
![]()

Trans. Nonferrous Met. Soc. China 22(2012) 731-739
Decomposition mechanism of pentlandite during electrochemical bio-oxidation process
LI Hong-xu1, 2, LI Chao1, 2, ZHANG Zhi-qian1, 2
1. School of Metallurgy and Ecology Engineering,
University of Science and Technology Beijing, Beijing 100083, China;
2. Key Laboratory of Ecological and Recycling Metallurgy (Education Ministry),
University of Science and Technology Beijing, Beijing 100083, China
Received 31 May 2011; accepted 26 December 2011
Abstract:
Electrochemical measurements were carried out to elucidate decomposition mechanism of pentlandite using modified powder microelectrode with Acidithiobacillus ferrooxidans attached or without on the mineral powder surface. Cyclic voltammetry (CV) results show that at a low potential of about -0.2 V (vs SCE), the pentlandite was transformed to an intermediated phase like Fe4.5-yNi4.5-xS8-z when Fe and Ni ions were evacuated from mineral lattice; when the potential was changed from -0.2 V to 0.2 V, the unstable violarite (Fe3Ni3S4) and FeNi2S4 were formed which was accompanied by element sulfur formed on the mineral surface; when the potential increased over 0.2 V, the unstable intermediated phase decomposed entirely; at a higher potential of 0.7 V, the evacuated ferrous ion was oxidized to ferric ion. The presence of Acidithiobacillus ferrooxidans made the oxidation peak current increase with initial peak potential negatively moving, and the bacteria also contributed to the sulfur removing from mineral surface, which was demonstrated by the reduction characteristic at potential ranging from -0.75 to -0.5 V. Leaching experiments and electrochemical results show that the solution acidity increasing when pH<2 may impede the oxidation process slightly.
Key words:
pentlandite; Acidthiobacillus ferrooxidans; bioleaching; powder modified microelectrode;
1 Introduction
The bioleaching method has been extended to nickel extraction from sulfide minerals. There have been several pilot projects of heap bioleaching established in Australia, Finland, and China in recent years, while the bioleaching process still gives slow kinetics and limited recovery [1-3]. Different influencing factors have been tested from different views, such as solution pH to get a maximum dissolution rate of nickel sulfide minerals [4,5], but it needs further studies on nickel sulfide self- oxidation decomposition behavior, especially oxidation in the presence of bacteria.
Most of metallic sulfide minerals are semiconductor and dissolved by the electrochemical mechanism to act anode. So, using the electrochemical method to elucidate their oxidation mechanism is reasonable [6-8]. At present, there are a number of studies on the anode dissolved mechanism of other metal sulfides, such as chalcopyrite in different media [9-11], while few measurements were conducted in the presence of microorganism [12,13]. Similar study has been focused on the pentlandite for the reason of hardly getting pure mineral crystal to be work electrode, even it is very abundant as the main nickel sulfide in the nature. WARNER et al [14] synthesized pentlandite (Fe4.5Ni4.5S8) artificially by vacuum technique, and used the electrochemical technique to clarify the dissolution mechanism of pentlandite in acidic iron(III) chloride solution. However, the tests were conducted in the absence of bacteria. The previous used massive sulfide electrode could not guarantee the affective attachment of microorganism on the polished crystal surface of sulfide mineral under quick scanning. So, in the present work, a novel bacteria modified powder microelectrode (PME) is provided as work electrode, which is in favor of detecting transient intermediate reaction under quick scanning [15-17].
2 Experimental
2.1 Mineral
The nature pentlandite (Fe4.5Ni4.5S8) mineral sample was obtained from Hunan Geology Museum, China, with a small quantity of pyrrhotite, magnetite and chalcopyrite. The massive specimen was crushed and milled to 50 μm (300 mesh) in N2 ambience in order to prevent surface from oxidation, then separated with dry magnetic tube. Wet magnetic separation was used for deep separation. So, the ferromagnetism of pyrrhotite and other weakly magnetic minerals like chalcopyrite were removed with. Table 1 shows the chemical element composition of the purified mineral.
Table 1 Chemical element composition of purified mineral

X-ray diffraction analysis was implemented to determine crystal composition and the result is shown in Fig. 1. Over 96% is pentlandite, only trace amount of chalcopyrite is included, which is approximate to the results of synthesized pentlandite powder by WARNER et al [14] by dry vacuum techniques with purity of 95%.
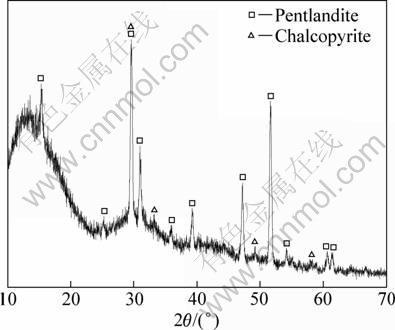
Fig. 1 XRD pattern of purified mineral sample
2.2 Bacterial culture
The mixed acidophilic bacteria were sampled from Jiangxi Yongping copper mine, China. Acidithiobacillus ferrooxidans were isolated from the mixed cultured bacteria in laboratory. The isolation method used was strictly abided by typical ones [16,18]. The abundance of Acidithiobacillus ferrooxidans species was obtained by sampling bacteria cultivated in 9K medium, and the nutritive composition of the 9K medium was as follows: 3.0 g/L (NH4)2SO4, 0.1 g/L KCl, 0.5 g/L K2HPO4, 0.5 g/L MgSO4·7H2O, 0.01 g/L Ca(NO3)2 [19]. The inoculated bacteria was then incubated in a water-bath shaker at 30 °C, and 125 r/min. Sulphate, biomass concentration and pH were measured over time for consecutive subcultures. The pH value of the culture medium was adjusted to 2 which shows the most appropriate environment for Acidithiobacillus ferrooxidans’s growth with 5 mol/L sulphuric acid [20,21]. After cultivating, the active and robust bacteria were obtained and selected by their growth curves on pentlandite at pH=2 and 30 °C. All adaption experiments were performed under optimum conditions as proposed by MAKAMOTO and TAKAHASHI [22] and MASON AND RICE [23].
