
Characterization of MnWO4 with flower-like clusters produced using spray pyrolysis
Somchai THONGTEM1, Surangkana WANNAPOP1, Titipun THONGTEM2
1. Department of Physics and Materials Science, Faculty of Science, Chiang Mai University,
Chiang Mai, 50200, Thailand;
2. Department of Chemistry and Center for Innovation in Chemistry, Faculty of Science,
Chiang Mai University, Chiang Mai, 50200, Thailand
Received 2 March 2009; accepted 30 May 2009
Abstract:
MnWO4 (huebnerite) with flower-like clusters of nano-plates was produced from the solutions containing MnCl2?4H2O and Na2WO4?2H2O by the 300 ℃ spray pyrolysis. The phase was detected by X-ray diffraction (XRD) and selected area electron diffraction (SAED), and is in accordance with the results characterized using energy dispersive X-ray (EDX) analysis. The flower-like clusters of nano-plates were characterized using scanning and transmission electron microscopes (SEM and TEM), and their parallel lattice planes using a high resolution transmission electron microscope (HRTEM). Vibration spectra of the huebnerite structured products were characterized using Raman and Fourier transform infrared (FTIR) spectrometers. Their photoluminescence (PL) emissions are in the same spectral region at 405-412 nm.
Key words:
spray pyrolysis; MnWO4; flower-like clusters;
1 Introduction
Wolframite (FexMn1-xWO4) is an iron manganese tungstate mineral, which is the intermediate between iron-rich ferberite (FeWO4) and manganese rich huebnerite (MnWO4)[1-2]. MnWO4 has bulk electrical conductivity, relatively low melting point and novel magnetic property[3], caused by its antiferromagnetic spin structure[4]. It can display photoluminescence (PL) emission with two main bands at 421 and 438 nm[3]. There are a number of processes used to produce MnWO4, such as microwave-assisted synthesis[1], melt solution process[5], solvothermal route[6], aqueous salt metathesis reaction[7], sol-gel technique[8], ambient template synthesis[9], solid state metathetic approach [10], and surfactant-assisted complexation-precipitation method[4]. The purpose of this research was to produce MnWO4 with flower-like clusters, from additive-free solution, using a spray pyrolysis method, which is simple and easy to handle, and more economical to process.
2 Experimental
Each 0.005 mol of MnCl2?4H2O and Na2WO4?2H2O was separately dissolved in 25 mL de-ionized water and mixed for 10 min. The mixture was sprayed on glass slides 10 times, which were placed in a 300 ℃ furnace for 10-40 h. No other additives were used in the process. The products were washed with de-ionized water and 95% ethanol, and dried at 60 ℃ for 10 h. Then they were intensively characterized using a X-ray diffractometer (XRD) operated at 20 kV, 15 mA and using the Ka line from a Cu target, a Fourier transform infrared (FTIR) spectrometer with KBr as a diluting agent and operated in the range 500-1 600 cm-1, a Raman spectrometer of 50 mW Ar laser with l=514.5 nm, a scanning electron microscope (SEM) equipped with an energy dispersive X-ray (EDX) analyzer operated at 15 kV, a transmission electron microscope (TEM) and a high resolution transmission electron microscope (HRTEM) as well as the use of selected area electron diffraction (SAED) technique operated at 200 kV, and a photoluminescence (PL) spectrometer using a 293 nm excitation wavelength at room temperature[3].
3 Results and discussion
The XRD spectra of the products (Fig.1) were indexed using Bragg’s law for diffraction. They correspond to that of the JCPDS software with reference code 74-1497[11]. They have P2/c space group of paramagnetic phase[5] and huebnerite structure[11]. It is composed of a number of edge-sharing (MnO6) and (WO6) octahedrons in a series of zigzags along c axis. Mn and W atoms are alternately arranged parallel to the (100) planes[5]. The broad XRD spectrum of glass (Fig.1), specified as amorphous phase, is also shown for comparison. Its spectrum was covered by the spectra of the products, showing that the deposited products on glass substrates are thick enough to prevent the X-ray beam from reflecting on them. The mists on glass slides are heated effectively, and a number of particles of controlled morphologies are produced. Atoms composing the products are arranged as systematic array in the crystal. Thus the spectra are very sharp. Their XRD intensities are also increased with the increase in the prolonged times which play the role in arranging atoms in crystal lattice.
