Trans. Nonferrous Met. Soc. China 22(2012) 3140-3146
Removal of cadmium from aqueous solutions using red mud granulated with cement
JU Shao-hua1,2,3, LU Shuai-dan1,2,3, PENG Jin-hui1,2,3, ZHANG Li-bo1,2,3, C. SRINIVASAKANNAN4, GUO Sheng-hui1,2,3, LI Wei1,2
1. State Key Laboratory Breeding Base of Complex Nonferrous Metal Resources, Cleaning Utilization in Yunnan Province, Kunming 650093, China;
2. Key Laboratory of Unconventional Metallurgy (Ministry of Education), Kunming University of Science and Technology, Kunming 650093, China;
3. Faculty of Metallurgical and Energy Engineering, Kunming University of Science and Technology, Kunming 650093, China;
4. Chemical Engineering Department, The Petroleum Institute, P.O. Box 2533, Abu Dhabi, United Arab Emirates
Received 6 January 2012; accepted 18 June 2012
Abstract:
A novel adsorbent was prepared from granular red mud mixed with cement and its potential to be a suitable adsorbent for the removal of cadmium ions from aqueous solutions was evaluated. The wet red mud was directly mixed up with cement at different mass fractions of 2%-8% and their properties were investigated. Based on the textural characteristics and strength, the granular red mud with 2% addition of cement maintaining for 6 d is identified to have better properties. The batch adsorption experiments for adsorption of Cd2+ ions from solution were performed at 30, 40 and 50 °C at different initial concentrations under the condition of constant pH of 6.5. The equilibrium adsorption was found to increase with the increase of temperature during the adsorption process. Langmuir adsorption isotherm model was found to match the experimental adsorption isotherm better. The kinetics of adsorption was modeled using a pseudo second order kinetic model and the model parameters were estimated.
Key words:
granular red mud; cadmium; waste water processing; adsorption; aqueous solutions; cement;
1 Introduction
Cadmium is an important metal, which can be used in many fields such as the manufacture of paints, plastic stabilizers, phosphors, Ni-Cd battery materials, electric contacts, fusible alloys and control rods for atomic reactor. However, cadmium and its compounds have been found to be very toxic and long-term exposure to cadmium can cause serious damage to human endocrine system, kidney, and bones [1,2]. Although the environmental discharge standards are very strict nowadays, the contamination of Cd in soil, lake and river is still a very serious problem due to the massive mining of zinc-lead [3]. Hence, it is imperative to identify the appropriate technology to control the Cd contamination in the irrigation water ways. One of the effective and time tested treatment techniques for control of water pollution is adsorption [4].
Red mud has been documented well that it can be used as a low cost absorbent for removing heavy metal ions, such as Zn2+[5], 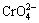 [6], Cd2+ [7], Pb2+ [8,9], Cu2+[7,10] and Ni2+ [11], and having high adsorption capacity for cadmium removal after specific thermal [12,13] or chemical pretreatment [14-16]. However, most of the earlier works pertaining to utilization of red mud employed was only powdered red mud. Powdered red mud, though endowed with a high specific adsorption area, it is not suitable for large scale commercial operation because it requires filtration for removal of the adsorbent.
[6], Cd2+ [7], Pb2+ [8,9], Cu2+[7,10] and Ni2+ [11], and having high adsorption capacity for cadmium removal after specific thermal [12,13] or chemical pretreatment [14-16]. However, most of the earlier works pertaining to utilization of red mud employed was only powdered red mud. Powdered red mud, though endowed with a high specific adsorption area, it is not suitable for large scale commercial operation because it requires filtration for removal of the adsorbent.
In the present work, the authors attempt to prepare a novel granular red mud adsorbent (GRM), and assess its ability to remove cadmium(II) ions from aqueous solution using batch tests. In addition, the adsorption isotherm and kinetics of adsorption are investigated.
2 Experimental
2.1 Preparation of adsorbent
Fresh red mud filter cake, containing 28.24% water with initial pH of about 13.3, was obtained from the Kaiman Aluminum Co. Ltd. in China, and was used as the principal raw materials for granulation. The high quality Portland cement was obtained from a cement plant of the Kunming city. The preparation procedures are described in Fig. 1.