2.3 Electrode and electrochemical measurements
The flowchart of preparing powder microelectrode is shown in Fig. 2. The first step was making a cavity microelectrode (CME) (Fig. 2(a)), then the powders of mineral grains were packed in. CME was made of a glass tube (5 mm in diameter and a total length of 100 mm) containing a Pt-wire (with diameter of 100 μm and length of 3 cm), with the latter corroded and ended at the near bottom of the glass tube providing a micro cylindrical cavity (Fig. 2(b)) with depth less then 100 μm. The cavity was obtained by a controlled dissolution of the Pt-wire in a highly concentrated hot solution of HCl+HNO3 for about 30 min. The diameter of the wire and the depth of the micro-cavity were measured with a microscope. The usual height—diameter ratio was between 0.4 and 1 to make grains well contact and prevent powder grains from easily escaping out of the cavity. Typically the whole resistance of prepared PME was less than 10 Ω [15,24]. The prepared pentlandite powders were saturated sufficiently by cultivated bacteria with micro cells of 1011/L to keep enough bacteria attaching on the powder surface absolutely, then pentlandite powders with and without bacteria attached were compressed respectively into the electrode cavity, as shown in Fig. 2(c).
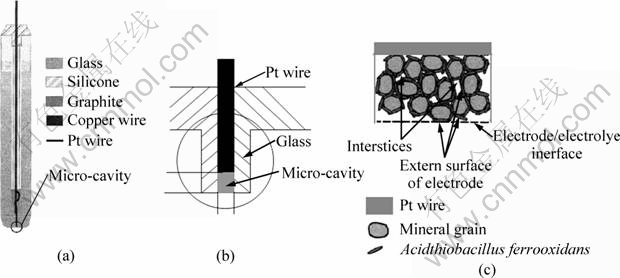
Fig. 2 Flowchart of preparing bacteria modified powder microelectrode: (a) Preparation of cavity microelectrode; (b) Packing mineral grains into microelectrode; (c) Compressing bacteria into micro-cavity
Such a thin electrode could provide more information about intermediate reaction during electrochemical decomposition process of sulfide anode as well as the kinetics process which needs to reduce the electrochemical interface area dramatically [25]. The electrochemical measurements were performed in a typical electrochemical cell (500 mL) with three electrode shown in Fig. 3, the working electrode PME (WE, with or without bacteria modified), the counter electrode (CE, Pt plate), and the reference electrode (RE, KCl-saturated calomel electrode). The cell temperature was kept at constant by connecting a circulating thermostatically controlled water loop. The electrolyte used in the experiment was standard 9K media without ferrous ion. The electrochemical measurements were carried out by Solartron 1287 with analyzing software. The first scan was started a few seconds after the electrode was immersed in the electrolyte solution. All the potentials value was vs SCE.
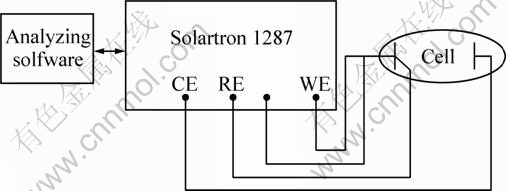
Fig. 3 Scheme of electrochemical measurement system
3 Results and discussion
3.1 Intermediate oxidation process of pentlandite
Pentlandite can be viewed as an alloy of iron, nickel and sulphur with an excess molar ratio of metal. In the acid leaching system there is extensive delocalization of electrons throughout the mineral lattice. So, assigning a formal valence state to various components is not valid. If a formal valence of -2 was assigned to sulfur in the phase, then this would arithmetically give both iron and nickel a mean formal valence less than +2 [14]. To investigate the occurrence of oxidation process of pentlandite at different potentials, a series of cyclic voltammetric (CV) experiments were performed in sulfuric acid media of pH 2, because around pH=2 usually is used as a proper solution system for sulfide [4,5]. The initial sweep potential started at rest potential of about 0.25 V. Figure 4 shows the CV results of anode oxidation and reverse cathode reduction process progressively. Apparently several anode oxidation peaks, A, B, C and H, and reverse cathode reduction peaks, D, E, F and G, are presented. Intuitively, the oxidation process of pentladite includes multi-intermediate steps implied by peaks A, B and C.
