Trans. Nonferrous Met. Soc. China 26(2016) 1439-1446
Corrosion behavior of T2 copper in 3.5% sodium chloride solution treated by rotating electromagnetic field
Peng ZHANG1, Qiang ZHU1, Qian SU2, Bin GUO2, Shu-kang CHENG3
1. School of Materials Science and Engineering, Harbin Institute of Technology at Weihai, Weihai 264209, China;
2. School of Materials Science and Engineering, Harbin Institute of Technology, Harbin 150001, China;
3. Institute of Electromagnetic and Electronic Technology, Harbin Institute of Technology, Harbin 150001, China
Received 19 July 2015; accepted 19 October 2015
Abstract:
Copper is susceptible to producing corrosion problems in corrosive environments, which leads to serious safety problems. Thus, investigating the corrosion behavior of copper is of great significance. The effects of rotating electromagnetic field on corrosion behavior of T2 copper in 3.5% sodium chloride solution with electrochemical measurements were investigated. The results showed that rotating electromagnetic field changed properties of 3.5% sodium chloride solution by increasing the values of temperature and pH and decreasing the values of conductivity and dissolved oxygen. The rotating electromagnetic field improved the corrosion resistance of T2 copper. The corrosion products of T2 copper in treated 3.5% sodium chloride solution were composed of Cu2O and CuCl. The low corrosion rate of T2 copper was resulted from the decrease of dissolved oxygen in 3.5% sodium chloride solution treated by rotating electromagnetic field.
Key words:
corrosion behavior; rotating electromagnetic field; electrochemical measurement; copper ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ;;
1 Introduction
Copper and its alloys are used widely in marine environments due to their excellent electrical and thermal conductivity, good machinability, corrosion resistance, and low cost etc [1-4]. Although copper and its alloys are resistant towards the influence of atmosphere and many chemicals, they are susceptible to corrosion problems such as pitting corrosion in aggressive media. Copper and its alloys are corroded easily in chloride containing aqueous solution, which may cause enormous economic loss and serious safety hazards [5]. Thus, investigating the anticorrosion behavior of copper and its alloys in seawater is necessary. The main method to slow down the corrosion rate in seawater is adding inhibitors, which include imidazole, thiazole and sulfoxide derivative. OTMACIC et al [6] found that methy- limidazole promoted the formation of protective film on copper surface in simulated seawater. Adding amino phenyl triazole (ATA) into simulated seawater reduced current of cathodic reaction process and the inhibition efficiency reached 98% [7]. DAFALI et al [8] discovered that sulfhydryl-methylimidazole had excellent inhibition effect on copper in simulated seawater. KHIATI et al [9] indicated that developed benzotriazole inhibitor had good inhibition effect on copper in seawater. However, there is a potential impact on the seawater environment by adding inhibitors. Thus, many scholars begin to research new inhibitors and methods which are non-toxic, biodegradable and environmentally friendly to slow down corrosion rate.
Many scholars have studied the influence of electromagnetic field on the corrosion behavior of metals. HU et al [10] discovered that the open circuit potentials (φop) of beryllium copper in sodium chloride solution were shifted by a horizontal magnetic field. The limiting current density was changed by a horizontal magnetic field with different concentrations. SUEPTITZ et al [11] proposed that superimposed magnetic field decreased the current density of copper in 0.05 mol/L H2SO4 solution in the transition and pre-passive region. CHOUCHANE
et al [12] indicated that the corrosion resistance of alloys with low alloy nickel content was improved significantly by magnetic field. HUANG et al [13] used electric field to slow down the corrosion rate of copper. KELLY [14] found that magnetic field increased the corrosion rate of some metallic materials such as titanium in sulphate solutions. The electromagnetic field has effect on the corrosion behavior of copper in seawater [15,16]. Although some conclusions about the influence of electromagnetic field on the corrosion have been obtained, significant inhibition effect has not yet been reached. GUO et al [17] found that rotating electromagnetic field had remarkable effect on the electrochemical corrosion of copper and the inhibition efficiency reached 89.14%. LU [18] proposed that magneto-electrochemistry could serve a novel approach for investigating reaction mechanisms and kinetics for complex electrode systems. CHEN et al [19] found that the presence of a static magnetic field can suppress effectively the corrosion of copper alloy. YUAN et al [20] proposed that the magnetic field affects the anodic dissolution of copper in chloride media. Researches indicated that electromagnetic field reduces the corrosion rate of some metals, but the influence of electromagnetic field on the corrosion behavior of metals has not been studied in detail.