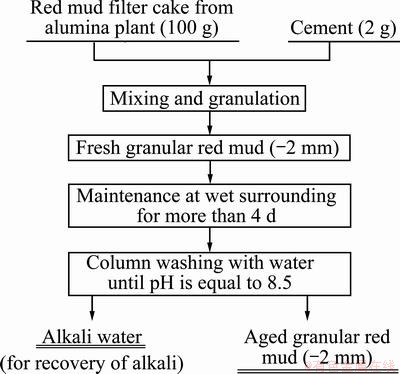
Fig. 1 Preparation procedure of granular red mud (GRM) adsorbent
The specific surface area of granular red mud was analyzed using Quanta Chrome Instruments, AUTOSORB-1.
2.2 Chemicals and reagents
All the chemicals and reagents used in the present work were of analytical grade. The CdCl2 stock solutions with a concentration of 100 mg/L were prepared from CdCl2. The metal ion solutions were subsequently diluted with deionized water to the desired concentration ranging from 1 to 10 mg/L.
2.3 Batch adsorption
Batch adsorption was performed under controlled experimental conditions at a desired concentration of Cd solution and pH. Granular red mud of about 10 g was added to 100 mL of Cd solution in a 250 mL sealed glass conical bottle. The sample bottles were placed in a shaker bath under controlled temperature for 24 h. The adsorption experiments were conducted at 30, 40 and 50 °C. In order to ensure sufficient interaction between Cd2+ and granular red mud adsorbent, the agitation speed of the shaker was kept at 100 r/min for all the batch experiments. After completion of adsorption, the solution was filtered directly.
The filtrate was analyzed using an atomic absorption spectrophotometer. The cadmium adsorption experiments were conducted at 5 different initial Cd(II) concentrations (1, 2, 5, 8 and 10 mg/L) added as CdCl2 at 30, 40 and 50 °C, respectively. The pH of solution was maintained at 6.5 for all the experiments.
The adsorption capacity of Cd(II) ions by granular red mud were calculated by the following mass balance equation:
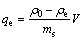 (1)
(1)
where ρ0 is the initial concentration, ρe is the equilibrium concentration, ms is the mass of adsorbent, and V is the volume of the solution.
3 Results and discussion
3.1 Characterization of adsorbent
The XRD pattern of red mud is shown in Fig. 2.
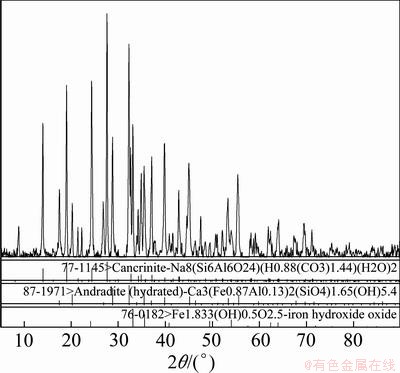
Fig. 2 XRD pattern of granular red mud adsorbent
The nitrogen adsorption isotherm of GRM was performed using surface area analyzer (Quantachrome Instruments AUTOSORB-1).
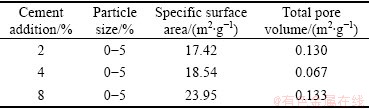
Table 1 lists the specific surface area and total pore volume of GRM at different cement additions ranging from 2% to 8%. It can be noted that the specific surface area and pore volume do not vary significantly with the increase of cement addition and the 2% addition is considered to be sufficient for the purposes of granulation.
Table 1 Textural characteristics of GRM with different contents of cement addition
The strength of GRM was assessed based on the efflorescence rate according to the following procedures: 1) 5 g of GRM with different cement additions were placed into a series of 250 mL conical flasks with water, for different time (either 3 or 6 d); 2) the flasks were placed in the shaker bath and shaken for 1 h; 3) filtering the resultant slurry with a 60 mesh screen; 4) drying the particles with a size 0.25 mm; 5) calculating the mass ratio of material in excess of 0.25 mm particles. The results of efflorescence rate are listed in Table 2.
