.
The responses of oxidative leaching of Ni, Cu, and Fe-bearing minerals of the nickel ore to the presence of Mg2+ or Na+ are similar, indicating that Mg2+ and Na+ affect the oxidative leaching of sulfide minerals analogously and the effects are nonselective in the experimental scope. The leaching efficiencies of Ni, Cu, and Fe-containing sulfide decrease with the increment of Mg2+ concentration in Fe3+-free or Fe3+-containing mediums. The Ni, Cu and Fe leaching is promoted by the addition of 1.0 g/L Na+ in the absence of Fe3+, but the leaching of those metals increases firstly then decreases with leaching time when 1.0 g/L Na+ was added to the Fe-containing solution. The negative influence of Na+ is subject to the increased concentration of the leached Fe3+ and Mg2+. In the presence of Fe3+, when added 4.0 g/L Mg2+ and Na+, the leaching ratios of Ni, Cu and Fe decreased by 41%, 36%, and 29% and 32%, 26%, and 26%, referred to the absence of Mg2+ and Na+ in the initial solutions for 2 h leaching test, respectively. While, the leaching ratios of Ni, Cu, and Fe reduced by 26%, 18%, and 22% and 14%, 5%, and 16% with the employment of 4.0 g/L Mg2+ and Na+ in the iron-free air atmosphere solutions, respectively. Moreover, the coupled use of Fe3+ and the preferential cations reduced the leaching ratios of Ni, Cu, and Fe by about 12%, 12%, and 13% relative to the single-use of Fe3+ for 6 h oxidative leaching. Those results suggest that the inescapable cations of Mg2+ and Na+, coupled with Fe3+, tend to have a synergistic effect.
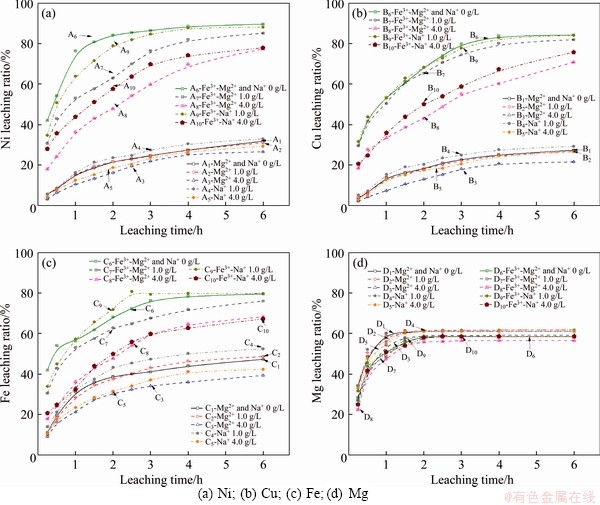
Figure 2 Effects of Mg2+ and Na+ on major elements leaching of low-grade nickel sulfide ore:(90 °C, agitation speed 400 r/min; sulfuric acid concentration 0.1 mol/L; initial added Fe3+ concentration 4.0 g/L; atmosphere)
It can be concluded that the preferential leached cations have a significant effect on sulfide mineral oxidation of the sulfide ore. During the oxidation leaching of the low-grade nickel sulfide ore, part of magnesium and iron could be preferentially dissolved to cations in lixivium, affecting the sulfide oxidation. There is an outstanding synergistic action between those ions. Therefore, the following work focused on the electrochemical behavior under the separated use of Mg2+ or Na+ and its coupled use with Fe3+ for the measurement of the cation stress on the nickel ore leaching.
3.2 Mg2+ and Na+ stress on oxidation leaching reactions
Figure 3 presents cyclic voltammograms (CV) of the low-grade sulfide nickel ore in different leaching solutions. The curves of the nickel ore mainly respond to the oxidative dissolutions of the metallic sulfide under test conditions, as shown in Figure 3. It can be seen that no new redox is detected in the presence of Mg2+, Na+, and Fe3+ in leaching solutions relative to the system without them, illustrating that cations of Mg2+, Na+, and Fe3+ cause no other redox reactions of the nickel ore. The closure areas of the ore redox curves decreased with the use of Mg2+ and Na+, suggesting that those priority dissolution cations lower the exchange of substances between ore electrode and leaching electrolyte. Mg2+ and Na+ affect the oxidation leaching of the sulfide minerals of the nickel ore primarily through the conversions between sulfur- containing species and Fe3+/Fe2+.
