J. Cent. South Univ. (2016) 23: 2792-2799
DOI: 10.1007/s11771-016-3342-3

Swelling behavior of hot preheated pellets in reduction roasting process
Han Gui-hong(韩桂洪)1, Zhang Duo(张多)1, Huang Yan-fang(黄艳芳)1, Jiang Tao(姜涛)2
1. School of Chemical Engineering and Energy, Zhengzhou University, Zhengzhou 450001, China;
2. School of Mineral Processing and Bioengineering, Central South University, Changsha 410083, China
 Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Abstract:
One kind of facile coal-based direct reduction process is using hot preheated pellets for reduction in grate kiln. In this work, effects of reduction parameters on swelling index of hot preheated pellets were investigated by photographic technique under isothermal conditions. Experimental results show that swelling index of pellets is firstly increased then gradually decreased with increasing reduction time, while that is found to be an obvious decrease from 175 % to 30% with the variation of temperature from 900 °C to 1100 °C. Results of XRD combined with SEM reveal that swelling behavior of pellets is decided by structure of newly formed metal iron grains. The formation and growth of fibers iron grains promote the increase in volume. Low temperature and low CO content are favored to the formation and orientated growth of metal iron grains in the one step process.
Key words:
direct reduction; hot preheated pellets; swelling index; fibers iron;
1 Introduction
Direct reduced iron has been considered as necessary feeds in the production of high quality steel. Compared with traditional iron-making process, direct- iron process is a facile process and of great significance to green metallurgy. Direct-iron process can be divided into coal-based process and gas-based process [1-2]. In China, coal-based direct reduction process was developed in last few years and the traditional coal direct process can be described as follows [3]. Firstly, dry balls prepared from iron concentrates are preheated at about 950 °C in oxidation atmosphere. Then, pellets are roasted in oxidizing atmosphere at about 1250 °C and oxidized pellets can be obtained. Finally, the oxidized pellets suffer a second roasting procedure under reduction conditions [4]. Different from the traditional coal direct process, a new process with advantages of fast reduction and low energy-consumption has been developed in China, which uses hot preheated pellets for reduction in rotary kiln [3, 5].
In order to ensure operation in rotary kiln reduction process, recued pellets should be with high mechanical strength. However, the swelling index of iron ore pellets during reduction roasting can always lead to the loss of strength and even degradation of pellets [6]. Literatures [6-10] have pointed out that the maximum swelling index should be no more than 20% during reduction which cannot cause problems in operation. Beyond 20%, it is named “abnormal swelling” or “catastrophic swelling”, which can result into the disintegration and weakening of the reduced pellets. Numerous studies have been conducted to make investigation on the swelling behavior of iron oxides and many causes have been proposed [6-18]. FU et al [11] ascribed the swelling of pellets to the changing in crystal lattice of iron oxides. It was figured out the volume changes along with reduction as Fe2O3 (100%) → Fe3O4 (124%)→FexO (131%)→Fe (126%). The reason for swelling was proposed as the disruptive stresses set-up during the transformation of Fe2O3 to Fe3O4 [12-14]. Some other researchers found out that swelling behavior only occurs when using CO as reduction agents and attributed the abnormal swelling to the carbon in metallic iron and their reactions with oxygen [9-10]. However, more and more researchers considered the reason as the formation of iron whiskers during the wustite-iron reduction step [15-21]. They considered that the growing whiskers pushed neighboring grains and led to swelling. Literatures have pointed out that the formation and growth of whiskers depend on a number of processing parameters including reduction temperature, firing time and so on [10, 16, 18-19]. FUWA and BANYA [16] studied the effects of reduction temperature on swelling index and it was shown that the swelling index firstly increased and then decreased with the increase of temperature. SHARMA et al [18-19] studied the effects of firing temperature and time on the swelling behavior of iron ore pellets. It was found that the maximum swelling index of pellets was decreased accompanied with the increase in firing temperature. EL-GLASSY et al [20] studied the effects of reducing gas on the volume change during the reduction of iron oxide compacts and it was found that a greater swelling was observed for the compacts reduced with CO than H2.