It can be see from Table 2 that the GRM with a cement addition of 2% (wet base) and after maintaining for 6 d under wet conditions has the highest strength.
The textural characteristics of the GRM with 2% cement addition (wet base) were investigated by SEM and EDS shown in Fig. 3.
It can be seen from Figs. 3 (a-c) that GRM exists on the porous surface. In addition, Fig. 3(b) shows that the pore size of the GRM is in the range of 3.2-6.8 μm. It can be supposed from Figs. 3(d-f) that the GRM prepared by present method has a characteristics of cement existence on the surface, while the red mud exists mainly inside, explaining the reason of minimal amount of 2% cement addition is sufficient to prepare high strength GRM.
Table 2 Efflorescence rates under different conditions
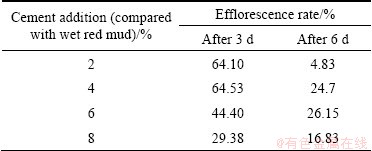
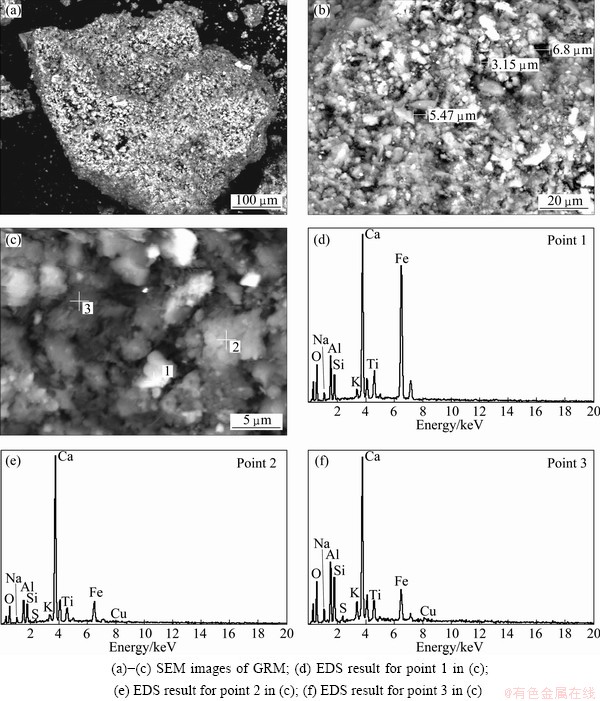
Fig. 3 SEM images and EDS results of cement granular red mud
3.2 Effect of contact time on adsorption
Figure 4 shows the effect of contact time on adsorption rate at different initial concentrations of Cd2+ solution, using adsorbent dosage of 0.1 g and at temperature of (30±1) °C.
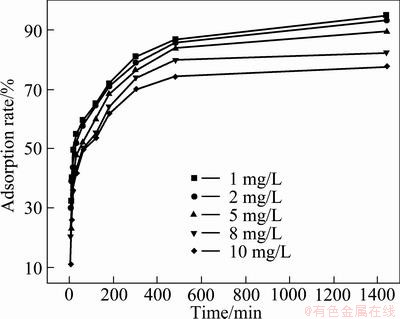
Fig. 4 Effect of contact time on adsorption rate
It is shown in Fig. 4 that the adsorption rate is very high initially, and it decreases when time increases, eventually reaches a steady state. The high initial rate of adsorption is due to the high concentration driving force available for mass transfer from the liquid to the adsorbent active sites. The removal rate of Cd2+ from solution decreases with the increase of initial concentration of Cd2+due to the limited availability of the adsorption sites, because the amount of adsorbent is constant in all the experiments.
3.3 Effect of initial Cd2+ concentration on batch adsorption
Figure 5 shows the effect of initial concentration of Cd2+ on adsorption amount, using adsorbent dosage of 1 g/L at temperature of (30±1) °C.