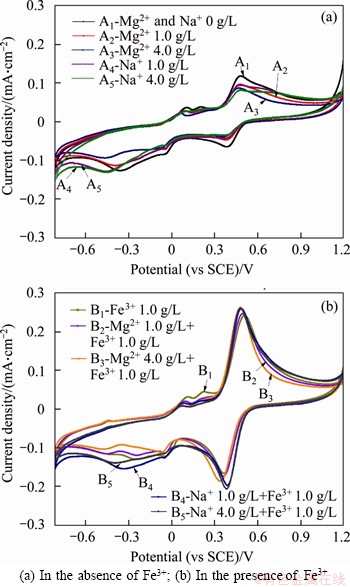
Figure 3 Effects of Mg2+ and Na+ on cyclic voltammetry curves of low-grade nickel sulfide ores in various solutions:(25 °C, sulfuric acid concentration 0.1 mol/L, scan range from -0.8 to 1.0 V(vs SCE), scan rate 25 mV/s)
The effects of Mg2+ and Na+ on the redox reactions of sulfide ore are roughly similar. In the iron-free acidic media (Figure 3(a)), the transfer responses of Fe3+/Fe2+ and sulfur-containing metal sulfide dissolution of the nickel ore are observed between 0.3 to 0.6 V and -0.1 to 0.2 V (vs SCE), respectively, which could be listed as Equations (1)-(6) [24-26]. The additions of Mg2+ and Na+ lead to the decrements in the redox peak current densities of those couples and the increments in the potential difference between redox peaks of the pairs with negative movement, corresponding to the cyclic transformation of Fe3+/Fe2+ and S0/Me(Ni,Cu, Co)S couples reduced, suggesting that the cations of Mg2+ and Na+ have a negative effect on the oxidation dissolution of sulfide minerals. Especially, the influences of Mg2+ and Na+ on the reductive reaction of S0/S2- are different. When added 1.0 g/L and 4.0 g/L Mg2+, the reductive current peak of S0/S2-, corresponding to Eq. (7), appears at -0.3 V, decreased by factors of 0.16 and 0.32, and the potential peak of S0/S2- has negative polarization by 0.07 and 0.1 V, respectively, but the use of 1.0 g/L or 4.0 g/L Na+ from the responses of S0/S2- shows 0.1 V negative potential movement with no decrement in the current densities. These suggest the presence of Mg2+ tends to combine with S2- for a steady sulfide and the usage of Na+ is inclined to impact the matter conductivity of the interface. The oxidative dissolution peak of SO32-/S0 (as Eq. (8)) at 0.21 V, related the intermediate oxidization to soluble products, reduced significantly with the employment of Mg2+ and Na+ referred to the absence of those cations, suggesting that those cations slow down the exchange of adsorbed S0 to solvable SO32- [27, 28].
Fe3++e=Fe2+, E0=0.54 V (1)
Ni2++S0+2e=NiS(γ), E0=0.14 V (2)
3Ni2++2S0+6e=Ni3S2, E0=-0.14 V (3)
Cu2++S0+2e=CuS, E0=0.35 V (4)
Co2++S0+2e=CoS, E0=-0.09 V (5)
S0+2H++2e=H2S, E0=-0.10 V (6)
S0+2e=S2-, E0=-0.75 V (7)
H2SO3+4H++4e=S0+3H2O, E0=0.21 V (8)
When there is the coupled use of Fe3+, influences of Mg2+, and Na+ on the nickel ore oxidation are present in Figure 3(b). It can be seen that the employment of Fe3+ leads to an acceleration of the redox reactions of the sulfide ores and facilitates the oxidation leaching of sulfide minerals significantly, due to the oxidation potential of Fe3+/Fe2+ higher than the redox potential of metal sulfide. The oxidative peak current of Fe3+/Fe2+is higher than the reductive one, suggesting that the oxidation of the ferrous-bearing mineral occurs rapidly. The performance of the use of Mg2+ in the Fe3+-containing solution is negative on the conversion of Fe3+/Fe2+, showing a reduction in the reductive peak current density and a negative movement of the reductive peak potential. The employment of Na+ leads to an increase of the current densities and a shortening of the potential difference of the Fe3+/Fe2+ couple, illustrating that Na+ is beneficial to the transformation of Fe3+/Fe2+ in the presence of Fe3+. However, the effect of Na+ on the conversation between sulfur-containing matters is negative, due to the oxidized reactions decreased and the reductive responses increased in the presence of Na+. Moreover, the absorbed sulfur oxidative dissolution of the characteristic peak of SO32-/S0 nearly at 0.2 V reduced with the coupled use of Fe3+ and the cations.
The oxidative leaching of the low-grade nickel sulfide ore is mainly about the transformation of Fe3+/Fe2+ and sulfur-containing substances. In the Fe3+-free and Fe3+-containing solutions, the transformation current responses of Mg2+ and Na+ to the sulfur-containing substances oxidation perform negatively, especially on the oxidative dissolution of the intermediate product of sulfur, which impacts the oxidation leaching remarkably. In the aspects of the exchange of Fe3+/Fe2+ pair, Mg2+ has a negative effect in each simulated medium and Na+ has a positive influence in the presence of Fe3+. Those suggest that Mg2+ has a trend to form magnesium-containing sulfide on the ore surface, which reduces the interchanges of materials between ore electrode and bath solutions and Na+ could enhance the redox activities on the interface of the ore and solution.