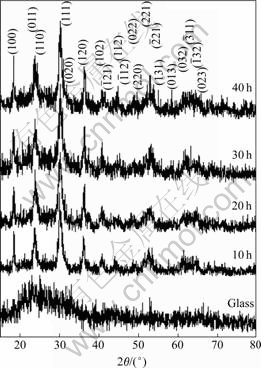
Fig.1 XRD spectra of glass and products on glass slides produced for 10, 20, 30 and 40 h
The FTIR spectra (Fig.2(a)) of MnWO4 with huebnerite structure show the inorganic modes in the range 556-983 cm-1 of the low wavenumber side at 556, 694, 826, 908 and 983 cm-1. The vibrations are in accordance with those of other researchers[2, 9]. These bands are assigned to be the internal stretching modes of ν3(Au) and ν3(Eu) transitions[9].
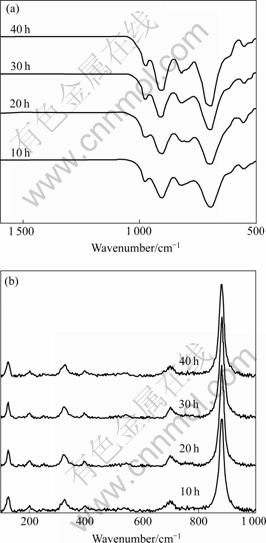
Fig.2 FTIR(a) and Raman spectra(b) of MnWO4 produced for 10, 20, 30 and 40 h
The Raman spectra (Fig.2(b)) of the huebnerite structure are very similar to those of KLOPROGGE et al[1]. A medium strong band is detected at 125 cm-1. The 200 cm-1 band is specified as ν(Ag) vibration involving the Mn cations, and the 245 cm-1 band as νdef(Ag) vibration of the cationic sublattices. Those of 324 and 393 cm-1 bands are respectively specified by FOMICHEV and KONDRATOV as deformation modes[12], and by DATURI et al as r(Bg) and δ(Ag) vibrations of terminal WO2 groups[13]. The 538 cm-1 band is specified as the symmetric Ag vibration. The 698 cm-1 band corresponds to the νas(Bg) vibration of (W2O4)n chain. The strongest intensity at 875 cm-1 belongs to symmetric Ag vibration of the terminal WO2 groups. At lower Raman wavenumber, the vibration frequency is smaller and the structure is in the state of closing to normal crystal lattice. When the vibration is at higher wavenumber, its structure becomes more distorted[14]. The higher amplitude of the atomic vibration does, the more the product structure distorts, and the opposite is also true.
The SEM images of MnWO4 (Fig.3) are characterized. The products are composed of a number of nano-plates in flower-like clusters. The flowers become larger, when the test is done in the longer period. They are the largest at 40 h test. For the present research, MnCl2?4H2O reacts with Na2WO4?2H2O to produce MnWO4 nuclei, which grows very rapidly by thermal heating. Their growths are anisotropic. Plate-like particles are produced, and simultaneously cluster to form flower-like colonies. They have different sizes, which are caused by random nucleation and growth processes.
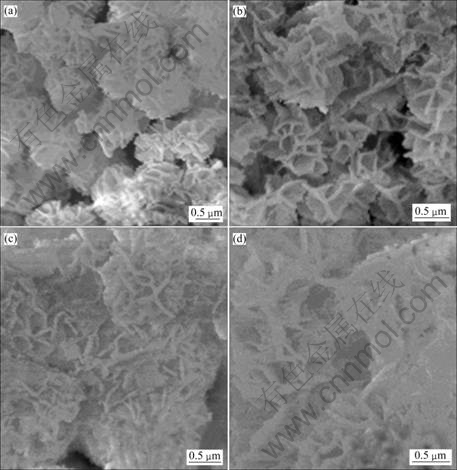
Fig.3 SEM images of MnWO4 produced for 10(a), 20(b), 30(c) and 40 h(d)
Each product was put into a beaker containing de-ionized water. After ultrasonic vibration, the product- dispersed water was dropped on a copper grid and dried in ambient atmosphere for further analysis. TEM images (Figs.4(a), (c) and (d)) show that the products are composed of a number of nano-plates with different orientations. The product of Fig.4(a) clusters together in irregular shape. But for those of Figs.4(c) and (d), they become more dispersive. HRTEM image (Fig.4(b)) show a number of (011) lattice planes in systematic arrays of crystal structure. Each array with the same orientation corresponds to a single crystal. SAED patterns (Figs.4(a), (c) and (d)) show several concentric rings. They are diffusive and hollow, showing that the products are composed of a number of nanosized crystals with different orientations. The calculated interplanar spaces[15] were compared with those of the JCPDS software[11]. They correspond to a variety of crystallographic planes labeled in parentheses. They are specified that the products are MnWO4.