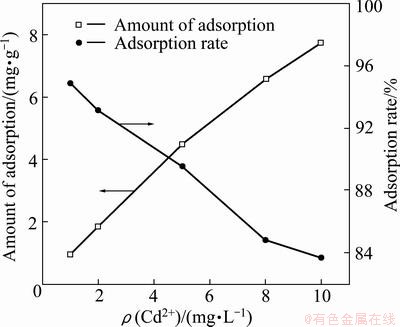
Fig. 5 Effect of initial concentration of cadmium ion on adsorption
The removal rate of Cd2+ from solution decreases with increase of the Cd2+ initial concentration, and the reduction was found to be in the range of 95%-84%. The amount of Cd2+ adsorbed by the adsorbent increases with increase of the initial concentration of Cd2+ in the solution. The higher initial concentration of Cd2+ with fixed amount of adsorbent will result in a higher equilibrium concentration of Cd2+ in the solution, which contributes to a higher amount of Cd2+ adsorbed by the adsorbent.
3.4 Effect of reaction temperature on batch adsorption
Figure 6 shows the effect of solution temperature on the removal of Cd2+ from solution at different initial concentrations of Cd2+ using adsorbent dosage of 1 g/L.
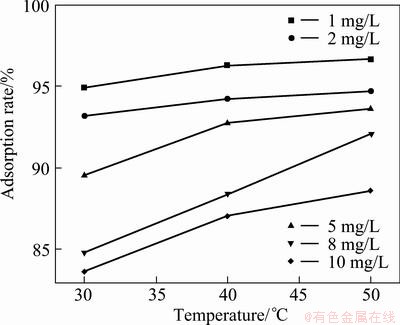
Fig. 6 Effect of reaction temperature on adsorption
It can be seen from Fig. 6 that as the adsorption temperature increases, the removal rate of Cd2+ also increases. A higher removal rate of cadmium from solution indicates a higher amount of transfer to the adsorbent phase, resulting in a higher adsorption capacity of the adsorbent. In other words, the adsorption capacity increases with increase in the temperature of the adsorption process. A physical adsorption is generally understood to be exothermic and the equilibrium adsorption will decrease with increase in the temperature of adsorption process. An increase of the adsorption capacity with increase in the temperature indicates a significant adsorption being endothermic reaction.
3.5 Adsorption isotherms and kinetics
The equilibrium adsorption of the Cd2+ was tested with the popular adsorption isotherms, Langmuir and Freundlich models. Both models are well detailed in Ref. [17] and hence only the representative model equations are provided. The Langmuir equation is expressed as follows:
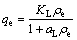 (2)
(2)
The linearized form of Eq. (2) is provided as follows:
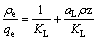 (3)
(3)
where qe is the equilibrium adsorption capacity of the adsorbent, ρe is liquid phase concentration at equilibrium , and KL (L/g) and aL (L/mg) are Langmuir constants. According to Fig. 4 and Eq. (3), a linear relationship between ρe and ρe/qe is shown in Fig. 7.
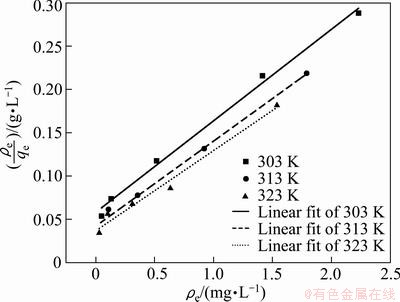
Fig. 7 Langmuir adsorption isotherm for Cd2+ onto GRM at different temperatures
From Fig. 7, the model constants, KL and aL along with the coefficient of determination (R2) are calculated and listed in Table 3.
Table 3 Langmuir and Freundlich parameters of GRM adsorbent at different temperatures
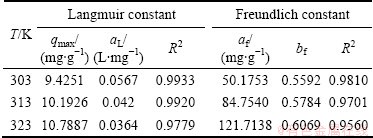
Freundlich isotherm is the semiempirical equation, with the assumption of multilayer absorption. The equation is expressed as
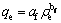 (4)
(4)
Transfer Eq. (4) into linear form as follows:
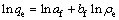 (5)
(5)
where af is the constant of Freundlich equation, to a certain extent reflecting the strength of the adsorption ability; bf is the component factor, which indicates the intensity of adsorption increasing with increase in the concentration and can also indicate the adsorption difficulty.