3.3 Mg2+ and Na+ stress on oxidation leaching rate
Figure 4 shows the potentiodynamic polarization plots of sulfide ore electrodes in various solutions. The corrosion of nickel ore is significantly affected by the oxidation reaction as the oxidative current density is much lower than the reductive current density. The corrosion current density increased with the use of Fe3+, illustrating that Fe3+ accelerates the sulfide ore corrosion [29]. The corrosion potential nearly appeared at 0.53 V, corresponding to the exchange potential of Fe3+/Fe2+, suggesting that the oxidative leaching of the low-grade nickel sulfide ore is more likely to the oxidation of ferrous-bearing minerals.
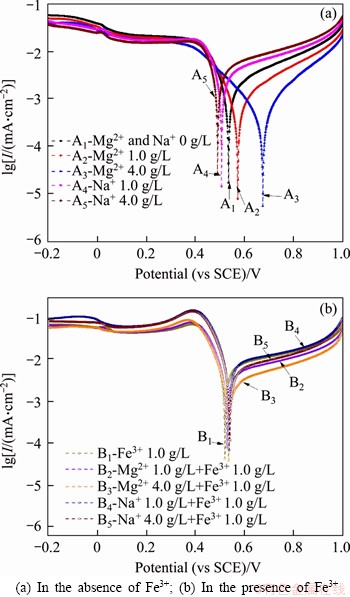
Figure 4 Effects of Mg2+ and Na+ on polarization curves of low-grade nickel sulfide ores in various solutions:(25 °C, sulfuric acid concentration 0.1 mol/L, scan range from -0.2 to 1.0 V(vs. SCE), scan rate 5 mV/s)
In the presence of Mg2+ (Figures 4(a) and (b)), the corrosion current of the nickel ore decreased with the concentration increase of Mg2+, illustrating that Mg2+ lowers the interchanges between the ore and leaching solution. Concretely, adding 1.0 g/L and 4.0 g/L Mg2+ to the Fe3+-free solutions, the corrosion potential moves positively by 0.03 and 0.14 V referred to that in the absence of Mg2+ solution, respectively. This positive polarization suggests the oxidation difficulty increases. The corrosion current densities decreased by factors of 0.28 and 0.27 with the additions of 4.0 g/L Mg2+ in Fe3+-free and Fe3+-containing solutions, severally, indicating that Mg2+ hinders the exchange of substances in ore leaching system. In the polarization transition range of oxidative reaction, the increase of the same potential leads to a greater increment in current density with the employment of Mg2+, suggesting that Mg2+ facilitates the electron change in the interface. While, the passivations appearing in the reduction polarization area become more noticeable with the use of Mg2+ to the leaching medium, suggesting that Mg2+ impacts the formation of the intermediate product film. It can be concluded that Mg2+ affects the oxidative dissolution of raw sulfide minerals and their intermediate products of the low-grade sulfide nickel ore.
Using Na+ can accelerate the oxidation of nickel ore in the Fe3+-free and Fe3+-containing solutions. The employment of Na+ lowers the current potential and increases the current potential in the Fe3+-free solutions. With the addition of Na+ to the Fe-free solutions, the oxidation Tafel slope related to the electron transfer rate at the stable oxidation region is smaller, suggesting that Na+ accelerates the electron transfer between the ore electrode and bath solution. In the Fe3+-containing solutions, the usage of 1.0 g/L Na+ increases the corrosion current density, corresponding to Na+ evoking an increase in the exchange of the species. When increasing Na+ in solution from 1.0 g/L to 4.0 g/L into the 1.0 g/L Fe3+, the oxidative current density decreased and the Tafel slope of oxidation increased. It suggests the increasing tendency that a high concentration of Na+ combines with Fe3+ to form a stabilized compound slowing down the oxidation rates. The second passivation occurring in the reductive range, corresponding to the oxidation products dissolution, responses to the increment of Na+ concentration slightly, indicating that Na+ mainly affects the metal sulfide oxidation reaction.
3.4 Mg2+ and Na+ stress on oxidation leaching impedance
Figure 5 presents Nyquist (Figures 5 (a) and (b)) and Bode (Figures 5(c) and (d)) plots for the nickel ore electrodes in various solutions. Table 2 shows the fitting results obtained by employing a simple equivalent circuit of Rsol (RactC1) (RpruCPE2) (Figure 5(e)) [30]. Rsol, Ract and Rpru reflect the solution resistance, the charge transfer resistance and the redox resistance of the intermediates during oxidation dissolution. C1 and CPE2 represent the double-layer capacitances between ore electrode/ intermediate electrode interfaces and surface product layer/electrolyte interfaces, respectively. Y0 and η2 relate to the parameters of CPE2.