In this work, the 3.5% sodium chloride solution was treated by rotating electromagnetic field. The properties such as pH, dissolved oxygen, temperature and conductivity of 3.5% sodium chloride solution after treatment of rotating electromagnetic field were investigated experimentally. Electrochemical measure- ment was used to investigate corrosion behavior of copper in magnetic treated solution. The mechanism of corrosion behavior of copper in treating solution was also revealed.
2 Experimental
Experimental T2 copper was selected to conduct corrosion experiments. The sample sizes were 20 mm × 20 mm × 2 mm. The chemical composition of T2 copper is shown in Table 1. The specimen surfaces were polished by 1500 grit waterproof abrasive papers, smoothed by polishing cloth at last, cleaned by acetone and alcohol, and dried successively.
A rotating electromagnetic device was proposed to obtain rotating electromagnetic field. The structure sketch of electromagnetic pyrogenic device referred to the work of LI et al [16]. The intensity of magnetic field with fixed direction changed with time in this work. The amplitude of magnetic field intensity was in the range of 0 to 0.25 T as shown in Fig. 1.
Table 1 Chemical composition of T2 copper (mass fraction, %)
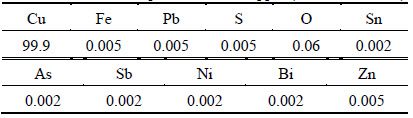
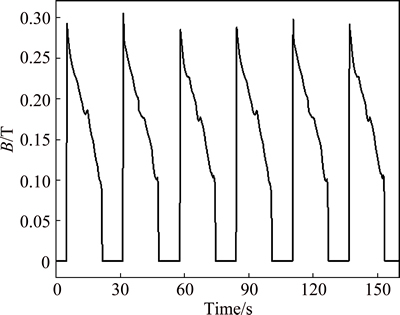
Fig. 1 Rotational magnetic field intensity with time
The medium of electrochemical corrosion experiment was two kinds of 3.5% sodium chloride solution. One is 3.5% sodium chloride solution without treatment. The other is 3.5% sodium chloride solution treated by rotating electromagnetic field with different frequencies. Experiments of testing water quality and electrochemical corrosion behavior of T2 copper were simultaneously carried out in the untreated and treated 3.5% sodium chloride solution. The treated 3.5% sodium chloride solution maintained the treated temperature when the corrosion test was carried out. The water quality of solution was measured by DDSJ-308A conductivity meters, JPSJ-605 dissolved oxygen meters and PHS-3C pH meter; the polarization curves and electrochemical impedance spectroscopy were measured by CHI660D electrochemical workstation. The frequencies parameters of rotating electromagnetic field were 50, 100, 150 and 200 Hz and treatment time was 3, 6, 9 and12 h.
3 Results and discussion
3.1 Properties of 3.5% sodium chloride solution treated by rotating electromagnetic field
Figure 2 shows the variation of temperature of 3.5% sodium chloride solution treated by rotating electromagnetic field. The temperature of solution firstly increases with the increase of treatment time, and then reaches a relatively stable value at a constant frequency. The higher the frequency is, the faster the temperature increases. The result shows that the 3.5% sodium chloride solution is heated by the rotating electromagnetic field. Figure 3 shows the variation of pH of 3.5% sodium chloride solution treated by rotating electromagnetic field. With the increase of treatment time, the pH of solution firstly increases quickly, and then the pH reaches a stable state after 3 h. This indicated that the effect of electromagnetic frequency on pH is not obvious. Figure 4 shows the variation of conductivity of 3.5% sodium chloride solution treated by rotating electromagnetic field. The conductivity of solution firstly reduces, then increases with the increase of treatment time. The conductivities of solution treated at 50 Hz and 100 Hz are always lower than that in untreated solution. The conductivities of solution treated at 150 Hz and 200 Hz for 9 h are higher than that in untreated solution. Figure 5 shows the variation of dissolved oxygen of 3.5% sodium chloride solution treated by rotating electromagnetic field. With the increase of treatment time, the dissolved oxygen in solution treated at 50 Hz and 100 Hz reduces, and then reaches a relatively stable value. The dissolved oxygen of solution treated at 150 Hz and 200 Hz firstly reduces, and then increases to a stable value. The effect of rotating electromagnetic field on water quality contains magnetized and thermal effects. The magnetized effect has influence on association state of solvent water molecules and hydration state of ion. The thermal effect has influence on solubility of CO2 and O2. Thus, the water quality parameters of 3.5% sodium chloride change as above. The properties of 3.5% sodium chloride solution are changed by the rotating electromagnetic field, such as the temperature, the pH, the conductivity and the dissolved oxygen. The changes of these parameters affect the corrosion behavior of T2 copper in 3.5% sodium chloride solution.