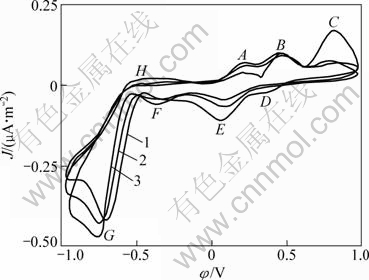
Fig. 4 Cyclic voltammograms of pentlandite in sulphuric acid of pH=2 (Microelectrode dish radius of 5×10-5 m, sweep speed of 5 mV/s, T=298 K, initial anode scan): 1—The first cycle; 2—The second cycle, 3—The third cycle
As shown in Fig. 4, at a low potential of about -0.2 V, the pentlandite begins to transform to an intermediated phase (Fe4.5-yNi4.5-xS8-z), referring the thermodynamic stability of pentlandite by φH—pH diagrams of iron-nickel-sulphur aqueous system [26]. At peak A, the precarious violarite (Fe3Ni3S4) and (FeNi2S4) are formed with Fe and Ni ions evacuating from mineral lattice, and the originally possible oxidation reaction is as follows:
![]() (1)
(1)
When potential increases approximately up to -0.15 V, Fe3Ni3S4 will transfer to intermediated ones, and the form of intermediate phase and the proportion of Ni-Fe-S released or still left in the lattice will depend on the anode oxidation potential and the solution composition, especially pH. It is referred that FeNi2S4 may be possible as further intermediate following reaction:
![]() (2)
(2)
When oxidation reaction goes further with more Fe2+ released, there will occur:
![]() (3)
(3)
When anode scan potential is up to 0.2 V, the unstable compounds begin to decompose entirely with the appearance of peak B:
![]() (4)
(4)
Subsequently the following reaction occurs
NiS2→Ni2++2S0 (5)
Apart from Ni and Fe ions released from pentlandite, the mechanism of S transforming has aroused more discussion rather than metallic ions evacuated from sulfide minerals during their decomposition process. WOLFGANG et al [27] considered that metal sulfides are degraded by a chemical attack of iron (III) ions and/or protons on the crystal lattice. However, the mechanism and chemistry of the degradation are determined by the mineral structure. The disulfides pyrite (FeS2), molybdenite (MoS2), and tungstenite (WS2) are degraded via the main intermediate thiosulfate. Exclusively, iron (III) ions are the oxidizing agents for the dissolution. Thiosulfate is, consequently, degraded in a cyclic process to sulfate, with elemental sulfur being only a side product not rooted in sulfide lattice. If the dissolved molecular oxygen or oxidants like ferric ion is provided, the sulfur transform process can be described as:
![]() (6)
(6)
In such degraded process, there is no intermediate multi-metallic sulfide phase formed other than the sulfur transform. Sulfide would be oxidized directly to liberate metal ion like iron or copper simultaneously with thiosulfate formed. However, some groups of metal sulfides like galena (PbS), sphalerite (ZnS), chalcopyrite (CuFeS2), hauerite (MnS2), orpiment (As2S3), and realgar (As4S4), are degradable by attack of iron (III) ion and proton during leaching in acid solution. The main intermediates are polysulfides and elemental sulfur [27,28], and the sulfur transforms along the process:
![]() (7)
(7)
From results of Fig. 4, we could assume that the pentlandite anode oxidized along polysulfides process. The difference from thiosulfate process is that during such process the intermediates polysulfides could be formed as well as element sulfur accumulated on the surface; however, formed sulfur is not crystalline but shows plastic and elastic properties, indicating that it comprises one or more allotropes [14]. On the process of chalcopyrite oxidation into polysulfides, many works have been done, including electrochemical tests and leached residues analysis, and the results demonstrated that the formation of element sulfur on the surface during the oxidation process in acid solution was a actual process [9,29-34]. In acid medium, the proton H+ in the solution would combine with intermediate S2- on the surface to form hydrogen sulfide:
![]() (8)
(8)
According to the results of WARNER et al [14], the oxidation of pentlandite with the evolution of hydrogen sulfide occurs at a potential up to about 0.2 V in the acid solution, and the peak B in Fig. 4 represents the formation reaction of hydrogen sulfide [14]. However, only partial S2- was transferred to hydrogen sulfide, part formed element sulfur, and the exact proportion of them is hard to be determined. It can further indicate that when potential is over 0.7 V at peak C, the formed intermediate polysulfide will continue to oxidize, and the element sulfur will also be transferred to sulfate, at the same time the released ferrous ion adsorbed on the minerals surface will be oxidized to higher valence state:
![]() (9)
(9)
The majority of iron ion will continue to diffuse into solution if the concentration of solution is not high. There are four obvious reduction wave peaks, D, E, F and G in Fig. 4, implying that during reverse cathode scanning the sulfur of different valence state will be reduced. The cathodic peaks mean the reduction of a sulfur-rich layer to form different amorphous metal sulfides [35]. But it is hard to determine that which metal sulfide has been produced during such instantaneously scanning, and the formation of NiS2, Ni2S3, Fe3Ni3S4 inferentially happened with potential varying from 0.5 V to -0.5 V. However, according to results WARNER et al [14,26] that the synthesis of pentlandite during cathode process is impossible, and the predominant cathode current below -0.5 V is due to the reduction of hydronium ions with the evolution of hydrogen gas shown by broad peak G:
![]() (10)
(10)
During positive scanning when potential is not up to -0.5 V, there is hardly other wave peak except peak H, because at this potential range the pentlandite is impossible to decompose. So, the hydrogen evolution is possible.
Multi-CV has been developed in order to get more information. The similar prewave indicated that the pentlandite decomposition steps were not changed, but the peak currents were intensified and initial reaction potential negatively moved and the oxidation reaction resistance changed especially at potential of peak C, further implying that the element sulfur was accumulated on the electrode surface with scanning repeatedly.