The characteristic Nyquist plots of the ore perform as semicircle curves in the high-frequency region (f ≥104 Hz) correspond to the oxidation of metal sulfides. The linear plots show slopes greater than 45° in the low-frequency region (f≤103 Hz), suggesting that the diffusion of the oxidative products is a significant factor in ore oxidative leaching. Combined with the results of resistance analysis with the previous performances of the metal sulfide, the oxidative dissolution of the low-grade nickel ore involves two electrochemical processes, the oxidation of the metal sulfide and the dissolution of the intermediates, and it is controlled by the oxidative diffusion of intermediates. With the use of Mg2+ and Na+, the minimum peak of phase angle moves to low frequency and the characteristic impedance of product dissolution increases, suggesting that the electron shuttle activity of the inner layer increases and the effects of the outer film products diffusion increase as shown in Bode plots.
The activation resistance of the interfacial chemical reactions of the metal sulfide decreased with the increase of Mg2+ and Na+ concentration, illustrating that those preferentially leached cations facilitate the electron transfer between the ore electrode and bath solution in the Fe3+-free solution. While there is a significant synergism of the mixed-use of Fe3+ and the cations of Mg2+ and Na+ on the electrochemical dissolution of metal sulfides. 4.0 g/L cations lead to an increase in the impedance of the metal sulfide referred to 1.0 g/L cations in Fe3+-containing solutions, suggesting that a high concentration of the cations coupled with Fe3+ is apt to form passivation layer leading to tardiness for sulfide oxidation leaching. Moreover, the solution impedance decreases with the addition of Mg2+ or Na+, suggesting that the cations enhance the electron exchange activity of the solution.
3.5 Mg2+ and Na+ stress on characterization of leaching residues
Figure 6 presents the SEM images of the nickel ore leached under the cation stress of Mg2+ and Na+ in the Fe3+-free and Fe3+-containing solutions. The images of the leached residues obtained from the sulfuric acid solution (Figure 6(a)), the sole use of Fe3+ acidic solution (Figure 6(b)) and the coupled use of Na+ with Fe3+ sulfuric acid solution (Figure 6(d)) are analogous,illustrating that the oxidative products of the ore response slightly to the presence of Fe3+ and Na+ under the operating conditions. However, it can be seen that the presence of Mg2+ leads to a more compact and viscous layer with a relatively high content of magnesium as shown in Figure 6(c). This layer performs a strong hydrophilic nature similar to hydroxide, suggesting that the relative enrichment of Mg2+ on the surface of the sulfide minerals generates an increase in hydroxyl ion concentration of the interface of the nickel ore, which leads to the diffusion of the oxidized Fe3+ from ferrous-containing minerals oxidation slowed down. The competitive adsorption between Na+ and Mg2+ to the surface of sulfide minerals causes the magnesium contents of the corrosion minerals to decrease under coupled use of Na+ with Fe3+ in leaching medium (Figure 6(d)). Those results suggest that Mg2+ affects more remarkably than Na+ in low-grade nickel sulfide ore oxidation leaching.
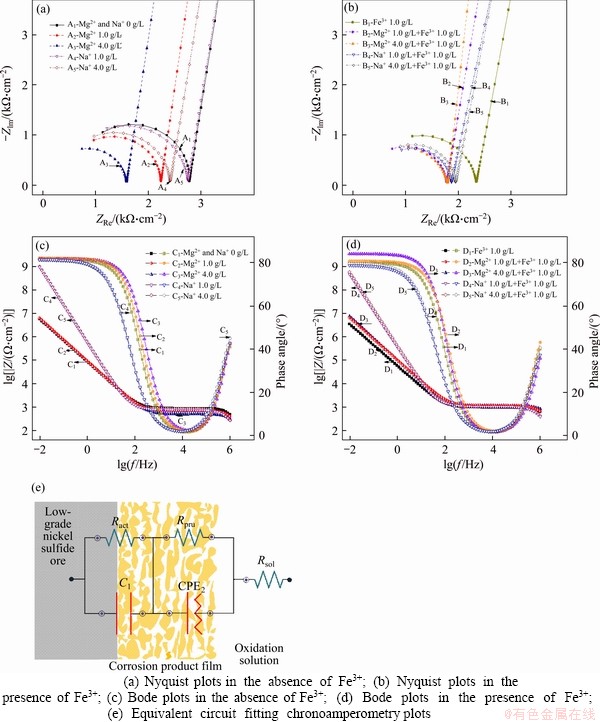
Figure 5 Effects of Mg2+ and Na+ on chronoamperometric plots of low-grade nickel sulfide ores in various solutions:(25 °C, sulfuric acid concentration 0.1 mol/L; frequency 0.01-105 Hz, potential perturbation OCP)
Table 2 Fitting results using equivalent circuit (Figure 5(e)) for low-grade nickel sulfide ore electrodes
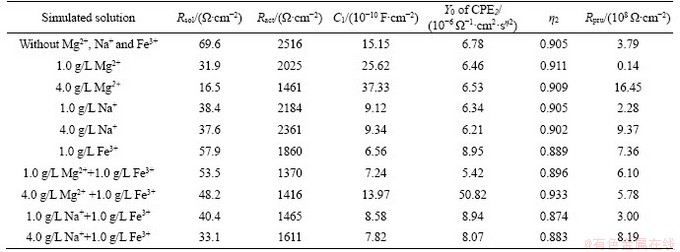
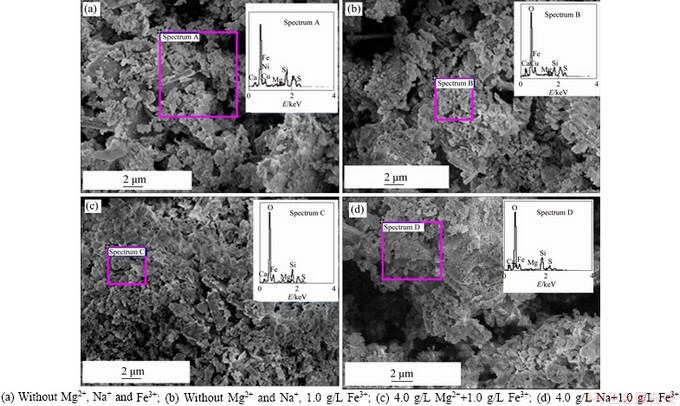
Figure 6 SEM micrographs of leaching residues after leaching for 1 h in various solutions:(90 °C, sulfuric acid concentration 0.1 mol/L, stirring speed 400 r/min, operation time 1 h)
3.6 Roles of Mg2+ and Na+ in nickel sulfide ores oxidation leaching
