- Abstract:
- 1 Introduction▲
- 2 Experimental▲
- 3 Results and discussion▲
- 4 Conclusions▲
- References
- Figure
- Fig.1 Fluorescence emission spectra of HA during agricultural waste composting (A, B, and C are the fluorescence spectra of all treatments at final stage of agricultural waste composting)
- Fig.2 Fluorescence excitation spectra of HA during agricultural waste composting
- Fig.3 Fluorescence synchronous scan spectra of HA during agricultural waste composting
J. Cent. South Univ. Technol. (2009) 16: 0440-0443
DOI: 10.1007/s11771-009-0074-7
![]()
Fluorescence spectroscopy characteristics of humic acid by inoculating white-rot fungus during different phases of agricultural waste composting
HUANG Hong-li(黄红丽), ZENG Guang-ming(曾光明), JIANG Rong-qing(蒋荣清), YUAN Xing-zhong(袁兴中), YU Man(喻 曼), HUANG Dan-lian(黄丹莲), ZHANG Jia-chao(张嘉超), FENG Chong-ling(冯冲凌)
(College of Environmental Science and Engineering, Hunan University, Changsha 410082, China)
Abstract:
The white-rot fungus, Phanerochaete chrysosporium (P. chrysosporium), was inoculated during different phases of agricultural waste composting, and its effect on the fluorescence spectroscopy characteristics of humic acid (HA) was studied. The results show that the emission spectra have a sharp peak at 400 nm and a broad shoulder with the maximum centered at 460 nm. The excitation spectra have two peaks and exhibit red shift (shift to longer wavelengths) at 470 nm. The synchronous scan spectra present a number of peaks and shoulders, and the peaks at shorter wavelengths disappear gradually and form a shoulder. At the final stage of composting, the fluorescence spectra have similar shapes, but the fluorescence intensities decrease. P. chrysosporium increases the degree of aromatization and polymerization of HA when it is inoculated during the second fermentation phase, while it does not produce an obvious change on the humification degree of HA when it is inoculated during the first fermentation phase. Compared with the fluorescence spectroscopy characteristics of HA from soil, the structure of HA from compost is simpler and the activity is higher.
Key words:
composting; inoculation; Phanerochaete chrysosporium; humic acid; fluorescence spectroscopy;
1 Introduction
The development of rural economics during recent decades has led to the increasing production of agricultural waste. Composting seems to be one of the most interesting proposals currently [1]. The most important factor affecting the successful application of compost for agricultural purposes is its degree of stability and maturity [2-5]. Because of these concerns, extensive researches have been conducted to evaluate methods used to describe the stability and maturity of compost [4-8].
During composting, humification process is involved in the decomposition of organic matter. Thereinto, the content and characteristic of humic acid (HA) are the important factors for evaluating the compost maturity [9]. Fluorescence spectroscopy is extensively adopted in compost due to the fluorescence structures in HA [9-13]. Fluorescence spectroscopy is classified into emission spectroscopy, excitation spectroscopy and synchronous scan spectroscopy, in which the synchronous scan spectroscopy can be taken as the finger printing spectroscopy of compounds. The messages involved in this method are incomparable with other traditional methods. However, there are some deficiencies in the identification of molecular structure as no standard spectra of HA can be referred. Currently, many researches about the fluorescence spectroscopy characteristics of HA from soil and dissolved organic matters from other sources provide the basis for the analysis of molecular structure of HA from compost [14-17].
There are quantities of lignocellulose in the agricultural waste, which is difficult to be biodegraded, hampering the maturity of compost. The effects of inoculation during composting have been reported [18-20]. It has been shown that inoculation can activate the biodegradation of organic matter and improve the quality of compost. In most of the previous cited work, only routine parameters during composting were profiled and compared between inoculated and non-inoculated treatments, but the effect of different inoculating time on the fluorescence spectroscopy characteristics of HA has not been reported.