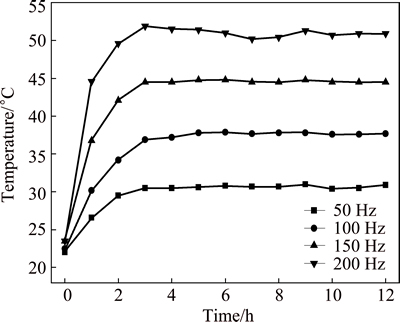
Fig. 2 Variation of temperature of 3.5% sodium chloride solution treated by rotating electromagnetic field
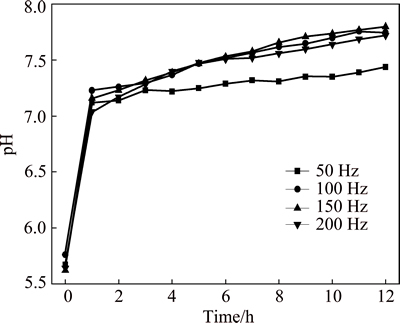
Fig. 3 Variation of pH of 3.5% sodium chloride solution treated by rotating electromagnetic field
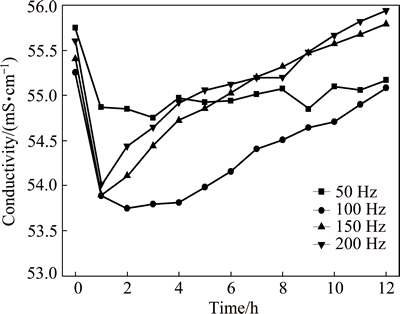
Fig. 4 Variation of conductivity of 3.5% sodium chloride solution treated by rotating electromagnetic field
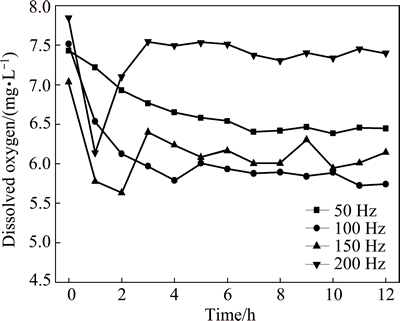
Fig. 5 Variation of dissolved oxygen of 3.5% sodium chloride solution treated by rotating electromagnetic field
3.2 Analysis of polarization curve
Figure 6 shows the polarization curves of copper in 3.5% sodium chloride solution treated with frequency of 50, 100, 150 and 200 Hz.
The corrosion rate vcorr is expressed as follows [17]:
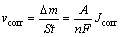 (1)
(1)
where A, n and F are atomic mass, number of electrons and Coulomb coefficient respectively, and Jcorr is the corrosion current density. It shows that the corrosion rate vcorr is proportional to the corrosion current density. Therefore, the corrosion current density is used to represent the corrosion rate.