The preferential cation of Mg2+, as well as Na+, affects the oxidation leaching of low-grade nickel sulfide significantly. Mg2+ and Na+ do not tend to participate in the redox of sulfide minerals directly; but as active species, the orientation movement of them on the surface of the minerals leads to accelerating the electron transfer of the ore/solution interface and impacting the diffusion of the oxidized intermediates.
The impedance test results show that Mg2+ and Na+ promote the electron transformation of ore oxidation, and the results of leaching tests, cyclic voltammetry curves, and polarization curves of those cations have negative influences on the sulfide oxidation leaching. Both results of the impedance and polarization curves show that in the presence of Mg2+ and Na+, the oxidative diffusion of middle species affects the nickel ore oxidation remarkably. Those suggest that Mg2+ and Na+ mainly impact on the interface dual layer of the ore electrode. It also can be concluded that the oxidation leaching of the nickel ore is controlled by the diffusion of the intermediate products, and the active particles of Mg2+ and Na+ move directly, evoking an acceleration in the transformation between the active species of the ores and solutions, which causes a promotion in the intermediate film formation.
The preferentially leached cations affect the whole leaching process of nickel ore. The oxidation leaching of sulfide minerals of the low-grade nickel sulfide ore is closely concerned with the oxidation leaching of ferrous-containing sulfides [31]. The peroxidation of Fe2+ from the ferrous-bearing sulfide minerals generates Fe3+, and then with the surface potential of metals sulfide increasing, the solid-state low valence sulfur-bearing substances are oxidized to soluble high valence sulfur- containing species, so the valuable metals are dissolved [32]. This process is affected by the presence of Mg2+ and Na+. In an acid environment, part of magnesium and/or sodium alkalescent gangues are preferentially dissolved into the leaching solution and produce Mg2+ and Na+. Under the effect of electrostatic interactions of sulfide minerals, Mg2+ and Na+ directly move and adsorb the electronegative sulfur active site on the surface of sulfide minerals. Those cation adsorptions enhance the electrochemical activities of the minerals with the promotion of the conversation of Fe3+/Fe2+ and electron transfer resistance. However, due to the adsorptions of Mg2+, the corrosion film of the oxidized intermediates is generated compactly, which slows down the leaching efficiencies of sulfide minerals. Figure 7 shows the probable roles of Mg2+ and Na+ in the nickel sulfide ores oxidation leaching.
4 Conclusions
The oxidation leaching of low-grade nickel ore includes mainly two courses, an interface electrochemical oxidation of metal sulfides and an intermediates oxidative diffusion, related to the electrochemical transformations of the Fe3+/Fe2+ couple and sulfur-containing substances, and it is controlled by the latter within the scope of tests. Mg2+ is leached preferentially and affects both processes.
The leaching performance of the ore shows that part of mafic magnesium gangues are preferentially leached in acidic solution, generating the cations of Mg2+, and the leaching efficiencies of Ni, Cu, and Fe decreased with the Mg2+ concentration increased. It is corroborated by the effect of Na+ on the leaching efficiencies of the main metals. The results of electrochemical measurements show that those cations of Mg2+ and Na+ are attracted by the anions and directionally adhere to the negative active sites of the surface of the metal sulfides, leading to an increase in the electrochemical activities of the minerals, which promote the electron transfer between the ores and solutions. On the other hand, the adsorptions of the cation of Mg2+ is inclined to generate a more compact intermediate film than that in the absence of the cations, which slows down the diffusion of the products and lowers the metals leaching efficiencies of the nickel ore.