The aim of the present investigation is to establish the differences in the fluorescence spectroscopy characteristics of HA caused by inoculating Phanerochaete chrysosporium (P. chrysosporium) during different phases of agricultural waste composting.
2 Experimental
2.1 Materials
The typical agricultural organic wastes were collected from the suburb of Changsha. Rice straw, which was dried and cut to 10-20 mm in length, was used as organic material difficult to be decomposed. Several kinds of vegetables chopped into 10-20 mm pieces were used as easily metabolized materials. Bran was used to adjust the initial mass ratio of carbon to nitrogen (m(C)/m(N)) of composting. Soil, which was air-dried and sieved through a 350 μm-screen to remove coarse plant debris, was added to increase microbial population.
2.2 Experimental methods
An experimental composting system was set up. Rice straw, vegetables, bran and soil at the mass ratio of 11?3?2?8 were fully mixed with an initial m(C)/m(N) of about 30/1 and packed loosely in an open box. Moisture content was adjusted to about 55% during the first fermentation phase, and about 45% during the second fermentation phase. To provide aerobic conditions, the mixture was turned twice a week in the first two weeks and then once a week afterwards. Three separate experiments (Samples A-C) were conducted. In Sample A, the heap was uninoculated with P. chrysosporium as control. In Sample B, the heap was inoculated with P. chrysosporium during the first fermentation phase. In Sample C, the heap was inoculated with P. chrysosporium during the second fermentation phase.
2.3 Microbial inoculum
The lignocellulolytic microorganism P. chrysosporium (AF 96007) was obtained from China Center for Type Culture Collection (CCTCC), and it was stored at 4 ℃ on potato dextrose agar (PDA). P. chrysosporium mycelium (and adherent spores) from a culture grown on PDA plate at 37 ℃ for 3 d was suspended in sterile water to reach the desired concentration of 1×109 CFU/mL (colony-forming units per mL). In Sample B, the heap was inoculated with 5 mL/kg of waste during the first fermentation phase (the second day). To avoid the increase of the moisture content of composting, the solid inoculant with a concentration of 1×109 CFU/g, which was P. chrysosporium cultivated in the mixture of rice straw and bran at 37 ℃ for 6 d, was inoculated with 10 g/kg of waste during the second fermentation phase in Sample C (the 15th day).
2.4 Analysis
Samples were taken from different places of heap and mixed at different stages of the composting process (0, 6, 15, 28 and 42 d) to extract the humic acid.
2.4.1 HA extraction
The extraction of HA was carried out according to Ref.[21]. 10 g fresh sample was treated with 200 mL pyrophosphate-NaOH solution (0.1 mol/L Na4P2O7?10H2O+0.1 mol/L NaOH, pH=13.0) in sealed bottles shaken at 200 r/min for 30 min and conserved at 4 ℃ for 12 h. The supernatant was acidified to pH=2.0 with H2SO4 solution. And then the HA was precipitated and purified.
2.4.2 Fluorescence spectroscopy
For fluorescence measurements, solutions of 20 mg/L of HA were prepared in 0.05 mol/L NaHCO3 solution. All spectra were performed on a Perkin-Elmer luminescence spectrometer LS55, with a scan speed of 200 nm/min, using excitation and emission slit bandwidths of 10 nm. Emission spectra were collected between 370 and 600 nm, with an excitation wavelength of 350 nm. Excitation spectra were collected between 300 and 500 nm, with an emission wavelength of 520 nm. Synchronous scan spectra were measured by scanning simultaneously over the range 300-600 nm with an optimized wavelength difference, Δλ=λem-λex=18 nm [22].