In the present work, an attempt was made to study the swelling behavior of pellets under isothermal conditions in the facile one-step process. For this purpose, the experiments were carried out using hot preheated pellets for reduction roasting with CO & CO2 gas mixture. The influences of reduction time, reduction temperature, CO concentration on swelling index of pellets were examined. Furthermore, the mechanisms of volume swelling of pellets were probed by means of XRD and SEM analysis.
2 Materials and methods
2.1 Materials
In this work, a kind of magnetite concentrates and a binder were used for pelletizing. The main chemical characteristics of raw materials were respectively shown in Tables 1 and 2. As seen from Table 1, TFe grade of iron concentrates is 67.15 % and the FeO content is high to 27.54 %, indicating that the iron concentrates used in this experiment is a kind of original magnetite.
Table 1 Main composition of iron ore concentrates (mass fraction, %)
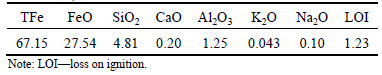
Table 2 Chemical composition of binder (mass fraction, %)
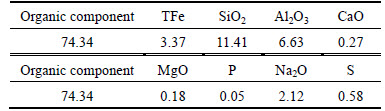
5 kg of the iron concentrates were mixed with 50 g binder and further rolled into green pellets with 12-15 mm diameter in a laboratory disc pelletizer. The produced green pellets were dried for 24 h at 120 °C. Thus, the resulting dried balls were preheated in air at 950 °C for 10 min and then roasted in reduction atmosphere. The porosity of preheated pellets is 21.98 % with average crushing strength of 974 N/pellet, which is close to that of the preheated pellets produced in industry. In addition, the FeO content in preheated pellets is 2.56%, indicating that the main iron-bearing mineral in preheated pellets is hematite. The morphology of preheated pellets is also observed by optical microscopy (Leica DMRXP, Switzerland) and the result is shown in Fig. 1. It is confirmed that most of magnetite has been oxidized into hematite. In addition, the particles are with large size and weak connected with each other.
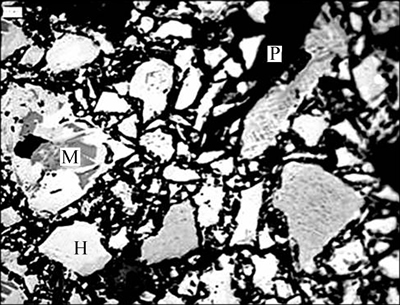
Fig. 1 Morphology of preheated pellets before reduction (M—Magnetite; H—Hematite; P—Pore)
2.2 Methods
2.2.1 Determination of swelling index
The swelling index of pellets was examined by visualizing physical changes of pellets in a horizontal tube furnace. The schematic diagram of the experimental setup is shown in Fig. 2.
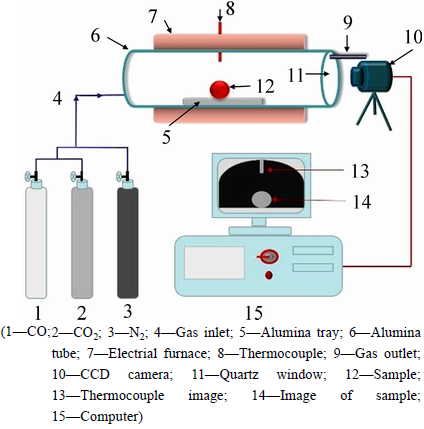
Fig. 2 Experimental measurement of swelling index
The experimental procedure can be described as follows. The hot preheated pellet was located on a specimen holder which could be pushed to a pre-determined location in the furnace. A CCD camera was used to capture the sample image every 5 min during reduction process. The output from camera was connected to a computer to record the entire process as a function of time. An image processing software was used to calculate cross-sectional area and the swelling index (RSI, Irs) was calculated on the basis of changes in the measured area using Eq. (1). The maximum swelling index can be named as Irs, max.