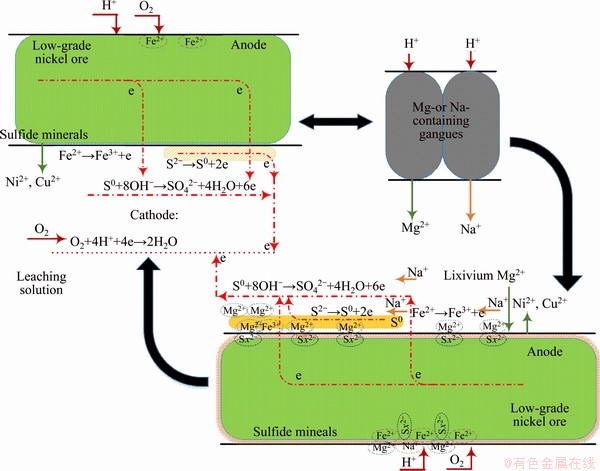
Figure 7 Effects of Mg2+ and Na+ on oxidation leaching of low-grade nickel sulfide ore
Compared the effects of Mg2+ and Na+ in the nickel ore oxidation leaching on the transformations of the Fe3+/Fe2+ couple and sulfur-containing species, it is found that Mg2+ negatively affects the oxidative diffusion of the intermediates through promoting a densification film generation, which lowers the leaching efficiencies of metal sulfides, and the unfavorable effect of Na+ tends to be the coupled effect of Mg2+ and Fe3+ from the dissolution of magnesium and ferrous minerals.
Contributors
KANG Jin-xing, WANG Xin and WANG Ya-yun provided the methodology, conducted the tests, wrote original draft, and revised and edited the manuscript. LIU Zhao-bo and HAN Guo-qiang revised the manuscript, and provided financial support. WANG Chuan-long, and LIU Zhi-Guo revised and edited the manuscript. All authors replied to reviewers' comments and revised the final version.
Conflict of interest
The authors declare no conflict of interest.
References
[1] KERFOOT D G E. Nickel [M]// Ullmann's Encyclopedia of Industrial Chemistry. 2000. DOI: 10.1002/14356007.a17_ 157.
[2] DAI Ta-gen, PAN Jun-qing, ZHANG De-xian. The 70-year progress of non-ferrous metal exploration in China [J]. The Chinese Journal of Nonferrous Metals, 2019, 29(9): 1817-1827. DOI: 10.19476/j.ysxb.1004.0609.2019.09.03. (in Chinese)
[3] TANG Zhong-li, REN Duan-jin. Types and metallogenic models of nickel sulfide deposits of China [J]. Acta Geologica Sinica, 1988, 1(2): 193-206. DOI: 10.1111/ j.1755-6724.1988.mp1002006.x.
[4] SUN Tao, WANG Deng-hong, QIAN Zhuang-zhi, FU Yong, CHEN Zheng-hui, LOU De-bo. Summary of metallogenic regularity for the nickel deposits, China [J]. Acta Geologica Sinica, 2014, 88(12): 2227-2251. DOI: 10.1002/pc. 750150203. (in Chinese)
[5] EKSTEEN J J, ORABY E A, NGUYEN V. Leaching and ion exchange based recovery of nickel and cobalt from a low grade, serpentine-rich sulfide ore using an alkaline glycine lixiviant system [J] Minerals Engineering, 2020, 145: 106073. DOI: 10.1016/j.mineng.2019.106073.
[6] CUI Fu-hui, MU Wen-ning, ZHAI Yu-chun, GOU Xue-yi. The selective chlorination of nickel and copper from low-grade nickel-copper sulfide-oxide ore: Mechanism and kinetics [J]. Separation and Purification Technology, 2020, 239: 116577. DOI: 10.1016/j.seppur.2020.116577.
[7] SUN Jian-zhi, CHEN Bo-wei, WEN Jian-kang, WANG Dian-zuo. Application and research progresses of hydrometallurgy technology for nickel ore [J]. The Chinese Journal of Nonferrous Metals, 2018, 28(2): 356-364. DOI: 10.19476/j.ysxb.1004.0609.2018.02.18. (in Chinese)
[8] LI Guang-shi, CHENG Hong-wei, XU Cong, LU Chang-yuan, LU Xiong-gang, ZOU Xing-li, XU Qian. Mineralogical analysis of nickel/copper polymetallic sulfide ore by X-ray diffraction using Rietveld method [C]// TMS 2016 Annual Meeting & Exhibition, Characterization of Minerals, Metals, and Materials 2016. Springer International Publishing, 2016. DOI: 10.1007/978-3-319-48210-1_8.
[9] PORTER T M. Regional tectonics, geology, magma chamber processes and mineralisation of the Jinchuan nickel- copper- PGE deposit, Gansu province, China: A review [J]. Geosience Frontiers, 2016,7(3): 431-451. DOI: 10.1016/ j.gsf.2015.10.005.