3 Results and discussion
3.1 Emission spectra
The emission spectra of HA are shown in Fig.1. At the initial stage of composting, HA has a sharp peak at 400 nm and a broad shoulder with the maximum centered at 460 nm. This indicates that HA from the initial compost has simple structure and low aromatization. At the final stage of composting, the peak at 400 nm weakens and the fluorescence intensity of 460 nm decreases by about 23%. This confirms that the fluorescence substances decrease and the aromatic
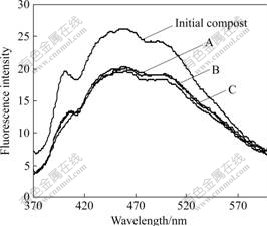
Fig.1 Fluorescence emission spectra of HA during agricultural waste composting (A, B, and C are the fluorescence spectra of all treatments at final stage of agricultural waste composting)
compounds increase in HA as reported by MIIKKI et al [12]. Nevertheless, the maximum absorption wavelength (460 nm) of HA from compost is shorter than that (470-500 nm) from soil, which shows that the structure of HA from compost is simpler and the activity is higher. The compost can stimulate the rejuvenation and activity of soil HA when it is added to soil.
At the final stage of composting, the shapes of emission spectra are similar in different samples, and the wavelengths of characteristic peaks do not change. But the fluorescence intensities are different. Compared with Sample A, the fluorescence intensities decrease by 0.95% and 4.52% at 400 and 460 nm respectively in Sample B, while they decrease 8.85% and 14.85% at these two wavelengths respectively in Sample C. The results show that P. chrysosporium, inoculated during the second fermentation phase, significantly stimulates the complication of HA structure. But when it is inoculated during the first fermentation phase, its effect is unobvious.
3.2 Excitation spectra
Fig.2 shows the excitation spectra of HA. The spectra of HA from initial compost have a sharp peak at 400 nm and a moderate peak at 470 nm, which are similar to HA from soil [23]. At the final stage of composting, the shapes of excitation spectra are similar in the three samples. The wavelength of peak presents red shift (shift to longer wavelengths) at 470 nm, and the fluorescence intensity significantly decreases. These results show that the aromatic structures increase after composting as the conclusion obtained from the emission spectra.
At the final stage of composting, the fluorescence intensities do not obviously change in Sample B, but they sharply decrease in Sample C compared with Sample A. Furthermore, the wavelengths of characteristic peak are 468.0, 468.5 and 472.0 nm in Samples A, B and
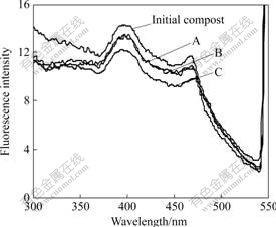
Fig.2 Fluorescence excitation spectra of HA during agricultural waste composting
C, respectively. That is to say, the wavelength of characteristic peak presents obvious red shift in Sample C, but this phenomenon is not clear in Sample B. These confirm that the inoculation of P. chrysosporium during the second fermentation phase can promote the degree of polycondensation in HA structure, but the inoculation during the first fermentation has no positive effect on the condensation of HA.
3.3 Synchronous scan spectra
The fluorescence synchronous scan spectra of HA are shown in Fig.3. At the initial stage of composting, the spectra exhibit a primary peak at 395 nm, a secondary peak at 475 nm, two minor peaks at 345 and 360 nm and a number of minor shoulders. At the final stage of composting, the fluorescence intensities decrease in all three samples, especially in the range of short wavelengths. The peaks at 345 and 360 nm disappear gradually and form a broad shoulder. In general, the short wavelengths and high fluorescence intensities may be attributed to simple structural components, a low degree of aromatic polycondensation and a low level of conjugated chromophores. The main peak at long wavelengths is associated with the presence of high molecular mass fractions in HA [24]. Therefore, the results show that the humification degree of HA increases at the final stage of composting.
