
Isothermal coarsening of primary particles during rheocasting
GUO Hong-min(郭洪民)1, LUO Xue-quan(罗学泉)1, ZHANG Ai-sheng(章爱生)1, YANG Xiang-jie(杨湘杰)2
1. School of Materials Science and Engineering, Nanchang University, Nanchang 330031, China;
2. School of Mechatronic Engineering, Nanchang University, Nanchang 330031, China
Received 1 September 2009; accepted 24 December 2009
Abstract:
The isothermal coarsening behavior of primary solid particles in A356 aluminum alloy semi-solid slurry produced by angular oscillation (AO) technique was investigated. The comparison between the calculation and experimental results shows good quantitative agreement with Lifshitz-Slyozov-Wagner theory. The results show that the variation in shape factor and solid fraction is not significant, the average particle size increases with increasing holding time at the expense of the particle density. Ostwald repining is most likely the predominant growth mechanism in the AO-treated semi-solid slurry during rheocasting. The differences of coarsening occurred in rheocasting and partial re-melting process were also discussed.
Key words:
rheocasting; semi-solid; coarsening; A356 alloy; angular oscillation technique;
1 Introduction
Semi-solid metal (SSM) processing has been developed as a promising technology which is capable of manufacturing high-integrity components with higher mechanical properties compared with conventional casting. Several reviews have been done[1-2]. The key factor in successful SSM processing is to obtain the thixotropic microstructure in the semi-solid state, in which the primary phase presents as fine and globular particles uniformly distributed in the liquid matrix. Such thixotropic microstructure can be produced by a number of techniques and can be typically classified into two routes: (1) Solid state route consisting of production of special feedstock through casting or plastic deformation, followed by partial re-melting. The final material of this route is the semi-solid slug used in thixoforming. (2) Liquid state route, in which thixotropic microstructure can be obtained directly from liquid alloy, and used as semi-solid slurry for rheocasting. In both cases, a coarsening process plays a significant role in the evolution of solid phase. The coarsening behavior of solid phase in the first route has been extensively investigated and comprehensively understood [3-7]. The kinetic of grain growth in the semi-solid state was
approximate to a diffusion-controlled coarsening, coalescence and Ostwald ripening generally control grain coarsening. The isothermal temperature, holding time and microstructure of special feedstock are the main factors affecting the evolution of solid phase in the semi-solid state.
Although, it is now evident that rheocasting offers significant advantages of processing semi-solid slurry directly from liquid alloy and recycling scrap metals in-house, no much attention was paid to the coarsening process happening in the second route. For most of modern rheocasting techniques [8-10], the semi-solid slurry is usually handled isothermally prior to subsequent component shaping, thus it is important to investigate the stability and extent of coarsening of the particles in order to control the flow behavior of semi-solid slurry in the die cavity. The main objective of the present work is to understand the kinetics and mechanisms for the fine and globular particles in an A356 alloy under such conditions. Before forming the semi-solid slurry, grain growth should be minimal so that the final component has a small grain size and acceptable mechanical properties. In addition, it is also important to identify if the coarsening kinetics in rheocasting are the same as reported previously in partial re-melting.
2 Experimental
The raw material used was a commercial aluminum alloy A356 (Al balance, 6.89% Si, 0.12% Fe, <0.05% Cu, <0.02% Mn, 0.35% Mg, 0.4% Zn, mass fraction). Disc samples were tested by differential scanning calorimetry (DSC) in an argon atmosphere. The samples were heated to 700 °C at 2.5 °C/min and cooled to room temperature at the same rate. The relationship between solid fraction and temperature was obtained by integrating under the curve of the heat flow vs temperature. The useful part of the curve (from full liquid to 49% solid fraction) is shown in Fig. 1. The liquidus temperature of the alloy is about 615.5 °C.