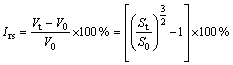 (1)
(1)
where V0 and Vt are the volumes of sample before and after reduction, while S0 and St are the corresponding cross-sectional areas. And reduction degree (R) of pellet sample at t min is evaluated as
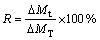 (2)
(2)
where △Mt refers to the mass loss at the moment of t, and △MT means the total mass loss in theory.
2.2.2 Structure characterization
The morphology of reduced pellets was observed by scanning electron microscope (FEI Quanta-200) equipped with EDS. The phase transformation of iron minerals during reduction was analyzed by X-ray diffraction (RIGAKU, D/Max 2500). The X-ray diffraction was performed on a D3 X-ray diffractometer equipped with a graphite monochromatized Cu Ka radiation source (λ=1.5406 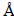 ). The sample was scanned in a 2θ range from 10° to 90° at a scan rate of 0.05 s-1.
). The sample was scanned in a 2θ range from 10° to 90° at a scan rate of 0.05 s-1.
3 Results and discussion
3.1 Factors affecting swelling index
3.1.1 Reduction time
Under the condition of CO of 95% and CO2 of 5% (volume fraction), the changing trend of swelling index with reduction time during reduction process is investigated and the results are shown in Fig. 3.
From Fig. 3, it is observed that swelling index rapidly increases and then gradually decreases with the extension of reduction time. The swelling index arrives to the maximum value of 35% when the pellets are reduced for approximately 20 min. With the proceeding of reduction roasting, however, the swelling index is decreased to 8% when the pellets are reduced for 85 min.
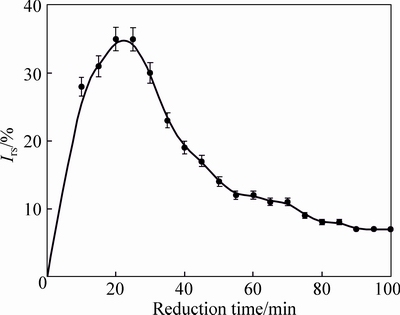
Fig. 3 Effects of reduction time on swelling index
3.1.2 Reduction temperature
When reduction temperature changes in the range from 900 °C to 1100 °C, the volume changes of pellets are researched under the condition of 95% CO. Experimental results are illustrated in Fig. 4. Meanwhile, the effects of reduction temperature on the maximum swelling index of pellets are also displayed in Fig. 5.
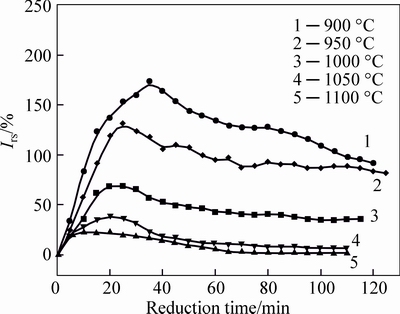
Fig. 4 Changing trends of swelling index
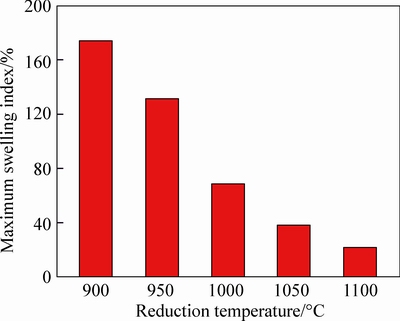
Fig. 5 Variation of maximum swelling index with temperature
Seen from the results in Figs. 4 and 5, it can be known that reduction temperature makes an obvious influence on the volume changing of pellets. It can be observed that the swelling index of pellets, especially the maximum swelling index, visibly decreases in a large range when temperature increases from 900 °C to 1100 °C. By comparison, the maximum swelling index at 900 °C reaches 175% when the pellets are reduced for 40 min, while the maximum swelling index at 950 °C, 1000 °C, 1050 °C and 1100 °C decreases to 132%, 69%, 39% and 22%, respectively. In addition, the time for pellets to reach the maximum expansion is shortened from 40 min to 10 min as temperature rises from 900 °C to 1100 °C. Figure 6 depicts the reduction degree of reduced pellets at different temperatures when swelling index arrives to the maximum value. From Fig. 6, the reduction degree for the maximum volume decreases accompanied with the increase of temperature. The reduction degree is about 50% at the moment of maximum swelling index at 900 °C, while that is about 30% at 1100 °C.