[10] RAO G V. Nickel and cobalt ores: Flotation [M]// Encyclopedia of Separation Science. Amsterdam: Elsevier, 2000: 3491-3500.
[11] BARSKII L A, RYBASV V, FAT YANOVA M A, PONOMAREV G P. Influence of sulfur-containing ions on selective flotation of copper-nickel ores [J]. Soviet Mining, 1986, 22(4): 310-316. DOI: 10.1007/BF02500860.
[12] YU Da-wei, UTIGARD T A, BARATI M. Fluidized bed selective oxidation-sulfation roasting of nickel sulfide concentrate: Part II. Oxidation roasting [J]. Metallurgical and Materials Transactions B, 2014, 45(2): 653-661. DOI: 10.1007/s11663- 013-9958-x.
[13] HUANG Kai-guo, CHEN Wan-xiong, PENG Xian-gan, ZENG Xiao-xi. A flotation technique for low-grade nickel ore [J]. The Chinese Journal of Nonferrous Metals, 1999, 9(3): 601-605. DOI: 10.19476/j.ysxb.1004.0609.1999.03. 030. (in Chinese)
[14] IMIDEEV V A, ALEKSANDROV P V, MEDVEDEV A S, BAZHENOVA O V, KHANAPIEVA A R. Nickel sulfide concentrate processing using low-temperature roasting with sodium chloride [J]. Metallurgist, 2014, 58(5, 6): 353-359. DOI: 10.1007/s11015-014-9915-1.
[15] HARRIS C T, PEACEY J G, PICKLESC A. Selective sulphidation and flotation of nickel from a nickeliferous laterite ore [J]. Minerals Engineering, 2013, 54: 21-31. DOI: 10.1016/j.mineng.2013.02.016.
[16] WATLING H R. The bioleaching of nickel-copper sulfides [J]. Hydrometallurgy, 2008, 91(1-4): 70-88. DOI: 10.1016/ j.hydromet.2007.11.012.
[17] DYSON N F, SCOTT T R. Acid leaching of nickel sulphide concentrates [J]. Hydrometallurgy, 1976, 1(4): 361-372. DOI: 10.1016/0304-386X(76)90037-2.
[18] BRYNER L C, JAMESON A K. Microorganisms in leaching sulfide minerals [J]. Applied Microbiology, 1958, 6(4): 281- 287. DOI: 10.1021/ ie50574a033.
[19] ZHEN Shi-jie, QIN Wen-qing, YAN Zhong-qiang, ZHANG Yan-sheng, WANG Jun, REN Liu-yi. Bioleaching of low grade nickel sulfide mineral in column reactor [J]. Transactions of Nonferrous Metals Society of China, 2008, 18(6): 1480-1484. DOI: 10.1016/s1003-6326(09)60029-7.
[20] SUN Jian-zhi, WEN Jian-kang, CHEN Bo-wei, WU Biao. Mechanism of Mg2+ dissolution from olivine and serpentine: Implication for bioleaching of high-magnesium nickel sulfide ore at elevated pH [J]. International Journal of Minerals Metallurgy and Materials, 2019, 26(9): 1069-1079. DOI: 10.1007/s12613-019-1823-8.
[21] KANG Jin-xing, FENG Ya-li, LI Hao-ran, DU Zhu-wei, DENG Xiang-yi, WANG Hong-jun. New understanding of the reduction mechanism of pyrolusite in the Acidithiobacillus ferrooxidans bio-leaching system [J]. Electrochimica Acta, 2019, 297: 443-451. DOI: 10.1016/ j.electacta.2018.12.031.
[22] SUN Chong, CHEN Xue-an, CHANG Xin-an, XIAO Wei-qiang, WANG Shao-hua, CHEN Yan-jun. Study on acid leaching conditions of Ni from tailings [J]. Inorganic Chemicals Industry, 2013, 45(8): 49-51, 54. DOI: 10.3969/ j.issn.1006-4990.2013.08.016. (in Chinese)
[23] DORADO A D, SOLE M, LAO C, ALFONSO P, GAMISANS X. Effect of pH and Fe(III) ions on chalcopyrite bioleaching by an adapted consortium from biogas sweetening [J]. Minerals Engineering, 2012, 39: 36-38. DOI: 10.1016/j.mineng.2012.06.009.
[24] BREDENHANN R, VUUREN C P J V. The leaching behavior of a nickel concentrate in an oxidative sulfuric acid solution [J]. Minerals Engineering, 1999, 12(6): 687-692. DOI: 10.1016/S0892- 6875(99)00051-5.
[25] LI Hong-xu, LI Chao, ZHANG Zhi-qian. Decomposition mechanism of pentlandite during electrochemical bio- oxidation process [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(3): 731-739. DOI: 10.1016/ s1003-6326(11)61238-7.