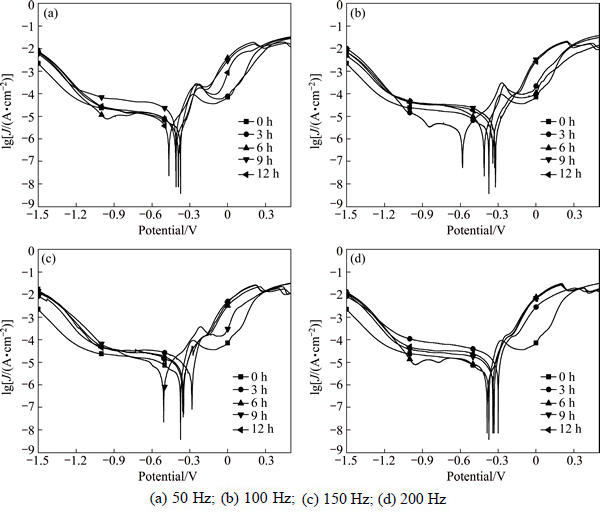
Fig. 6 Polarization curves of T2 copper in 3.5% sodium chloride solution treated with different frequencies: (a) 50 Hz; (b) 100 Hz; (c) 150 Hz; (d) 200 Hz
The corrosion current densities of different polarization curves were obtained by Tafel extrapolation method as shown in Fig. 7. It can be found that the corrosion current density of T2 copper in 3.5% sodium chloride solution treated by rotating electromagnetic field changes significantly. The corrosion current density of T2 copper in treated 3.5% sodium chloride solution is smaller than that in untreated solution except the results at 200 Hz for 3 h. The lower the electromagnetic frequency is, the smaller the corrosion current density is when the solution is treated for 3 h. When the treatment time is 6 h and 9 h, the corrosion current density increases sharply at 50 Hz. The corrosion current density in solution treated at 200 Hz is the minimum for 9 h. When the treatment time is 12 h, the minimum corrosion current density is 0.075 A/m2 in solution treated at 100 Hz. Based on all the experimental data, the optimal processing parameter is 50 Hz treated for 3 h.
Typically, inhibition efficiency in electrochemical corrosion is expressed as follows [17]:
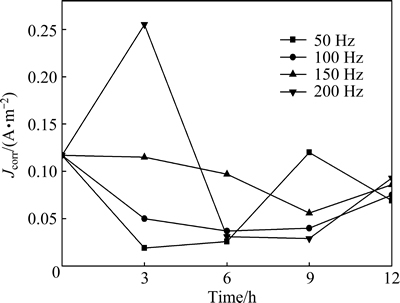
Fig. 7 Corrosion current densities of T2 copper in 3.5% sodium chloride solution treated by rotating electromagnetic field with different frequencies
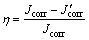 (2)
(2)
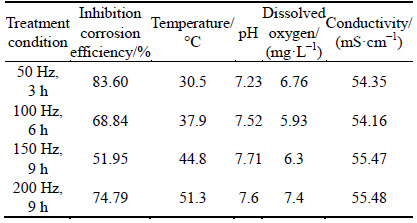
where Jcorr and J′corr are the corrosion current densities in untreated solution and treated solution, respectively. The inhibition efficiency is obtained according to Eq. (2), and those results are shown in Table 2. Table 2 also shows other properties of 3.5% sodium chloride.
Table 2 Properties of 3.5% sodium chloride solution with different treatment conditions
3.3 Analysis of electrochemical impendence spectroscopy (EIS)
Figure 8 shows the Nyquist plots of T2 copper in 3.5% sodium chloride treated by rotating electromagnetic field. Nyquist plots of T2 copper are composed by two approximately semicircles. There is a big difference between maximum imaginary part and the span of real part of the impedance from these results. The maximum imaginary part and the real part span of the impedance of the first semicircular is more than the second when the solution is treated at 100 Hz for 6 h and 200 Hz for 3 h. The difference is smaller than that in the untreated solution.
Figure 9 shows the equivalent circuit of Fig. 8. The equivalent circuit is composed by three units in series; the three units are resistance Rs, constant phase angle element Qdl and resistor Rpo in parallel, constant phase angle element Qox and resistor Rpo in parallel respectively. Constant phase angle element Qdl is dominated by electric double layer capacitor and constant phase angle element Qox is dominated by oxide capacitance.
The values of solution resistance Rs under the different treatment conditions are shown in Table 3. The values of solution resistance Rs increase at 50 and 100 Hz from 3 to 12 h. The values of solution resistance Rs decrease firstly and then increase at 150 and 200 Hz from 3 to 12 h.