At the final stage of composting, the fluorescence synchronous scan spectra of HA exhibit a number of peaks at 350, 395 and 475 nm in all treatments, while the fluorescence intensities vary due to different inoculating time. Compared with Sample A, the fluorescence intensities do not change in Sample B, but they obviously decrease in Sample C. Therefore, Fig.3 shows that P. chrysosporium can increase the degree of polycondensation and aromatization of HA when it is inoculated during the second fermentation phase, while it cannot take this effect when it is inoculated during the
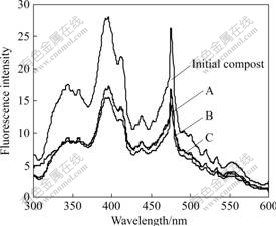
Fig.3 Fluorescence synchronous scan spectra of HA during agricultural waste composting
first fermentation phase. This indicates the inoculation of P. chrysosporium during the second fermentation phase of composting will improve the humification process of composting.
4 Conclusions
(1) At the final stage of composting, the fluorescence emission spectra have a sharp peak at 400 nm and a broad shoulder with the maximum centered at 460 nm. The excitation spectra have two peaks and present red shift at 470 nm. The synchronous scan spectra present a number of peaks and shoulders. The peaks at 345 and 360 nm disappear gradually and form a shoulder.
(2) At the final stage of composting, the fluorescence spectra have similar shapes, but the fluorescence intensities decrease. Three fluorescence spectra show that P. chrysosporium increases the degree of aromatization and molecular polymerization of HA when it is inoculated during the second fermentation phase, while it does not produce an obvious change on the humification degree of HA when it is inoculated during the first fermentation phase.
References
[1] HACHICHA S, SALLEMI F, MEDHIOUB K, HACHICHA R, AMMAR E. Quality assessment of composts prepared with olive mill wastewater and agricultural wastes [J]. Waste Management, 2008, 28(12): 2593-2603.
[2] S?NCHEZ-MONEDERO M A, CEGARRA J, GARC?A D, ROIG A. Chemical and structural evolution of humic acids during organic waste composting [J]. Biodegradation, 2002, 13(6): 361-371.
[3] YU H Y, ZENG G M, HUANG H L, XI X M, WANG R Y, HUANG D L, HUANG G H, LI J B. Microbial community succession and lignocellulose degradation during agricultural waste composting [J]. Biodegradation, 2007, 18(6): 793-802.
[4] WU L, MA L Q, MARTINEZ G A. Comparison of methods for evaluating stability and maturity of biosolids compost [J]. Journal of Environmental Quality, 2000, 29(2): 424-429.
[5] BREWER L J, SULLIVAN D M. Maturity and stability evaluation of composted yard trimmings [J]. Compost Science and Utilization, 2003, 11(2): 96-112.
[6] CHICA A, MOHEDO J J, MARTIN M A, MARTIN A. Determination of the stability of MSW compost using a respirometric technique [J]. Compost Science and Utilization, 2003, 11(2): 169-175.
[7] ZMORA-NAHUM S, MARKOVITCH O, TARCHITZKY J, YONA C. Dissolved organic carbon (DOC) as a parameter of compost maturity [J]. Soil Biology and Biochemistry, 2005, 37(11): 2109-2116.
[8] CASTALDI P, ALBERTI G, MERELLA R, MELIS P. Study of the organic matter evolution during municipal solid waste composting aimed at identifying suitable parameters for the evaluation of compost maturity [J]. Waste Management, 2005, 25(2): 209-213.
[9] WEI Z M, XI B D, ZHAO Y, WANG S P, LIU H L, JIANG Y H. Effect of inoculating microbes in municipal solid waste composting on characteristics of humic acid [J]. Chemosphere, 2007, 68(2): 368-374.
[10] PLAZA C, XING B S, FERN?NDEZ J M, SENESI N, POLO A. Binding of polycyclic aromatic hydrocarbons by humic acids formed during composting [J]. Environmental Pollution, 2009, 157(1): 257-263.
[11] DROUSSIA Z, D′ORAZI V, HAFIDI M, OUATMANE A. Elemental and spectroscopic characterization of humic-acid-like compounds during composting of olive mill by-products [J]. Journal of Hazardous Materials, 2009, 163(2/3): 1289-1297.