According to Fig. 4 and Eq. (5), a linear relationship of lnρe and lnqe is shown in Fig. 8. From Fig. 8, the model parameters af and bf along with the R2 value are listed in Table 3.
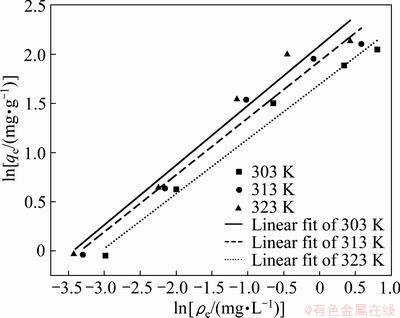
Fig. 8 Freundlich adsorption isotherm for Cd2+ onto GRM at different temperatures
The R2 value listed in Table 3 clearly indicates that the Langmuir mode fits better than the Freundlich model. The value of qmax indicates the monolayer adsorption capacity of the GRM, which increases with the increase of the temperature during the adsorption process.
The adsorption kinetics is generally modeled using the pseudo first order or pseudo second order model, while the second order model fits the experimental data better compared with the first order model for the adsorption of metal ions in solution using solid adsorbent [16]. The suitability of the pseudo second order model to match the experimental data was based on the R2 value. The quasi second order kinetic model is given as
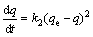 (6)
(6)
Equation (7) can be linearized to the following form:
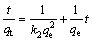 (7)
(7)
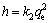 (8)
(8)
where h (mg·g-1·min-1) is initial adsorption rate, k2 (g·mg-1·min-1) is the quasi second order model of rate constant, and qt is the amount adsorbed at any given time, while qe is the equilibrium adsorption capacity.
Figure 9 shows the closeness of the model equation with the experimental data. The R2 value is presented in Table 4 along with the model kinetic parameters. The microanalysis of the GRM surface after Cd adsorption is shown in Fig. 10.
It can be seen in Fig. 10(a) that the porous surface of GRM after adsorption of Cd still exists, the EDS analysis in Fig.10(b) also clearly indicates the presence of Cd in the GRM.
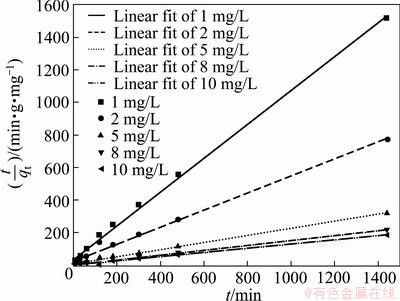
Fig. 9 Fitting curves of pseudo-second-order kinetic model with experimental data
Table 4 Pseudo-second-order model parameters at different concentrations
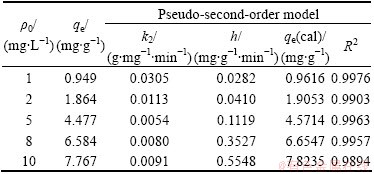
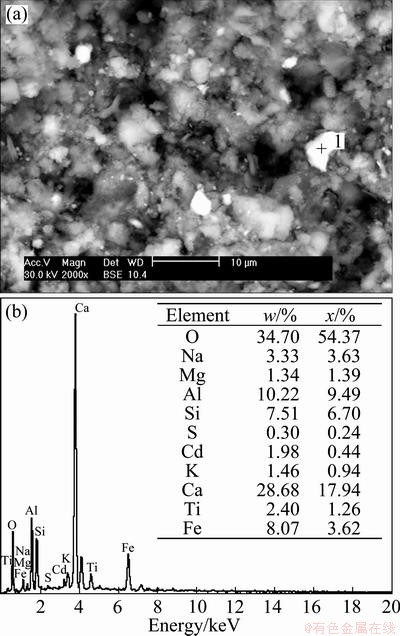
Fig. 10 SEM image of GRM (a) and EDS result (b) for point 1 in (a)
4 Conclusions
1) The wet red mud was directly mixed up with cement at different mass fractions ranging from 2% to 8% and their physical properties were estimated. Based on the textural characteristics and the physical strength, the 2% addition of cement is identified to have better properties.