3.4 Analysis of surface morphology
Figure 10 shows surface morphologies of electrochemical corrosion specimens in both corrosion media. The surface morphologies of specimen corroded in treated 3.5% sodium chloride are uniform compared with those in untreated solution. Surface morphologies of specimen corroded are different in solutions under different electromagnetic frequencies. Figure 10(b) shows the least pitting defects treated by 50 Hz, and those minimum defects improve corrosion resistance of that T2 copper specimen.
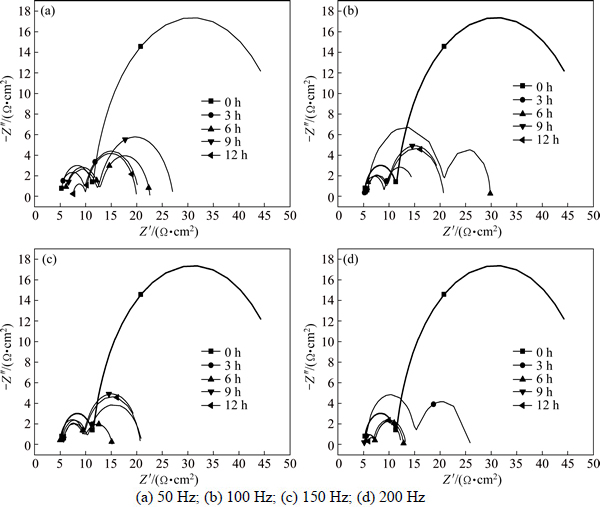
Fig. 8 Nyquist plots of T2 copper in 3.5% sodium chloride solution treated by rotating electromagnetic field with different frequencies: (a) 50 Hz; (b) 100 Hz; (c) 150 Hz; (d) 200 Hz
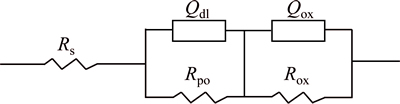
Fig. 9 Equivalent circuit of electrochemical impendence spectroscopy
3.5 Analysis of phase components
Figure 11 shows phase components of corrosion products of T2 copper in 3.5% sodium chloride solution treated by rotating electromagnetic field. The corrosion product of T2 copper in untreated 3.5% sodium chloride solution is Cu2O. The corrosion products of T2 copper in treated 3.5% sodium chloride solution are Cu2O and CuCl. There is new phase component of CuCl among corrosion products in treated solution. The influence of treatment time on the formation of Cu2O and CuCl is slight.
Table 3 Solution resistance Rs under different treatment conditions
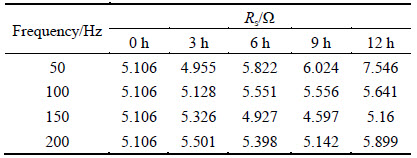
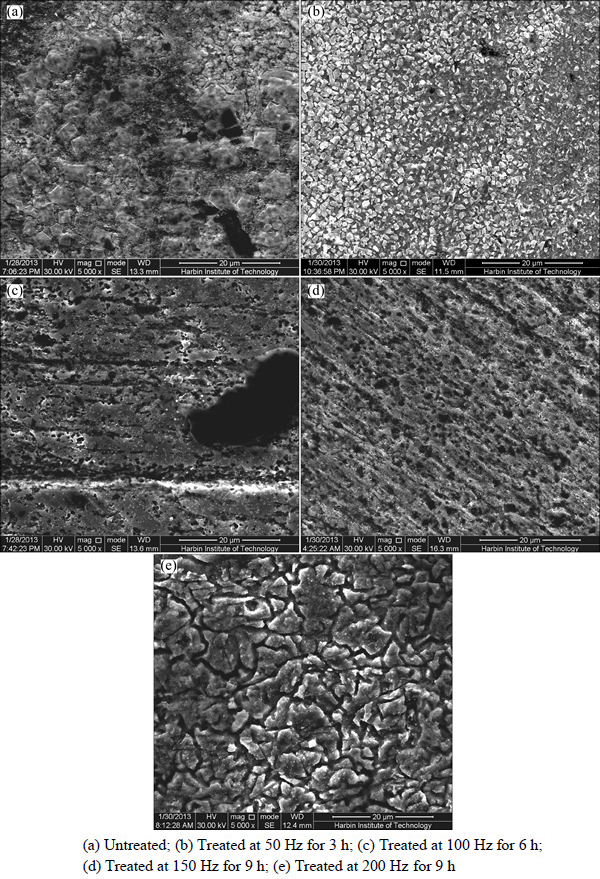
Fig. 10 Surface morphologies of T2 copper corroded in 3.5% sodium chloride solution treated under different conditions: (a) Untreated; (b) Treated at 50 Hz for 3 h; (c) Treated at 100 Hz for 6 h; (d) Treated at 150 Hz for 9 h; (e) Treated at 200 Hz for 9 h