3.2 Effect of Acidithiobacillus ferrooxidans on pentlandite decomposition
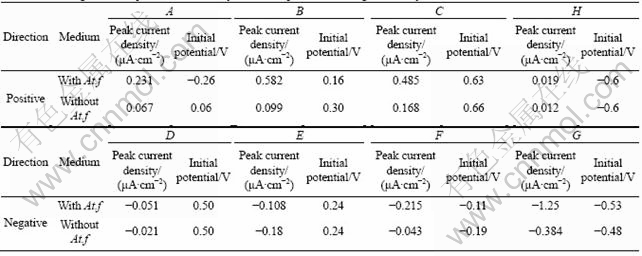
Figure 5 shows multi-CVs with Acidithiobacillus ferrooxidans attached on the PMME surface. Compared with Figure 4, there is no significant change in the anode oxidation course of pentlandite decomposition and reverse cathode reduction, besides anode oxidation reactions occurring at more negative initial potential. Overall process was conducted at higher reaction currents, and the results are summarized in Table 2. The attachment of Acidithiobacillus ferrooxidans on the mineral surface did not change the step of reaction with only intensified pentlandite.
From Table 2, the current densities of anode oxidation and cathode reduction were increased and initial potentials of anode process were negatively removed, which demonstrates that the presence of Acidithiobacillus ferrooxidans enhanced anode reactions and made them happen more easily and drastically. Although anode oxidation course of pentlandite was not changed, the presence of Acidithiobacillus ferrooxidans could be attributed to the intermediate process enhanced and the kinetics resistance factors eliminated, especially the ploysulfide process accelerated and element sulfur removed.
The role of bacteria during the sulfide bioleaching and the interaction of bacteria with sulfide have been discussed a lot in past decades, especially on the controversy of direct or indirect effect of bacteria on the sulfide surface. Gradually, the indirect effect was proved and recognized as the only way for bacteria reaction on the sulfide decomposition during bioleaching process [6, 27-29]. According to viewpoint of SCHIPPER and SAND [28], metal sulfides are degraded by a chemical attack of iron (III) ions and/or protons on the crystal lattice. The primary iron (III) ions are supplied by the bacterial extracellular polymeric substances (EPS),where they are complex to glucuronic acid. The mechanism and chemistry of the degradation were determined by the mineral structure in two ways, thiosulfate and polysulfides processes [27,36]. Acidithiobacillus ferrooxidans could oxidize ferrous to ferric ions to get energy for metabolism, as well as oxidize element sulfur, thiosulphate and tetrathionate [37]. The ferrous oxidation can be illustrated by chemical reaction as following pathway:
![]() (11)
(11)
The sulfur oxidation is more complex, includes many steps and cannot be depicted by concise chemical equations. But this does not hinder to determine the pentlandite oxidation process even in the presence of Acidithiobacillus ferrooxidans if indirect mechanism of bacteria is possible. However the results from Fig. 5 and Table 2 were conducted that iron ion in the solution was free, which excludes the possibility of iron (III) attacking on pentlandite by ion adsorption on the mineral surface. At pH=2 during scanning, the enhancement of pentlandite decomposition in the presence of Acidithiobacillus ferrooxidans was mainly contributed to the element removed and surface ion diffusion accelerated. Although the bacteria were washed many times before they were modified on the electrode, there were still many iron ions concentrated on the cell surface. During the scanning, the released iron ion from pentlandite could be oxidized to iron (III) and accumulated in EPS instantaneously, due to the additional supply of protons by EPS which has —SH group to liberate H+ when bacteria absorbed on the sulfide surface [27,29,36]. The effects of all these above reasons could enhance pentlandite decomposition obviously.
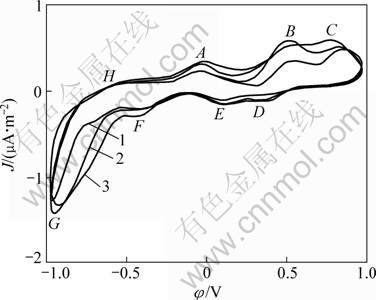
Fig. 5 Cyclic voltammograms of pentlandite with bacteria on surface (Microelectrode dish radius of 5×10-5 m, sweep speed of 5 mV/s, T=298 K, pH=2, initial sweep direction of anode): 1—The first cycle; 2—The second cycle; 3—The third cycle
Table 2 Scanning results of peak current density and initial potentials during the first cycle
3.3 Effect of pH
Due to pentlandite as metal sulfide oxidized process in the light of chemical attack of iron (III) ions and/or protons on the crystal lattice in acid solution as polysulfides process, the increase of concentration of protons or iron (III) ions could enhance the pentlandite oxidation and resolution processes. Figure 6 shows the CVs of the pentlandite in the presence of bacteria under more intensive acid conditions of pH from 2 to 1.5. The decrease of pH means the increase of protons. Because the more extensive acid system is not proper to bacteria living, the scanning under lower pH was not conducted. From Fig. 6 the anode reaction peak, like peak C at pH=1.5, is obviously broader than that at pH=2, especially at potential over 0.65 V. However, during the cathode process it is much different, the peaks E and F were intensified while peak G at the potential under -0.65 V was reduced significantly. The enlarged peak C revealed that the process of element sulfur previously
formed on the pentlandite surface transforming to sulfide hydrogen is intensively strengthened:
![]() (12)
(12)
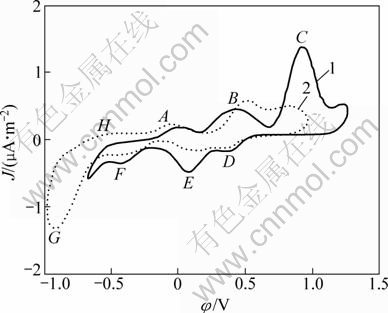
Fig. 6 Cyclic voltammograms of pentlandite with bacteria at different pH values (Microelectrode dish radius of 5×10-5 m, sweep speed of 5 mV/s, T=298 K, pH=2, initial sweep direction of anode): 1—pH=2; 2—pH=1.5
The width of the peak G was shrunk apparently, because the sulfur hydrogen formed during anode oxidation process escaped and could not be reduced on the penlandite surface. And the change of peaks C and G, further illustrated that the step of sulfur hydrogen formation has appeared during pentlandite anode oxidation process in the presence of bacteria, and with decreasing the solution acidity the sulfur hydrogen generated increases. The enlarged peak E means the enhancement of intermediate steps of anode process, that is to say, the increase of H+ concentration including the liberated EPS of Acidithiobacillus ferrooxidans would enhance pentlandite oxidation reactions, and H2S would be generated more heavily.