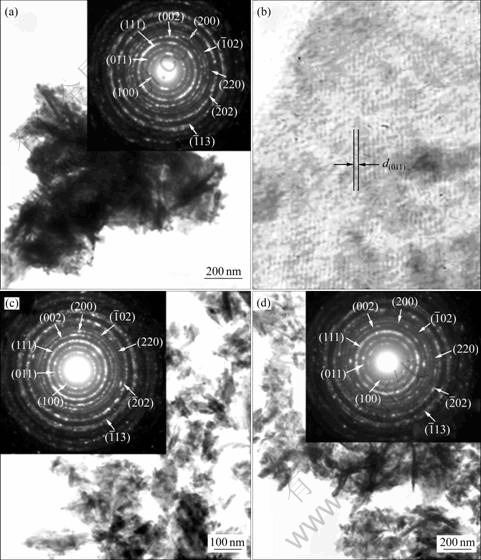
Fig.4 TEM and HRTEM images, and SAED patterns of MnWO4 produced for 10 h(a, b), 20 h(c) and 30(d)
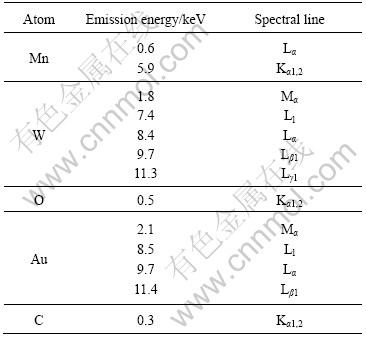
The EDX spectra (Fig.5) reveal the presence of Mn, W and O in the products[16]. They are in good accordance with the phase detected using XRD and SAED. Au and C are also detected. They are caused by the sputtered Au on the products to improve the quality of SEM images, and by C tape used for sample mounting. Different energy peaks are detected due to the electronic transition of atoms as summarized in Table 1[16].

Fig.5 EDX spectra of MnWO4 produced for 10, 20, 30 and 40 h
Table 1 Emission energies of atoms
By using a 293 nm excitation wavelength[3], photoluminescence (PL) spectra (Fig.6) show electronic transition within (WO4)2- anion molecular complex, associated with the intrinsic emission[17-18]. The intrinsic luminescence is caused by a nonlinear two-photon stimulation process[18]. They can be excited either in the excitonic absorption band or in the recombination process[19], resulting from the huebnerite-structured products. The intrinsic emission peaks are in the spectral region at 405-412 nm although the products are produced using different prolonged times. The results are in accordance with those detected by other researchers[3, 10]. PL intensities are increased with the increase in time. It is the highest at 40 h test. The shoulders or green bands, depended on the excitation type and sample quality, are caused by some defects and impurities, and are specified as the extrinsic emission[18].
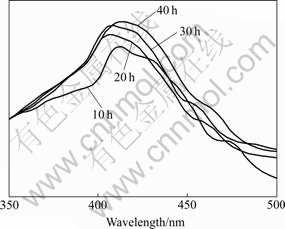
Fig.6 PL spectra of MnWO4 produced for 10, 20, 30 and 40 h
4 Conclusions
MnWO4 on glass slides were successfully produced by the 300 ℃ spray pyrolysis method. The phase are MnWO4, composing of Mn, W and O. The products are flower-like clusters of nano-plates, each of which is composed of a number of crystallographic planes aligning in lattice array. Vibration wavenumbers provide the evidence of huebnerite structure, corresponding to the product phase. PL emission shows the narrow central peaks in the same spectral region at 405-412 nm. The highest intensity peak is emitted from the product of 40 h test.
Acknowledgements
We are extremely grateful to the Thailand Research Fund (TRF) and Center for Innovation in Chemistry (PERCH-CIC), Commission on Higher Education (CHE), Ministry of Education, Thailand, for financial support.