[26] AHMADI A, SCHAFFIE M, PETERSEN J, SCHIPPERS A, RANJBAR M. Conventional and electrochemical bioleaching of chalcopyrite concentrates by moderately thermophilic bacteria at high pulp density [J]. Hydrometallurgy, 2011, 106(1,2): 84-92. DOI: 10.1016/ j.hydromet.2010.12.007.
[27] ALMEIDA C M V B, GIANNETTI B F. The electrochemical behavior of pyrite–pyrrhotite mixtures [J]. Journal of Electroanalytical Chemistry, 2003, 553: 27-34. DOI: 10.1016/S0022-0728(03)00254-7.
[28] MARAPE G, VEMAAK M K G. Fundamentals of pentlandite mineralogy and its effect on its electrochemical behavior [J]. Minerals Engineering, 2012, 32: 60-67. DOI: 10.1016/j.mineng.2012.03.031.
[29] LI Y, KAWASHIMA N, LI J, CHANDRA A P, GERSON A R. A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite [J]. Advances in Colloid & Interface Science, 2013, 197-198: 1-32. DOI: 10.1016/j.cis.2013.03.004.
[30] KARIMI S, GHAHREMAN A, RASHCHI F. Kinetics of Fe(III)-Fe(II) redox half-reactions on sphalerite surface [J]. Electrochimica Acta, 2018, 281: 624-637. DOI: 10.1016/ j.electacta.2018.05.132.
[31] YANG Cong-ren, JIAO Fen, QIN Wen-qing. Leaching of chalcopyrite: An emphasis on effect of copper and iron ions [J]. Journal of Central South University, 2018, 25(10): 2380- 2386. DOI: 10.1007/s11771-018-3922-5.
[32] TAMURA H, KAWAMURA S, HAGAYAMA M. Acceleration of the oxidation of Fe2+ ions by Fe(III)- oxyhydroxides [J]. Corrosion Science, 1980, 20(8, 9): 963- 971. DOI: 10.1016/ 0010-938X(80)90077-3.
(Edited by YANG Hua)
中文导读
阳离子胁迫下低品位硫化镍矿的氧化浸出行为
摘要:本文模拟研究了镁离子和钠离子胁迫对低品位硫化镍矿氧化浸出的影响。主要研究阳离子对有价值金属的浸出效率的影响以及含镍硫化矿石的电化学氧化行为。浸出试验结果表明,部分含镁脉石和含亚铁硫化物会优先溶解在浸出液中,浸出过程中Ni和Cu的浸出效率下降与Mg2+的浸出浓度增加相关性较大。电化学研究结果表明,低品位硫化镍矿的氧化浸出受中间产物氧化扩散的控制。Mg2+和Na+影响Fe3+/Fe2+电对和含硫物质的转化,这些阳离子易受硫化矿物表面阴离子吸引并定向粘附在金属硫化物表面荷负电的活性位点,从而增加界面电子转移的电化学活性,促进矿石与浸出介质之间的电子转移。通过比较Mg2+和Na+的作用,发现Mg2+通过促成致密钝化膜的形成,降低了金属矿物的浸出效率,对反应中间产物的氧化扩散不利,而Na+的不利影响往往是与优先被浸出的Mg2+和Fe3+协同作用引起。
关键词:镁离子;钠离子;中间产物扩散;定向吸附;钝化膜
Foundation item: Projects(2019M650972, 2017M621034 ) supported by China Postdoctoral Science Foundation
Received date: 2020-03-04; Accepted date: 2020-07-13
Corresponding author: KANG Jin-xing, PhD, Engineer; Tel: +86-18810641067; E-mail: kangjx88@126.com; ORCID: https://orcid.org/ 0000-0001-6268-6157
Abstract: The effects of cations stress of magnesium ion and sodium ion on the low-grade nickel sulfide ore oxidative leaching in simulated sulfuric acid solutions were investigated. This study was performed in two courses, including the effect of the cations on the valuable metals leaching efficiencies of the nickel ore and its influences on the electrochemical oxidation behavior of the nickel ore. The leaching results present that parts of magnesium-containing gangues and ferrous sulfide are preferentially dissolved into lixivium, and the leaching efficiencies of Ni and Cu decreased much related to the leached concentrations of Mg2+ increased. The results of electrochemical measurements show that the oxidation leaching of the low-grade nickel sulfide ore is controlled by the intermediates oxidative diffusion. Mg2+, as well as Na+, affects the transformations of the Fe3+/Fe2+ couple and sulfur-containing species, and those cations are apt to be attracted by the anions and directionally adhere to the negative active site of the metal sulfide surface, causing an increase in the electrochemical activities, which facilitates the electron transfer between the ore and leaching mediums. By comparative study of the role of Mg2+ and Na+, it is found that Mg2+ negatively affects the oxidative diffusion of the intermediates through promoting the generation of a compact film, which lowers the metals leached efficiencies, and the unfavorable effect of Na+ tends to be the coupled effect of the leached Mg2+ and Fe3+.