3.4 Effect of ferric ion
Figure 7 shows the CVs of pentlandite with ferric ions (Fe3+) of different concentrations. After ferric ions are added, CV was changed a lot with increase of anode and cathode peak currents. Some anode intermediate oxidation reactions were enhanced as well as their reverse reduction cathode process. Peaks A and B were enhanced much than peak C which is the most obviously enhanced anode peak when solution H+ concentration increased. The increase of acidity was mainly contributed to the surface sulfur transformation to sulfide hydrogen, while the increase of ferric ion would dedicate to oxidation process as equations (2) and (4).
Especially, the remarkable current increase of peak A demonstrates that the addition of ferric ion enhanced the initial oxidation reaction, and made intermediate phase of Fe3Ni3S4, Ni3S4 or NiS2 transform more quickly. This is consistent with the point view of SCHIPPERS and SAND [28]. They thought that the ferric ion is the primary reagent which broke down the pentlandite crystal with metallic ion released and intermediate matter formed including element sulfur. If the element sulfur could not be removed promptly, it would accumulate on the surface acting as the diffusion kinetic resistant.
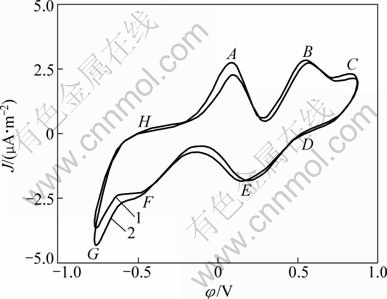
Fig. 7 Cyclic voltammograms of pentlandite with bacteria and different ferric concentrations (Microelectrode dish radius of 5×10-5 m, sweep speed of 5 mV/s, T=298 K, pH=2, initial sweep direction of anode): 1—c(Fe3+)=0.050 mol/L; 2—c(Fe3+)=0.150 mol/L3
So, increasing ferric ion is obviously more effective to enhance the oxidation reaction than decreasing pH with Acidithiobacillus ferrooxidans attached under such quick electrochemical scanning. Under this condition it can be considered that the ferric ion is the key factor to break down the chemical bond of lattice of pentlandite. Although SCHIPPERS and SAND [28] have shown that the EPS of attached bacteria concentrated a lot of ferric ions on the minerals surface during bioleaching process. The above results show that the attached Acidithiobacillus ferrooxidans are not more active than ferric ion added in electrolyte, especially to the oxidation reaction represented by peaks A and B. The possible one of reasons is that the attached bacteria have no abundant iron source during quick anode scanning, which is different from bacterium cultivation or practical bioleaching process. In the process the ferrous ion is abundant. So, we know that the ferric ion plays a pivotal role in the oxidative resolution process of pentlandite, which would be accelerated dramatically with ferrous iron for sulfide was oxidized to ferric ones with Acidithiobacillus ferrooxidans:
![]() (13)
(13)
That is demonstrated by the increase of current density of reverse direction scanning corresponding to the anode oxidation reaction and positively moving of the initial potentials.
3.5 Bioleaching and XRD analysis
The bioleaching experiments were carried out to further illustrate the effect of Acidithiobacillus ferrooxidans and ferric ion on the penlandite oxidation process. During leaching process, Acidithiobacillus ferrooxidans attached on the mineral or suspended in solution, and ferric ion was added in the solution. In order to make bacterium strains separate and not adsorb on the mineral surface when suspension condition for bacteria was needed, a cellulose dialysis bag was used with molecular entrapment 8000 which has been bag immersed with 50% ethanol and washed with deionized water and 1 g sterilized pentlandite mineral powders were packed in the bag. The pulp density was 1% (mass fraction), temperature was 25 °C, and pH was 2. Under those conditions, leaching solution was contained in the flask and put in shake incubator (HZQ-F160) and shaken at 150 r/min. The leaching results are shown in Fig. 8.