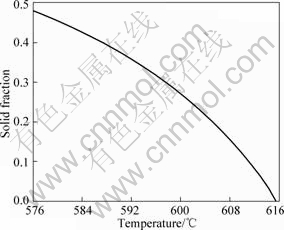
Fig.1 Temperature vs solid fraction curve of A356 aluminum alloy calculated from DSC
The semi-solid slurry was produced using angular oscillation (AO) technique. A diagram of the AO apparatus and details was given in Ref.[9]. As-received A356 alloy ingots were melted in a resistance furnace at 710 °C. A predetermined dose of melt was then cooled to 660 °C followed by bottom pouring from a crucible over the oscillating barrel. Then the melt was collected in a slurry holder (cylindrical graphite mould, 90 mm outside diameter, 60 mm inside diameter, 100 mm height, coated with boron nitride), cooled to 589 °C within 40 s, and then held at 589 °C and coarsened under a quiescent state in a given holding time up to 90 min. The specimens were taken from the slurry holder at different time intervals and immediately quenched into salt water. The oscillating barrel was kept at 120 °C. The oscillating amplitude and frequency were π/9 and 4.0 Hz, respectively. The oscillating cycle was the same as that used in Ref.[9].
Specimens for microstructure examination were prepared and etched with a 0.5% HF solution. The microstructure was examined by a Zeiss optical microscope equipped with image quantitative analysis system. The particle size, solid fraction, shape factor and particle density of the primary α(Al) were measured. The diameter (D) was calculated as the diameter of a circle with the same area as that of a particle in the micrograph. The shape factor is defined as SF=4πA/P2, where A and P are the average area and average perimeter, respectively. SF increases with increasing sphericity of primary α(Al) and turns to 1 for a perfect circle. The solid fraction after quenching (φ) was calculated as
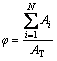 (1)
(1)
where Ai is the area for particle i, AT is the total analyzed area and N is the total number of the examined solid particles. The particle density (Nv) was calculated as:
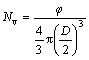 (2)
(2)
3 Results and discussion
3.1 Microstructure evolution during isothermal treatment
Figs.2 and 3(a) show the microstructures of the as-received ingot of A356 alloy solidified under normal condition and semi-solid slurry produced by AO from a pouring temperature of 660 °C, respectively. The bright phase in both microstructures is primary α(Al). A predominant presence of dendritic forms of primary α(Al) with grain size of several millimeters was observed in the normal cast sample. In contrast, most of the primary α(Al) in the rheocast sample were present as fine and globular particles with an average diameter of 58 μm and a shape factor of 0.81, and featured zero-entrapped eutectic.
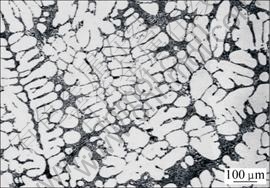
Fig.2 OM image of as-received ingot of A356 alloy
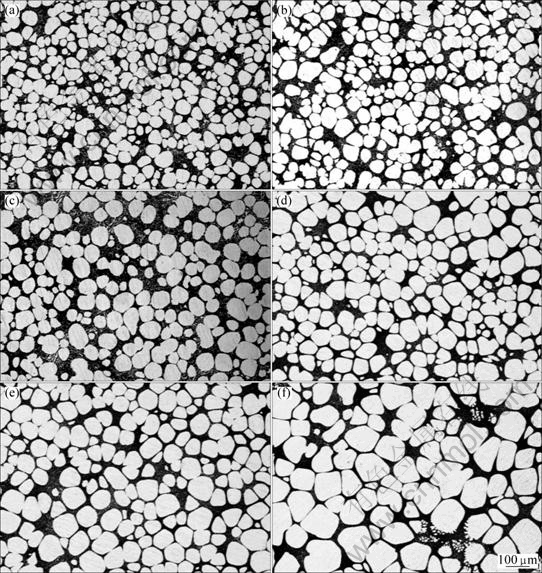
Fig.3 Evolution of primary α(Al) rheocast during isothermal treatment at 589 °C for: (a) 0 min; (b) 2 min; (c) 10 min; (d) 20 min; (e) 35 min; (f) 90 min
Fig.3 shows the rheocast microstructures of A356 quenched after isothermal holding at 589 °C for 0, 2, 10, 20, 35 and 90 min, respectively. With prolonged holding time, no agglomeration was observed and most solid particles were isolated. These particles showed no sign of entrapped eutectic at different stages. The effects of holding time on the particle size, particle density, solid fraction and shape factor are shown in Figs. 4-5. The variation in shape factor and solid fraction was not significant. The average particle size increased with increasing holding time, which was at the expense of the particle density.