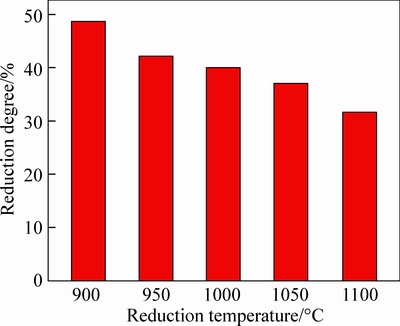
Fig. 6 Reduction degree when swelling index arrived to maximum value
3.1.3 Reduction gas
The volume change of pellet during reduction with different reducing gas at 1050 °C is given in Fig. 7. As shown in Fig. 7, when CO concentration is 85%, the maximum swelling index is 54% while that decreases to 39% if CO concentration increases to 95%. The results indicate that the maximum swelling index of pellets decreases with increasing CO concentration, which means that increase in reducing atmosphere may prefer to restrain the volume increasing.
On the whole, temperature makes an obvious effect on swelling behavior of pellets reduced. Particular attention should be taken onto that abnormal swelling or catastrophic swelling takes place if reduction temperature is lower than 1000 °C. In order to reveal the reasons for this phenomenon, effects of temperature on the phase transformation and microstructure of iron ore minerals in reduced pellets were both investigated by SEM and XRD.
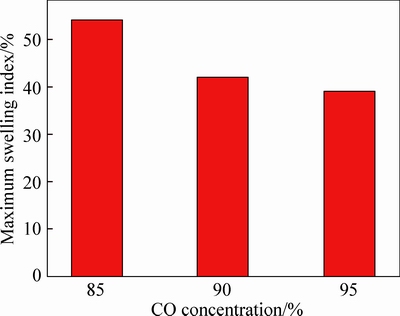
Fig. 7 Effects of CO concentration on maximum swelling index of pellets
3.2 Phase transformation of iron minerals
When the pellets were respectively reduced for 10, 20, 40 and 60 min at 900 °C, the phase transformation of iron minerals in reduced pellets was investigated. The XRD patterns of reduced pellets are given in Fig. 8.

Fig. 8 XRD patterns of reduced pellets at 900 °C
As shown in Fig. 8, hematite in preheated pellets rapidly disappears once reduced. The main iron-bearing minerals during the reduction process are separately magnetite, wustite, and metal iron and fayalite. There is still a little SiO2 existing in the phase of free silicon dioxide. The content of iron-bearing minerals was further calculated by XRD patterns and listed in Table 3.
Table 3 Content of iron-bearing minerals in reduced pellets at 900 °C
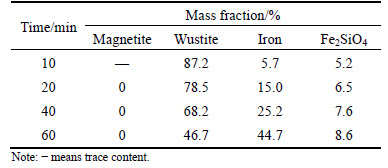
The results in Table 3 demonstrate that wustite content in pellets arrives to 87.2% when pellets are reduced for 10 min and it is gradually decreased with increasing time. When pellets are reduced for 40 min, wustite content can decrease to 68.2% which is still the dominant iron-bearing mineral in the pellets. If reduction time is longer than 40 min, wustite content rapidly decreases accompanied with the increasing metal iron content. For metal iron content, it gradually increases to 25.2% with increasing reducing time until 40 min. If time extending, metal iron content increases quickly and could arrive to 44.7% after reduced for 60 min.
Comparatively, while the reduction temperature increases to 1050 °C, the phase transformations of pellets with different reduction time are displayed in Fig. 9.