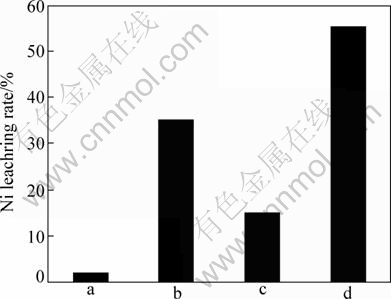
Fig. 8 Leaching pentlandite results under conditions of pH=2, 25 °C, shake speed of 150 r/min, pulp density of 1% (mass fraction): a—At.f suspension in solution without ferric ion; b—At.f attached on mineral without ferric ions in solution; c—At.f suspension in solution with ferric ions of 2.5 g/L; d—At.f attached on mineral with ferric ions on 2.5 g/L
When Acidithiobacillus ferrooxidans suspended in the solution without ferric ions, the leaching rate is only 2% after leaching for 4 d. Under this condition, the oxidation of pentlandite only relies on the proton of sulfate acid, and the released ferrous ions spread through the dialysis bag into solution and will be oxidized to ferric ions, ferric ions will rediffuse back to the mineral surface and attack on the mineral. But this process is relatively slow. However, if Acidithiobacillus ferrooxidans have attached on the mineral surface without ferric ions in the solution for 4 d, the leaching rate of pentlandite reaches 35%. It is demonstrated that the Acidithiobacillus ferrooxidans are not only contributed to the oxidation of ferrous ions to ferric ions but also contributed to the oxidation of intermediate phase like element sulfur produced on the surface, and bacteria can also supply some organic acidity and protons by EPS. They are also able to break down chemical bond of pentlandite surface structure in the polysulfides process [28]. When ferric ion was added with concentration up to 2.5 g/L and bacteria were also suspended in the solution but separated to the mineral surface, after 4 d, the leaching rate of pentlandite was up to 15%, much lower and nearly half leaching rate when Acidithiobacillus ferrooxidans attached on the mineral surface. So, the role of bacteria is only to regenerate ferric ion. When Acidithiobacillus ferrooxidans attached on mineral surface and ferric ions were added in the solution, the 4 d-leaching rate reaches 55%, higher than in the case of only Acidithiobacillus ferrooxidans attached or suspended with ferric ions. It demonstrates that the combination of Acidithiobacillus ferrooxidans and ferric ions is the crucial way to accelerate the pentlandite decomposition and leaching.
Figure 9 shows XRD pattern of leached residue with Acidithiobacillus ferrooxidans attached on the surface. By comparing with Fig. 1, after bioleaching, a large number of structure of pentlandite surface has been destroyed, especially the left range in the figure. In this range there is no element sulfur detected, meaning that the element sulfur on the mineral surface has been taken away by Acidithiobacillus ferrooxidans. If the mechanism of pentlandite oxidation follows the polysulfides process proposed by SAND et al [28,36], it should be detected the results of WARNER et al [14] to leaching pentlandite in ferric chloride. This is consistent with the electrochemical results that we got above. XRD result also shows the existing of intermediate nickel sulfides, such as Ni3S2 and NiS, on the mineral surface, meaning that in the given sufficient time the surface with bacteria has adequately oxidized intermediate for pentlandite decomposition. That is unlike using the electrochemical scanning to get detail simultaneous information of intermediate reaction during nickel sulfide oxidation process.
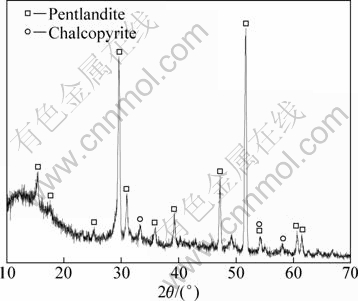
Fig. 9 XRD pattern of leached residue of pentlandite mineral sample
4 Conclusions
1) With powder micro electrode, CV tests show that pentlandite decomposes in many intermediate steps with nickel, iron and sulfur complex compounds formed and existed on the mineral surface.
2) The formation of precarious intermediate metal sulphide like violarite (Fe3Ni3S4) or (FeNi2S4) is possible, and the sulfur transforms along polysulfides process:
![]()
3) In acid medium, partial intermediate S2- on the surface would combine with absorbed proton H+ to form hydrogen sulfide at a lower potential:
![]()
4) Ferrous ions are released when pentlandite decomposes, and oxidize to ferric ions at a higher potential. This reaction will be strengthened by Acidithiobacillus ferrooxidans attached on the sulfide mineral surface. The significant important role of Acidithiobacillus ferrooxidans is oxidation of the intermediate element sulfur formed.
References
[1] CAMERON R A, LASTRA R, MORTAZAVI S, BEDARD P L, MORIN L, DOUGLAS G W, KENNEDY K J. Bioleaching of a low-grade ultramafic nickel sulphide ore in stirred-tank reactors at elevated pH [J]. Hydrometallurgy, 2009, 97(3-4): 213-220.
[2] RIEKKOLA-VANHANEN M. Talvivaara black schist bioheapleaching demonstration plant [J]. Adv Mater Res, 2007, 20-21: 30-33.
[3] QIN W Q, ZHEN S J, YAN Z Q, CAMPBELL M, WANG J, LIU K, ZHANG Y S. Heap bioleaching a low grade nickel-bearing sulphide ore containing high levels magnesium as olivine, chlorite and antigorite [J]. Hydrometallurgy, 2009, 98(1-2): 58-65.
[4] AHONEN L, TUOVINEN O H. Bacterial leaching of complex sulfide ore samples in bench-scale column reactors [J]. Hydrometallurgy, 1995, 37(1): 1-21.
[5] PLUMB J J, MUDDLE R, FRANZMANN P D. Effect of pH on rates of iron and sulphur oxidation by bioleaching organisms [J]. Minerals Engineering, 2008, 21(1): 76-82.