References
[1] KLOPROGGE J T, Weier M L, Duong L V, Frost R L. Microwave-assisted synthesis and characterization of divalent metal tungstate nanocrystalline minerals: Ferberite, hübnerite, sanmartinite, scheelite and stolzite[J]. Mater Chem Phys, 2004, 88: 438-443.
[2] Ingham B, Chong S V, Tallon J L. Layered tungsten oxide-based hybrid materials incorporating transition metal ions[J]. Curr Appl Phys, 2006, 6: 553-556.
[3] Xing Y, Song S, Feng J, Lei Y, Li M, Zhang H. Microemulsion-mediated solvothermal synthesis and photo- luminescent property of 3D flowerlike MnWO4 micro/ nanocomposite structure[J]. Solid State Sci, 2008, 10: 1299-1304.
[4] Lei S, Tang K, Fang Z, Huang Y, Zheng H. Synthesis of MnWO4 nanofibres by a surfactant-assisted complexation- precipitation approach and control of morphology[J]. Nanotech, 2005, 16: 2407-2411.
[5] Heyer O, Hollmann N, Klassen I, Jodlauk S, Bohat? L, Becker P, Mydosh J A, Lorenz T, Khomskii D. A new multiferroic material: MnWO4[J]. J Phys: Condens Matter, 2006, 18: L471-L475.
[6] Chen S J, Chen X T, Xue Z, Zhou J H, Li J, Hong J M, You X Z. Morphology control of MnWO4 nanocrystals by a solvothermal route[J]. J Mater Chem, 2003, 13: 1132-1135.
[7] Montemayor S M, Fuentes A F. Electrochemical characteristics of lithium insertion in several 3D metal tungstates (MnWO4, M = Mn, Co, Ni and Cu) prepared by aqueous reactions[J]. Ceram Internat, 2004, 30: 393-400.
[8] Qu W, Wlodarski W, Meyer J U. Comparative study on micromorphology and humidity sensitive properties of thin-film and thick-film humidity sensors based on semiconducting MnWO4[J]. Sensors Actuat B, 2000, 64: 76-82.
[9] Zhou H, Yiu Y, Aronson M C, Wong S S. Ambient template synthesis of multiferroic MnWO4 nanowires and nanowire arrays[J]. J Solid State Chem, 2008, 181: 1539-1545.
[10] Parhi P, Karthik T N, Manivannan V. Synthesis and characterization of metal tungstates by novel solid-state metathetic approach[J]. J Alloys Comp, 2008, 465: 380-386.
[11] Powder diffraction files[S]. JCPDS-ICDD. USA, PA 19073-3273, 2001.
[12] Fomichev V V, Kondratov O I. Vibrational spectra of compounds with the wolframite structure[J]. Spectrochim Acta, 1994, 50A: 1113-1120.
[13] Daturi M, Busca G, Borel M M, Leclaire A, Piaggio P. Vibrational and XRD study of the system CdWO4-CdMoO4[J]. J Phys Chem B, 1997, 101: 4358-4369.
[14] Fu H, Pan C, Zhang L, Zhu Y. Synthesis, characterization and photocatalytic properties of nanosized Bi2WO6, PbWO4 and ZnWO4 catalysts[J]. Mater Res Bull, 2007, 42: 696-706.
[15] Andrews K W, Dyson D J, Keown S R. Interpretation of electron diffraction patterns[M]. 2nd ed. New York: Plenum Press, 1971: 14-15.
[16] X-ray Absorption and Emission Energies[M]. Oxford Instruments Analytical, Halifax Rd., High Wycombe Bucks HP12 3SE, UK.
[17] Treadaway M J, Powell R C. Luminescence of calcium tungstate crystals[J]. J Chem Phys, 1974, 61: 4003-4011.
[18] Mikhailik V B, Bailiff I K, Kraus H, Rodnyi P A, Ninkovic J. Two-photon excitation and luminescence of a CaWO4 scintillator[J]. Radiat Measur, 2004, 38: 585-588.
[19] Pankratov V, Grigorjeva L, Millers D, Chernov S, Voloshinovskii A S. Luminescence center excited state absorption in tungstates[J]. J Luminesc, 2001, 94/95: 427-432.
Corresponding author: Titipun THONGTEM; Tel: +66-53-943345; Fax: +66-53-892277; E-mail: ttpthongtem@yahoo.com; ttpthongtem@hotmail.com
(Edited by YUAN Sai-qian)