According to Figs. 8 and 9, it can be concluded that hematite and magnetite both rapidly disappear once pellets are reduced at 1050 °C. Free silicon dioxide disappears and most of SiO2 exists in the phase of fayalite (Fe2SiO4). According to the XRD patterns in Fig. 9, the main iron-bearing minerals during the reduction process are separately wustite, metal iron and fayalite, the content of which in reduced pellets listed in Table 4.
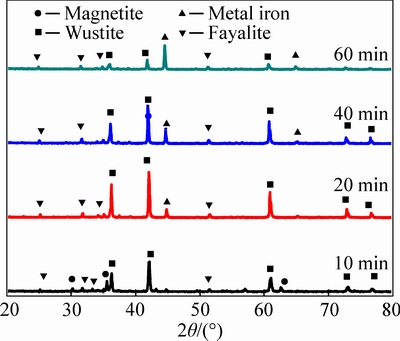
Fig. 9 XRD patterns showing phase transformations of reduced pellets at 1050 °C
Table 4 Content of iron-bearing minerals in reduced pellets at 1050 °C
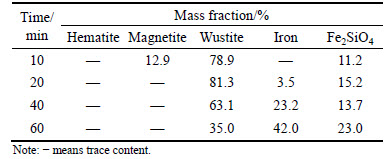
Table 4 shows that pellets are mainly composed of wustite, magnetite and Fe2SiO4 after reduced for 10 min. The content of iron-bearing minerals is separately 78.9%, 12.9% and 11.2%. With increasing time, magnetite fleetly disappears and wustite content gradually arrives to the maximum value when pellets are reduced for 20 min. When reduction time is longer than 20 min, wustite content rapidly decreases to 63.1% when time extends to 40 min. Accompanied with decreasing wustite content, metal iron appears and its content is quickly increased with a faster rate than that at 900 °C. For Fe2SiO4, its content increases from 11.2% to 23.0% with extending reducing time, which is a little higher than that listed in Table 3.
3.3 Morograph of iron minerals
As known to all, reduction process is preceded from the outer layer to the internal. Therefore, morphology of external and internal pellets is all observed by means of SEM. When pellets are reduced at 900 °C with different time, the morphology of pellets is given in Fig. 10.
Figure 10 vividly describes the micro-morphology of minerals in the reduced pellets with different reduction time at 900 °C. When pellets are reduced for 10 min, particles in the external are coarse, which are in the form of rod, as shown in Fig. 10(a). Combined Fig. 10(a) and Table 3, it could be concluded that most of particles in the out layer are wustite. In the core of reduced pellets, there is a few Fe3O4 with large size and in the form of granulate, which is wrapped by wustite. However, it is worth noting that there are obvious cracks on Fe3O4 particles. As mentioned in the former literatures, the transformation stress generated during the reduction of Fe2O3 to Fe3O4 would lead to the rupture of particles and destruction of crystal structure [21].
With increasing reduction time, more and more wustite transforms into metal iron and metal iron grains start to grow up gradually. In Fig. 10(b), there are many pores in pellets after reduced for 20 min and a few of iron grains grow up in the form of fibers along the pores. In Fig. 10(c), when pellets are reduced for 40 min, the average pore size in the reduced pellets increases. External metal iron grains start to weakly connect with each other. In the internal, wustite particles are still in the form of flaky and grainy. The newly formed metal iron grains grow up in the form of fibers. While reduction time extends to 60 min, the fibers metal iron grains are much bigger and closely connected with others, as displayed in Fig. 10(d). In the internal, wustite grains can be divided into two parts. One is fibrous metal iron grains orientated growing up, the other is silicon-bearing minerals moving in the opposite direction and finally separated with metal iron grains.
For comparing and analyzing the swelling behavior at different tempratures, the microstructure evolvement of pellets is also investigated by means of SEM when pellets were reduced at 1050 °C. The results are given in Fig. 11.