2) The granular red mud (GRM) mixed with cement has potentials to be a suitable adsorbent for the removal of cadmium ions from aqueous solutions. The batch experiments for adsorption of Cd2+ ions with the GRM from solution were performed at 30, 40 and 50 °C at different initial concentrations under the condition of constant pH of 6.5. The results show that the GRM has good adsorption capacities. The equilibrium adsorption increases with the increase of temperature during the adsorption process, indicating the adsorption is an endothermic reaction.
3) The Langmuir adsorption isotherm model matches the experimental adsorption isotherm better. The adsorption capacity of adsorbent for Cd 2+ is more than 9 mg/g. The pseudo second order model matches the experimental data well. R2 values are more than 0.99.
4) The SEM and EDS results of the GRM surface after Cd adsorption clearly indicate the presence of Cd in the GRM.
References
[1] AN Li-hui, WANG Jing-bo, FU Qing, WANG Chun-yan, ZHANG Lei, LI Zi-cheng, ZHEN Bing-hui, SHANG Jing-jing. Impacts of environmental cadmium pollution on reproduction system: A review of recent researches [J]. Journal of Environment and Health, 2011, 28(1): 89-91. (in Chinese)
[2] MCDOWELL L R. Mineral in animals and human nutrition [M]. New York: Academic Press Inc, 1992: 359-364.
[3] RAN Lie, LI Hui-he. Progress in the research of present situation of soil cadmium pollution and its hazards [J]. Journal of Chongqing University of Arts and Sciences: Natural Science Edition, 2011, 30(4): 69-73. (in Chinese)
[4] WANG Xin-gang, LIU Xue-qing, CHEN Fang-yan, TANG Yu-bin, LV Xi-wu, ZHANG Dong, WANG Shao-xiang. Research on emergency treatment of accidental cadmium pollution of raw water by powdered activated carbon [J]. Technology of Water Treatment, 2011, 37(7): 73-77. (in Chinese)
[5] SAHU R C, PATEL R, RAY B C. Adsorption of Zn(II) on activated red mud: Neutralized by CO2 [J]. Desalination, 2011, 266: 93-97.
[6]  U. Chromate removal from water using red mud and crossflow microfiltration [J]. Desalination, 2005, 181: 135-143.
U. Chromate removal from water using red mud and crossflow microfiltration [J]. Desalination, 2005, 181: 135-143.
[7] MA Y, LIN C, JIANG Y, LU W, SI C, LIU Y. Competitive removal of water-borne copper, zinc and cadmium by a CaCO3-dominated red mud [J]. Journal of Hazardous Materials, 2009, 172: 1288-1296.
[8] GUPTA V K, GUPTA M, SHARMA S. Process development for the removal of Lead and chromium from aqueous solutions using red mud an aluminium industry waste [J]. Water Resources, 2001, 35: 1125-1134.
[9] LUO Hui-li, HUANG Sheng-sheng, LUO Lin, LIU Yan, WEI Jian-hong. Effect of chemical fixation of Pb in mine contaminated soil using red mud-phosphorus composition particles [J]. The Chinese Journal of Nonferrous Metals, 2011, 21(9): 2277-2284. (in Chinese)
[10] NADAROGLU H, KALKAN E, DEMIR N. Removal of copper from aqueous solution using red mud [J]. Desalination, 2010, 251: 90-95.
[11]  A. Rinsed and thermally treated red mud sorbents for aqueous Ni2+ ions [J]. Chemical Engineering Journal, 2010, 162: 75-83.
A. Rinsed and thermally treated red mud sorbents for aqueous Ni2+ ions [J]. Chemical Engineering Journal, 2010, 162: 75-83.
[12] MONTANARO L, BIANCHINI N, RINCON J M. Sintering behaviour of pressed red mud wastes from zinc hydrometallurgy [J]. Ceramics International, 2001, 27: 29-37.