[12] MIIKKI V, SENESI N, HAENNINEN K. Characterization of humic material formed by composting of domestic and industrial biowastes: Part 2. Spectroscopic evaluation of humic acid structures [J]. Chemosphere, 1997, 34(8): 1639-1651.
[13] WEI Zi-min, XI Bei-dou, ZHAO Yue, WANG Shi-ping, XU Jing-gang, ZHANG Di, LIU Hong-liang. Study on fluorescence spectroscopy characteristics of humic acid by inoculating microbes on municipal solid waste composting [J]. Acta Scientiae Circumstantiae, 2005, 25(10): 1349-1354. (in Chinese)
[14] KWIATKOWSKA J, PROVENZANO M R, SENESI N. Long term effects of a brown coal-based amendment on the properties of soil humic acids [J]. Geoderma, 2008, 148(2): 200-205.
[15] HUO S L, XI B D, YU H C, HE L S, FAN S L, LIU H L. Characteristics of dissolved organic matter (DOM) in leachate with different landfill ages [J]. Journal of Environmental Sciences, 2008, 20(4): 492-498.
[16] SHIRSHOVA L T, GHABBOUR E A, DAVIES G. Spectroscopic characterization of humic acid fractions isolated from soil using different extraction procedures [J]. Geoderma, 2006, 133(3/4): 204-216.
[17] DOU Sen, CHEN En-feng, XU Xiang-cheng, ZHANG Ji-hong. Effect of application of organic manures on the structural characteristics of humic acids in soils—The optical properties of HAs [J]. Acta Pedologica Sinica, 1995, 32(1): 41-49. (in Chinese)
[18] GAIND S, PANDEY A K, LATA. Biodegradation study of crop residues as affected by exogenous inorganic nitrogen and fungal inoculants [J]. Journal of Basic Microbiology, 2005, 45(4): 301-311.
[19] XI B D, ZHANG G J, LIU H L. Process kinetics of inoculation composting of municipal solid waste [J]. Journal of Hazardous Materials, 2005, 124(1/3): 165-172.
[20] BARRENA R, PAGANS E, FALTYS G, SANCHEZ A. Effect of inoculation dosing on the composting of source-selected organic fraction of municipal solid wastes [J]. Journal of Chemical Technology and Biotechnology, 2006, 81(3): 420-425.
[21] ZENG G M, HUANG H L, HUANG D L, YUAN X Z, JIANG R Q, YU M, YU H Y, ZHANG J C, WANG R Y, LIU X L. Effect of inoculating white-rot fungus during different phases on agricultural waste compost maturity [J]. Process Biochemistry, 2009, 44(4): 396-400.
[22] TLOMBARDI A, FJARDIM W. Fluorescence spectroscopy of high performance liquid chromatography fractionated marine and terrestrial organic materials [J]. Water Research, 1999, 33(2): 512-520.
[23] PROVENZANO M R, SENESI N, PICCONE G. Thermal and spectroscopic characterization of composts from municipal solid wasters [J]. Compost Science and Utilization, 1998, 6(3): 67-73.
[24] SENESI N, MIANO T M, PROVENZANO M R, BRUNETTI G. Characterization, differentiation, and classification of humic substances by fluorescence spectroscopy [J]. Soil Science, 1991, 152(4): 259-270.
(Edited by CHEN Wei-ping)
Foundation item: Project(2005CB724203) supported by the Major State Basic Research and Development Program of China; Project(IRT0719) supported by the Program for Changjiang Scholars and Innovative Research Team in University of China; Projects(50608029, 50808073) supported by the National Natural Science Foundation of China; Project(2007185) supported by the Environmental Protection Technology Research Program of Hunan Province, China
Received date: 2008-08-29; Accepted date: 2008-11-06
Corresponding author: ZENG Guang-ming, Professor, PhD; Tel: +86-731-8822754; E-mail: zgming@hnu.cn
- Fluorescence spectroscopy characteristics of humic acid by inoculating white-rot fungus during different phases of agricultural waste composting