As presented in Fig. 11, when reduction temperature increases to 1050 °C, there are fewer pores in the pellets than that shown in Fig. 10. And the mineral particles are in different forms. Once reduced at 1050 °C for 10 min, the external particle grains are closed and in the form of particulate. The internal particles are with large size. Combined with XRD curves, it can be known that the internal particles are mostly granular magnetite particles, wrapped in flake wustite. While reduction time increases to 20 min, there are some metal iron grains in the form of flake in the external (Fig. 11(b)). At the same time, magnetite disappears in the inner pellets and wustite particles presents as obvious large-sized lamellar structure. With the continued increasing of time, metal iron grains rapidly increase and dominate in the outer layer of reduced pellets. What is more, metal iron grains fastly grow up until connected with each other. In the internal, metal iron particles are formed and grow up from the edge of wustite particles.
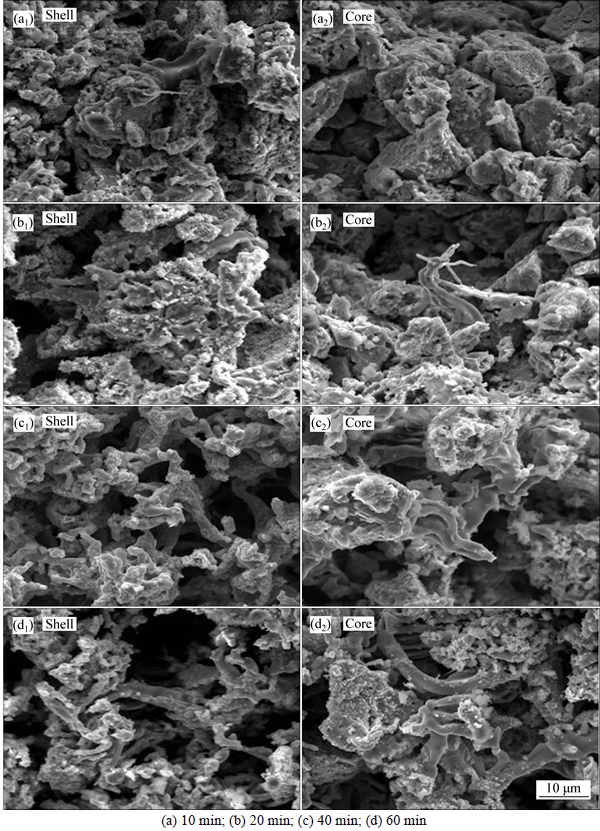
Fig. 10 SEM images of pellets showing shell (a1), (b1), (c1), (d1) and core (a2), (b2), (c2), (d2) reduced at 900 °C for different reduction time:
Through the evolution of iron ore minerals in reduced pellets and their microstructure at different temperatures, it indicates that the swelling index of pellets is mainly decided by the shape of newly formed metal iron grains, which is agreed with the former literatures [16-19]. It has been mentioned that hot preheated pellets are used for reduction [16]. And preheated pellets are characterized with high porosity and low crushing strength, which will lead to the low diffusive resistance to CO. According to the thermodynamics of ferrous iron oxide and CO, Fe2O3 is very easy to transform into Fe3O4. The stress generated by the transformation of Fe2O3 to Fe3O4 results in the fracture or destruction of crystal structure and formation of crystal defects [13-14]. At the initial formation stages of metallic iron, Fe2+ would be easy to gather along the edge of cracks due to crystal defects and the nucleation of metallic is formed at the crystal defects. However, when pellets are reduced at low temperature, the growth rate of irons is limited and the newly formed Fe2+ would move to nucleus to make metal ferrous grains grow up. Because of the large porosity of reduced pellets, metal ferrous grains gradually grow up along the pore which will prompt the formation of fibers iron. Finally, fibrous metal iron grains lead to high swelling index. With increasing temperature, the transformation rate of wustite to metal iron increases. Metal iron grains can be rapidly formed and connected with each other. Closed metal iron layers will be easy to form. Consequently, most iron grains are grown up in lamellar structures, restraining the formation of fibers iron and leading to the low swelling index.