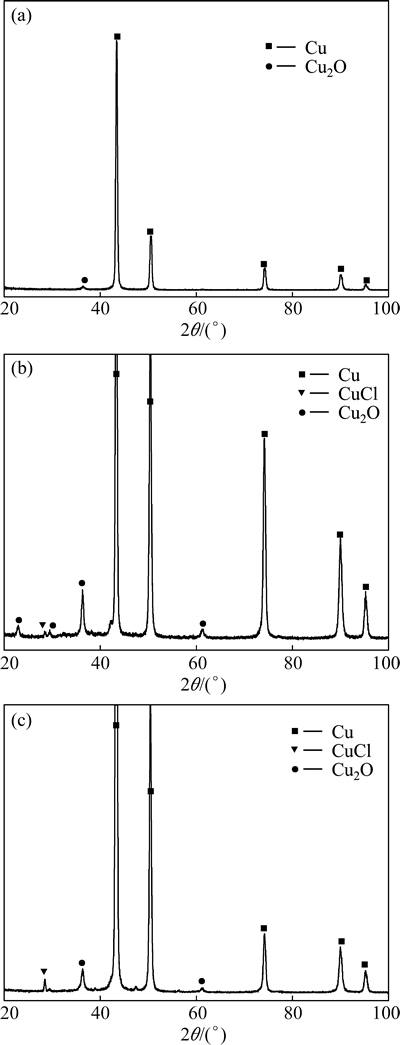
Fig. 11 Phase components of corrosion products of T2 copper in 3.5% sodium chloride solution treated by rotating electromagnetic field for 0 h (a), 3 h (b) and 12 h (c)
3.6 Analysis of corrosion products
According to the corrosion morphology, XRD and electrochemical corrosion results, the electrochemical corrosion mechanisms of T2 copper in treated 3.5% sodium chloride solution can be described as follows [17].
Firstly, processes controlled by cathode reaction of oxygen depolarized are expressed as follows:
Cu+OH-→Cu(OH)(ads)+e (3)
2Cu(OH)(ads)→Cu2O+H2O (4)
2CuCl+H2O→Cu2O+2HCl (5)
Secondly, process controlled by oxidation reaction of dissolved oxygen is expressed as follows:
4Cu+O2→2Cu2O (6)
Thirdly, processes controlled by complex reaction of chloride are expressed as follows:
Cu2O+Cl-+H2O→Cu2Cl(OH)2 (7)
Cu+Cl-→CuCl(ads)+e (8)
CuCl(ads)→CuCl(film) (9)
CuCl+e→Cu+Cl- (10)
CuCl(ads)+Cl-→CuCl2- (11)
CuCl2-→Cu2++2Cl+e (12)
The reactions of T2 copper in 3.5% sodium chloride solution contain the above three processes. The processes are hindered in treated 3.5% sodium chloride solution, which are controlled by cathode reaction of oxygen depolarized and oxidation reaction of dissolved oxygen. It is because that the reduction of dissolved oxygen in treated 3.5% sodium chloride solution results in cathodic reaction slowing down. Further, it causes the anodic reaction slowing down. It is one of the reasons for the low corrosion rate of T2 copper in treated 3.5% sodium chloride solution.
4 Conclusions
1) Temperature and pH of treated 3.5% sodium chloride solution are higher than those of untreated solution. The maximum values of temperature and pH are 52 °C and 7.8, respectively.