[6] HANSFORD G S, VARGAS T. Chemical and electrochemical basis of bioleaching processes [J]. Hydrometallurgy, 2001, 59(2-3): 135-145.
[7] TRIBUTSCH H. Direct versus indirect bioleaching [J]. Hydrometallurgy, 2001, 59(2-3): 177-185.
[8] MOEHL T, KUNST M, W?NSCH F, TRIBUTSCH H. Consistency of photoelectrochemistry and photoelectrochemical microwave reflection demonstrated with p- and n-type layered semiconductors like MoS2 [J]. Journal of Electroanalytical Chemistry, 2007, 609(1): 31-41.
[9] C?MEZ C, FIGUEROA M, MU?OZ J, BL?ZQUEZ M L. Electrochemistry of chalcopyrite [J]. Hydrometallurgy, 1996, 43(1-3): 331-344.
[10] LU Z Y, JEFFREY M I, LAWSON F. An electrochemical study of the effect of chloride ions on the dissolution of chalcopyrite in acidic solutions [J]. Hydrometallurgy, 2000, 56(2): 145-155.
[11] ARCE E M, GONZ?LEZ I. A comparative study of electrochemical behavior of chalcopyrite, chalcocite and bornite in sulfuric acid solution [J]. International Journal of Mineral Processing, 2002, 67(1-4): 17-28.
[12] CHOI W K, TORMA A E, OHLINE R W, GHALI E. Electrochemical aspects of zinc sulphide leaching by Thiobacillus ferrooxidans [J]. Hydrometallurgy, 1993, 33(1-2): 137-152.
[13] BEVILAQUA D, DI?Z-PEREZ I, FUGIVARA C S, SANZ F, BENEDETTI A V, GARCIA O Jr. Oxidative dissolution of chalcopyrite by Acidithiobacillus ferrooxidans analyzed by electrochemical impedance spectroscopy and atomic force microscopy [J]. Bioelectrochemistry, 2004, 64(1): 79-84.
[14] WARNER T E, RICE N M, TAYLOR N. An electrochemical study of the oxidative dissolution of synthetic pentlandite in aqueous media [J]. Hydrometallurgy, 1992, 31(1-2): 55-90.
[15] CHA C S, LI C M. Powder microelectrodes [J]. Journal of Electroanalytical Chemistry, 1994, 368(1-2): 47-54.
[16] NORRIS P R, BRIERLEY J A, KELLY D P. Physiological characteristics of two facultatively thermophilic mineral-oxidising bacteria [J]. FEMS Microbiology Letters, 1980, 7(2): 119-122.
[17] BRIERLEY J, BRIERLEY C. Present and future commercial applications of biohydrometallurgy [J]. Hydrometallurgy, 2001, 59(2-3): 233-239.
[18] DAS A, BHATTACHARYYA S, BANERJEE P C. Purification of Thiobacillus ferrooxidans cultures by single colony isolation and influences of agarose on the colony morphology [J]. Journal of Microbiological Methods, 1989, 10 (4): 281-287.
[19] LIU Hsuan-Liang, LAN Yann-Wen, CHENG Yang-Chu. Optimal production of sulphuric acid by Thiobacillus thiooxidans using response surface methodology [J]. Process Biochemistry, 2004, 39(12): 1953-1961.
[20] TUOVINEN O H, KELLY D P. Studies on the growth of Thiobacillus ferrooxidans. IV. Influence of monovalent metal cations on ferrous iron oxidation and uranium toxicity in growing cultures [J]. Arch Microbiol, 1974, 98: 167-174.
[21] CURUTCHET G, DONATI E. Iron-oxidizing and leaching activities of sulphur-grown Thiobacillus ferrooxidans cells on other substrates: Effect of culture pH [J]. Journal of Bioscience and Bioengineering, 2000, 90(1): 57-61.
[22] MAKAMOTO T K, TAKAHASHI N T. Adaptation of Thiobacillus ferrooxidans to nickel ion and bacterial oxidation of nickel sulphide [J]. Biotechnology Letters, 1995, 17(2): 229-232.
[23] MASON L J, RICE N M. The adaptation of Thiobacillus ferrooxidans for the treatment of nickel-iron sulphide concentrates [J]. Minerals Engineering, 2002, 15(11): 795-808.
[24] CACHET-VIVIER C, VIVIER V, CHA C S, NEDELEC J Y, YU L T. Electrochemistry of powder material studied by means of the cavity microelectrode (CME) [J]. Electrochimica Acta, 2001, 47(1-2): 181-189.
[25] LAU Y Y, WONG D K Y, EWING A G. Intracellular voltammetry at ultrasmall platinum electrodes [J]. Microchemical Journal, 1993, 47(3): 308-316.
[26] WARNER T E, RICE N M, TAYLOR N. Thermodynamic stability of pentlandite and violarite and new EH-pH diagrams for the iron-nickel sulphur aqueous system [J]. Hydrometallurgy, 1996, 41(2-3): 107-118.
[27] WOLFGANG S, TILMAN G, PETER-GEORG J, AXEL S. (Bio) chemistry of bacterial leaching-direct vs. indirect bioleaching [J]. Hydrometallurgy, 2001, 59(2-3): 159-175.
[28] SCHIPPERS A, SAND W. Bacterial leaching of metal sulfides proceeds by two indirect mechanisms via thiosulfate or via polysulfides and sulfur [J]. Appl Environ Microbiol, 1999, 65: 319-321.