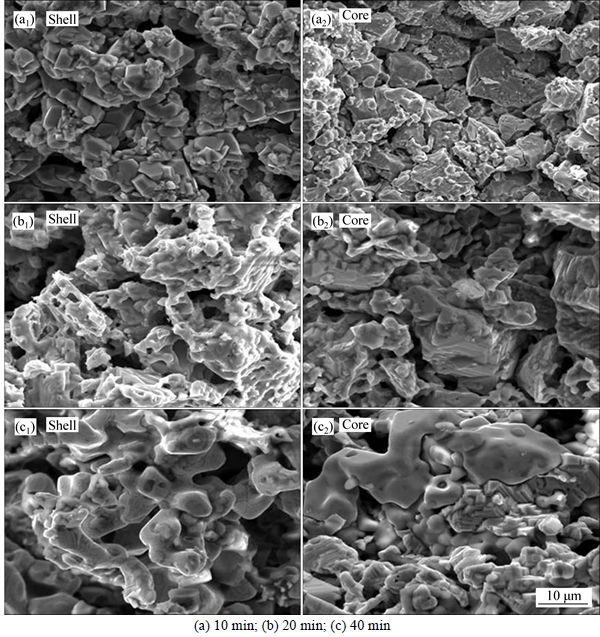
Fig. 11 SEM images of pellets showing shell (a1), (b1), (c1) and core (a2), (b2), (c2) reduced at 1050 °C for different reduction time:
4 Conclusions
1) Reduction temperature seems to have a significant effect on the maximum swelling index. With increasing temperature, the maximum swelling index is rapidly decreased. The highest maximum swelling index of 175% can be obtained when hot preheated pellets are reduced at 900 °C.
2) According to the microstructure and XRD patterns of reduced pellets with different temperature and time, it can be concluded that the swelling behavior of pellets is decided by the generated rate and morphology of metal irons. Once preheated pellets are reduced at low temperature, there is not enough time for the cracks generated during the transformation of hematite to magnetite to disappear and the newly formed iron crystal nucleus is prior to form and gather at lattice imperfections, resulting in orientated growth of metal irons. Finally, obvious swelling is obtained. When pellets are reduced at high temperature, the formation rate of metal iron increases and the newly formed metal iron grains are easy to connect with each other, resulting in the formation of a closed shell. Eventually, metal iron grains grow up in the form of flake, restraining the increasing volume.
References
[1] KASAI E, KITAJIMA T, KAWAGUCHI T. Carbothermic reduction in the combustion bed packed with composite pellets of iron oxide and coal [J]. ISIJ international, 2000, 40 (9): 842-849.
[2] DANCY T. The evolution of direct reduction [C]// 50th Ironmaking Conference Proceedings. Washington, USA: Iron and Steel Society, 1991: 611-624.
[3] LI Jian. A study on mechanism and process of direct reduction of pellets made from concentrate and composite binder [D]. Changsha: Central South University, 2007. (in Chinese)
[4] ZHU De-qing, QIU Guan-zhou, JIANG Tao, XU Jing-cang. An innovative process for direct reduction of cold-bound pellets from iron concentrate with a coal-based rotary kiln [J]. Journal of Central South University of Technology, 2000, 7(2): 68-71.
[5] ZHU De-qing, MENDES V, CHUN Tie-jun, PAN Jian, LI Qi-hou, LI Jian, QIU Guan-zhou. Direct reduction behaviors of composite binder magnetite pellets in coal-based grate-rotary kiln process [J]. ISIJ International, 2011, 51 (2): 214-219.
[6] SHARMA T, GUPTA R, PRAKASH B. Effect of gangue content on the swelling behaviour of iron ore pellets [J]. Minerals Engineering, 1990, 3 (5): 509-516.
[7] WANG Hai-tao, SOHN H Y. Effects of firing and reduction conditions on swelling and iron whisker formation during the reduction of iron oxide compact [J]. ISIJ International, 2011, 51 (6): 906-912.