2) Conductivity and dissolved oxygen of treated solution are lower than those of untreated solution. The minimum values of conductivity and dissolved oxygen are 53.75 mS/cm and 5.74 mg/L, respectively. The decrease of dissolved oxygen slows down corrosion rate of T2 copper in 3.5% sodium chloride solution.
3) Surface morphologies of specimen corroded in treated solution are uniform compared with that in untreated solution. The corrosion products of T2 copper are Cu2O and CuCl in treated 3.5% sodium chloride solution.
4) The maximum inhibition efficiency of 83.60% is obtained under the condition of 50 Hz treated for 3 h.
References
[1] NUNEZ L, REGUERA E, CORVO F, GONZALEZ E, VAZQUEZ C. Corrosion of copper in seawater and its aerosols in a tropical island [J]. Corrosion Science, 2005, 47: 461-484.
[2] WANG Hong-xing, ZHANG Yan, CHENG Jia-lin, LI Yu-shan. High temperature oxidation resistance and microstructure change of aluminized coating on copper substrate [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(1): 184-190.
[3] ZHU An-yin, CHEN Jing-lin, ZHOU Li, LUO Li-yang, LEI Qian, ZHANG Liang, ZHANG Wan. Hot deformation behavior of novel imitation-gold copper alloy [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(5): 1349-1355.
[4] AKINLABI E T, ANDREWS A, AKINLABI S A. Effects of processing parameters on corrosion properties of dissimilar friction stir welds of aluminium and copper [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(5): 1323-1330.
[5] ZHAO Yue-hong, LIN Le-yun, CUI Da-wei. Localized corrosion of copper alloys in China seawater for 16 years [J]. Transactions of Nonferrous Metals Society of China, 2004, 14(6): 1082-1090.
[6] OTMACIC H, TELEGDI J, PAPP K, STUPNISEK-LISAC E. Protective properties of an inhibitor layer formed on copper in neutral chloride solution [J]. Journal of Applied Electrochemistry, 2004, 34: 545-550.
[7] ES-SALAH K, KEDDAM M, RAHMOUNI K, SRHIRI A, TAKENOUTI H. Aminotriazole as corrosion inhibitor of Cu-30Ni alloy in 3% NaCl in presence of ammoniac [J]. Electrochimica Acta, 2004, 49: 2771-2778.
[8] DAFALI A, HAMMOUTI B, TOUZANI R, KERTIT S, RAMDANI A, EL-KACEMI K. Corrosion inhibition of copper in 3 percent NaCl solution by new bipyrazolic derivatives [J]. Anti-Corrosion Methods and Materials, 2002, 49: 96-104.
[9] KHIATI Z, OTHMAN A A, SANCHEZ-MORENO M, BERNARD M C, JOIRET S, SUTTER E, VIVIER V. Corrosion inhibition of copper in neutral chloride media by a novel derivative of 1,2,4-triazole [J]. Corrosion Science, 2011, 53: 3092-3099.
[10] HU Jing, DONG Chao-fang, LI Xiao-gang, XIAO Kui. Effects of applied magnetic field on corrosion of beryllium copper in NaCl solution [J]. Journal of Materials Science and Technology, 2010, 26: 355-361.
[11] SUEPTITZ R, TSCHULIK K, UHLEMANN M, SCHULZ L, GEBERT A. Effect of high gradient magnetic fields on the anodic behavior and localized corrosion of iron in sulphuric acid solutions [J]. Corrosion Science, 2011, 53: 3222-3230.
[12] CHOUCHANE S, LEVESQUE A, ZABINSKI P, REHAMNIA R, CHOPART J P. Electrochemical corrosion behavior in NaCl medium of zinc-nickel alloys electrodeposited under applied magnetic field [J]. Journal of Alloys and Compounds, 2010, 506: 575-580.
[13] HUANG Hua-liang, GUO Xing-peng, ZHANG Guo-an, DONG Ze-hua. Effect of direct current electric field on atmospheric corrosion behavior of copper under thin electrolyte layer [J]. Corrosion Science, 2011, 53: 3446-3449.
[14] KELLY E J. Magnetic field effects on electrochemical reactions occurring at metal/flowing-electrolyte interfaces [J]. Journal of the Electrochemical Society, 1977, 124: 987-994.