[29] TRIBUTSCH H, ROJAS-CHAPANA J A. Metal sulfide semiconductor electrochemical mechanisms induced by bacterial activity [J]. Electrochimica Acta, 2000, 45(28): 4705-4716.
[30] FARQUHAR M L, WINCOTT P L, WOGELIUS R A, VAUGHAN D J. Electrochemical oxidation of the chalcopyrite surface: An XPS and AFM study in solution at pH 4 [J]. Applied Surface Science, 2003, 218(1-4): 34-43.
[31] BEVILAQUA D, ACCIARI H A, ARENA F A, BENEDETTI A V, FUGIVARA G S, FILHO G T, J?NIOR O G. Utilization of electrochemical impedance spectroscopy for monitoring bornite (Cu5FeS4) oxidation by Acidithiobacillus ferrooxidans [J]. Minerals Engineering, 2009, 22(3): 254-262.
[32] NAVA D, GONZ?LEZ I, LEINEN D, RAMOS-BARRADO J R. Surface characterization by X-ray photoelectron spectroscopy and cyclic voltammetry of products formed during the potentiostatic reduction of chalcopyrite [J]. Electrochimica Acta, 2008, 53(14): 4889-4899.
[33] NAVA D, GONZ?LEZ I. Electrochemical characterization of chemical species formed during the electrochemical treatment of chalcopyrite in sulfuric acid [J]. Electrochimica Acta, 2006, 51(25): 5295-5303.
[34] PARKER G K, WOODS R, HOPE G A. Raman investigation of chalcopyrite oxidation [J]. Colloids and Surfaces A, 2008, 318(1-3): 160-168.
[35] TIAN M, HE Y N, WONG J L. Subspeciation of NiS in sulfidic nickel by carbon paste electrode voltammetry [J]. Talanta, 2001, 55(2): 349-356.
[36] KINZLER K, GEHRKE T, TELEGDI J, SAND W. Bioleaching—A result of interfacial processes caused by extracellular polymeric substances (EPS) [J]. Hydrometallurgy, 2003, 71(1-2): 83-88.
[37] PRONK J T, MEULENBERG R, HAZEU W, BOS P, KUENEN J G. Oxidation of reduced inorganic sulphur compounds by acidophilic thiobacilli [J]. FEMS Microbiology Letters, 1990, 75(2-3): 293-306.
镍黄铁矿电化学生物氧化过程的分解机理
李宏煦,李 超,张祉倩
1. 北京科技大学 冶金与生态工程学院,北京 100083;
2. 北京科技大学 教育部生态与循环冶金重点实验室,北京 100083
摘 要:应用表面粘附和没有粘附Acidithiobacillus ferrooxidans的镍黄铁矿粉末微电极进行电化学测试,以说明镍黄铁矿氧化分解的机理。循环伏安CV结果显示,在-0.2 V的低电位区域,在镍、铁离子析出时镍黄铁矿转变为中间相Fe4.5-yNi4.5-xS8-z;当电位在-0.2 V到 0.2 V区间时,有不稳定的紫硫镍矿Fe3Ni3S4和 FeNi2S4形成并在表面伴有元素硫的产生;当电位增加到0.2 V以上时,不稳定相将全部分解;在高电位0.7 V时,析出的亚铁离子被氧化为高铁离子。嗜酸氧化亚铁硫杆菌Acidithiobacillus ferrooxidans的存在使峰电位增高,反应起始电位负移,并对表面形成的元素硫有氧化去除作用。这一过程可通过-0.75到-0.5 V电位区间发生的的还原反应证实。生物浸出和电化学实验结果均表明当pH<2时溶液酸度的增加对氧化过程有轻度的阻碍作用。
关键词:镍黄铁矿;嗜酸氧化亚铁硫杆菌;生物浸取;改性微电极
(Edited by LI Xiang-qun)
Foundation item: Project (20876014) supported by the National Natural Science Foundation of China
Corresponding author: LI Hong-xu; Tel: +86-10-82376226; Fax: +86-10-62332786; E-mail: lihongxu@ustb.edu.cn; lihongxu2001@126.com
DOI: 10.1016/S1003-6326(11)61238-7
Abstract: Electrochemical measurements were carried out to elucidate decomposition mechanism of pentlandite using modified powder microelectrode with Acidithiobacillus ferrooxidans attached or without on the mineral powder surface. Cyclic voltammetry (CV) results show that at a low potential of about -0.2 V (vs SCE), the pentlandite was transformed to an intermediated phase like Fe4.5-yNi4.5-xS8-z when Fe and Ni ions were evacuated from mineral lattice; when the potential was changed from -0.2 V to 0.2 V, the unstable violarite (Fe3Ni3S4) and FeNi2S4 were formed which was accompanied by element sulfur formed on the mineral surface; when the potential increased over 0.2 V, the unstable intermediated phase decomposed entirely; at a higher potential of 0.7 V, the evacuated ferrous ion was oxidized to ferric ion. The presence of Acidithiobacillus ferrooxidans made the oxidation peak current increase with initial peak potential negatively moving, and the bacteria also contributed to the sulfur removing from mineral surface, which was demonstrated by the reduction characteristic at potential ranging from -0.75 to -0.5 V. Leaching experiments and electrochemical results show that the solution acidity increasing when pH<2 may impede the oxidation process slightly.