[8] WANG Hai-tao, SOHN H Y. Effect of CaO and SiO2 on swelling and iron whisker formation during reduction of iron oxide compact [J]. Ironmaking & Steelmaking, 2011, 38 (6): 447-452.
[9] YI Ling-yun, HUANG Zhu-cheng, JIANG Tao, WANG Li-na, QI Tao. Swelling behavior of iron ore pellet reduced by H2–CO mixtures [J]. Powder Technology, 2015, 269(1): 290-295.
[10] NASR M, OMAR A, HESSIEN M, EI-GEASSY A A. Carbon monoxide reduction and accompanying swelling of iron oxide compacts [J]. ISIJ international, 1996, 36 (2): 164-171.
[11] FU Ju-ying, JIANG Tao, ZHU De-qing. Sintering and palletizing [M]. Changsha: Central South University Press, 1996: 175-178. (in Chinese)
[12] WRIGHT J, MORRISON A. Changes in diffusivity due to sintering in metallized iron oxide pellets [J]. Metallurgical Transactions B, 1982, 13(3): 518-520.
[13] HAYES P, GRIEVESON P. Microstructural changes on the reduction of hematite to maanetite [J]. Metallurgical Transactions B, 1981, 12(3): 579-587.
[14] HAYES P, GRIEVESON P. The effects of nucleation and growth on the reduction of Fe2O3 to Fe3O4 [J]. Metallurgical Transactions B, 1981, 12(2): 319-326.
[15] CHANG M, de JONGHE L C. Whisker growth in reduction of oxides [J]. Metallurgical Transactions B, 1984, 15(4): 685-694.
[16] FUWA T, BANYA S. Swelling of iron ore pellets during reduction [J]. Trans Iron Steel Inst Japan, 1969, 9(2): 137-147.
[17] MONN J, WALKER R Swelling of iron oxide compacts during reduction [J]. Ironmaking Steelmaking, 1975, 2(1): 30-35.
[18] SHARMA T, GUPTA R, PRAKASH B. Effect of firing condition and ingredients on the swelling behaviour of iron ore pellets [J]. ISIJ International, 1993, 33(4): 446-453.
[19] SHARMA T, GUPTA R, PRAKASH B. Swelling of iron ore pellets by statistical design of experiment [J]. ISIJ International, 1992, 32(12): 1268-1275.
[20] EL-GEASSY A A, NASR M, HESSIEN M. Effect of reducing gas on the volume change during reduction of iron oxide compacts [J]. ISIJ International, 1996, 36(6): 640-649.
[21] TANIGUCHI S, OHMI M, NAKAJIMA T. Crushing strength of metallised iron pellets after hydrogen reduction under rising temperature conditions [J]. Transactions of the Japan Institute of Metals, 1981, 22(5): 309-314.
(Edited by FANG Jing-hua)
Foundation item: Projects(51404213, 51404214, 51674225) supported by the National Natural Science Fundation of China; Project(1421324065) supported by the Development Fund for Outstanding Young Teachers of Zhengzhou University, China
Received date: 2015-11-03; Accepted date: 2015-12-20
Corresponding author: HUANG Yan-fang, Associate Professor, PhD; E-mail: hlele114@163.com
Abstract: One kind of facile coal-based direct reduction process is using hot preheated pellets for reduction in grate kiln. In this work, effects of reduction parameters on swelling index of hot preheated pellets were investigated by photographic technique under isothermal conditions. Experimental results show that swelling index of pellets is firstly increased then gradually decreased with increasing reduction time, while that is found to be an obvious decrease from 175 % to 30% with the variation of temperature from 900 °C to 1100 °C. Results of XRD combined with SEM reveal that swelling behavior of pellets is decided by structure of newly formed metal iron grains. The formation and growth of fibers iron grains promote the increase in volume. Low temperature and low CO content are favored to the formation and orientated growth of metal iron grains in the one step process.