[13]  S, ERGUN O N. Use of fly ash, phosphogypsum and red mud as a liner material for the disposalof hazardous zinc leach residue waste [J]. Journal of Hazardous Materials, 2010, 173: 468-473.
S, ERGUN O N. Use of fly ash, phosphogypsum and red mud as a liner material for the disposalof hazardous zinc leach residue waste [J]. Journal of Hazardous Materials, 2010, 173: 468-473.
[14] BERTOCCHI A F, GHIANI M, PERETTI R. Red mud and fly ash for remediation of mine sites contaminated with As, Cd, Cu, Pb and Zn [J]. Journal of Hazardous Materials, 2006, B134: 112-119.
[15] APAK R,  K, TURGUT M H. Modeling of copper(II) , cadmium(II) , and lead(II) adsorption on red mud [J]. Journal of Colloid and Interface Science, 1998, 203: 122-130.
K, TURGUT M H. Modeling of copper(II) , cadmium(II) , and lead(II) adsorption on red mud [J]. Journal of Colloid and Interface Science, 1998, 203: 122-130.
[16] ZHU C, LUAN Z, WANG Y, SHAN X. Removal of cadmium from aqueous solutions by adsorption on granular red mud (GRM) [J]. Separation and Purification Technology, 2007, 57: 161-169.
[17] KUMAR K V. Pseudo-second order models for the adsorption of safranin onto activated carbon: Comparison of linear and null-linear regression methods [J]. Journal of Hazardous Materials, 2007, 142: 1-2.
采用水泥制粒的赤泥脱除水溶液中的镉离子
巨少华1,2,3, 卢帅丹1,2,3, 彭金辉1,2,3, 张利波1,2,3, C. SRINIVASAKANNAN4, 郭胜惠1,2,3 , 李 玮1,2
1. 云南省复杂有色金属资源清洁利用国家重点实验室,昆明 650093;
2. 昆明理工大学 非常规冶金教育部重点实验室,昆明 650093;
3. 昆明理工大学 冶金与能源工程学院,昆明 650093;
4. Chemical Engineering Department, The Petroleum Institute, P.O. Box 2533, Abu Dhabi, United Arab Emirates
摘 要:采用湿赤泥添加水泥作为粘结剂的方法制备出一种新型粒状吸附剂材料,并评价这种粒状赤泥材料脱除水溶液中镉离子的潜力。直接向湿赤泥中添加2%-8%的水泥,研究不同水泥添加量和保养时间对粒状赤泥基吸附剂材料的粉化率和比表面积的影响。结果表明,在2%的水泥添加量下保养6 d后的粒状赤泥的结构特性和强度等参数达到了较好的水平。在pH为6.5,温度分别为30、40和50 °C及不同 Cd2+浓度溶液的条件下,进行吸附实验研究。结果表明,随着温度的升高吸附性能提高,并以此采用伪二级动力学模型进行吸附动力学模拟,得到了相关的模型参数。
关键词:粒状赤泥;镉;废水处理;吸附;水溶液;水泥
(Edited by LI Xiang-qun)
Foundation item: Project (51264022) supported by the National Natural Science Foundation of China
Corresponding author: PENG Jin-hui; Tel: +86-871-5147924; Fax: +86-871-5192076; E-mail: jhpeng72@hotmail.com
DOI: 10.1016/S1003-6326(12)61766-X
Abstract: A novel adsorbent was prepared from granular red mud mixed with cement and its potential to be a suitable adsorbent for the removal of cadmium ions from aqueous solutions was evaluated. The wet red mud was directly mixed up with cement at different mass fractions of 2%-8% and their properties were investigated. Based on the textural characteristics and strength, the granular red mud with 2% addition of cement maintaining for 6 d is identified to have better properties. The batch adsorption experiments for adsorption of Cd2+ ions from solution were performed at 30, 40 and 50 °C at different initial concentrations under the condition of constant pH of 6.5. The equilibrium adsorption was found to increase with the increase of temperature during the adsorption process. Langmuir adsorption isotherm model was found to match the experimental adsorption isotherm better. The kinetics of adsorption was modeled using a pseudo second order kinetic model and the model parameters were estimated.