[15] ZHANG Peng, GUO Bin, JIN Yong-ping, CHENG Shu-kang. Corrosion characteristics of copper in magnetized sea water [J]. Transactions of Nonferrous Metals Society of China, 2007, 17(S1): s189-s193.
[16] LI Ji-nan, ZHANG Peng, GUO Bin. Effects of rotating electromagnetic on flow corrosion of copper in seawater [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(2): s489-s493.
[17] GUO Bin, ZHANG Peng, JIN Yong-ping, CHENG Shu-kang. Effects of alternating magnetic field on the corrosion rate and corrosion products of copper [J]. Rare Metals, 2008, 27: 324-328.
[18] LU Zhan-peng. Effects of magnetic fields, solution composition and electrode potential on anodic dissolution and passivation [J]. ECS Transactions, 2014, 59: 429-438.
[19] CHEN Bi, ZHENG Bi-juan, ZHANG Fan, LIU Hong-fang. Corrosion behavior of HSn70-1 copper alloy in SRB containing medium in atatic magnetic field [J]. Journal of Chinese Society for Corrosion and Protection, 2014, 34(4): 339-345.
[20] YUAN Bo-yu, WANG Chao, LI Liang, CHEN Shen-hao. Investigation of the effects of the magnetic field on the anodic dissolution of copper in NaCl solutions with holography [J]. Corrosion Science, 2012, 58: 69-78.
T2紫铜在旋转电磁场处理的3.5%氯化钠溶液中的腐蚀行为
张 鹏1,朱 强1,苏 倩2,郭 斌2,程树康3
1. 哈尔滨工业大学(威海) 材料科学与工程学院,威海 264209;
2. 哈尔滨工业大学 材料科学与工程学院,哈尔滨 150001;
3. 哈尔滨工业大学 电磁与电子技术研究所,哈尔滨 150001
摘 要:铜在腐蚀环境中容易产生腐蚀问题,这会带来非常严重的安全问题。因此,铜的腐蚀行为的研究具有重大的意义。通过电化学测量方法研究了旋转电磁场对T2紫铜在3.5%氯化钠溶液中腐蚀行为的影响。结果表明:旋转电磁场改变了3.5%氯化钠溶液的性质,提高了溶液的温度和pH值,降低了溶液的电导率和溶氧量。旋转电磁场改善了T2紫铜的耐腐蚀性能。T2紫铜在旋转电磁场处理的3.5%氯化钠溶液中腐蚀产物由Cu2O和CuCl 组成。T2紫铜在旋转电磁场处理过的3.5%氯化钠溶液的低腐蚀速率是由于旋转电磁场导致溶液中溶氧量减少造成的。
关键词:腐蚀行为;旋转电磁场;电化学测量;紫铜
(Edited by Yun-bin HE)
Foundation item: Projects (51207031, 51177022) supported by the National Natural Science Foundation of China; Project (2013M541368) supported by the China Postdoctoral Science Foundation; Project (BS2011NJ002) supported by the Promotive Research Fund for Excellent Young and Middle-Aged Scientists of Shandong Province, China; Project (2008DFR60340) supported by the International Science and Technology Cooperation of China
Corresponding author: Peng ZHANG; Tel: +86-631-5687324; E-mail: pzhang@hit.edu.cn
DOI: 10.1016/S1003-6326(16)64249-8
Abstract: Copper is susceptible to producing corrosion problems in corrosive environments, which leads to serious safety problems. Thus, investigating the corrosion behavior of copper is of great significance. The effects of rotating electromagnetic field on corrosion behavior of T2 copper in 3.5% sodium chloride solution with electrochemical measurements were investigated. The results showed that rotating electromagnetic field changed properties of 3.5% sodium chloride solution by increasing the values of temperature and pH and decreasing the values of conductivity and dissolved oxygen. The rotating electromagnetic field improved the corrosion resistance of T2 copper. The corrosion products of T2 copper in treated 3.5% sodium chloride solution were composed of Cu2O and CuCl. The low corrosion rate of T2 copper was resulted from the decrease of dissolved oxygen in 3.5% sodium chloride solution treated by rotating electromagnetic field.