3.2 Growth kinetics and evolution mechanism
The coarsening observed in globular particles is usually described by Lifshitz-Slyozov-Wagner (LSW) theory. A simple form of the theory can be written as[11-12]:
![]() (3)
(3)
where d0 is the initial particle size, d is the particle size at time t, n is the coarsening exponent and K is the coarsening rate constant. The experimental data in Fig.3 are reconstructed according to Eq.(3) at n=3 and the result is shown in Fig.6. The solid line represents curve fitted to classical SLW equation at n=3 and R2 is regression coefficient. The coarsening rate constant is found to be 269 μm3/s. The basic LSW assumption of no overlapped diffusion fields surrounding the particles[13] can not be justified in most real cases of the ripening of semi-solid alloys. Recently, many investigations have
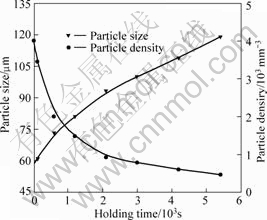
Fig.4 Effect of isothermal holding time on particle density and particle size
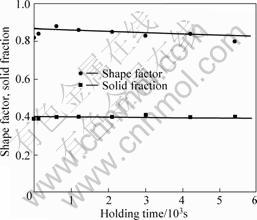
Fig.5 Effect of isothermal holding time on shape factor and solid fraction
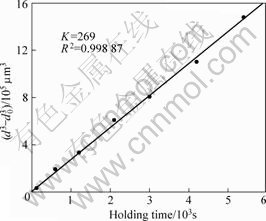
Fig.6 Evolution of average particle diameter as function of isothermal holding time
been made to modify the classical LSW theory to allow the overlapping of diffusion fields. In the case of purely diffusion-driven Ostwald ripening, VOORHEES and GLICKSMAN[14-15] yielded the following relation for the coarsening rate constant K:
![]() (4)
(4)
where Г is the Gibbs-Thomson coefficient, DL is the liquid interdiffusion coefficient, mL is the slope of liquidus curve, CS and CL are the equilibrium composition of solid-liquid interface at the coarsening temperature, f(φ) is the function of the volume solid fraction φ. Using the following values for Al-Si alloy at 589 °C with φ=0.4: Г=2×10-7 m?K, DL=3×10-9 m2/s, mL=6.0 K/% Si , CS=1.3% Si, CL=10.8% Si (molar fraction), f(φ)=3.12, a calculated coarsening rate constant K of 229 μm3/s is obtained. Although this value is slightly lower than the experimental observed value, the experiment result shows a very good agreement with the theory calculation. In addition, the resulted regression coefficient value R2 is very close to 1. Therefore, both the coarsening exponent n and the coarsening rate constant K suggest that the coarsening of primary α(Al) particles in the semi-solid slurry of A356 alloy obeys Ostwald ripening, and the coarsening is mainly volume diffusion-controlled.
The differences between coarsening process occurring in the current work and the reported partial re-melting[3-7, 18-21] can be summarized as: (1) solid fraction almost remains constant in the current work, but changes significantly at initial stages then becomes constant for partial re-melting; (2) shape factor almost remains constant during coarsening in the current work, but usually increases with increasing holding time of partial re-melting; (3) no entrapped liquid is observed in particles in the current work, but entrapped liquid in particles is a common phenomenon for partial re-melting. Fig.7 shows the cubic coarsening rate constant K vs temperature of A356 alloy produced in this work and partial re-melting of special feedstock of other studies[6, 18-20]. Considerably higher K values during partial re-melting were reported in A536 alloy feedstock produced by direct chill cast (DC cast, 723 μm3/s at 580 °C), strain-induced melt activation (SIMA, 483 μm3/s at 580 °C), conventional cast (953 μm3/s at 586 °C), ultrasonic treatment (UST, 534 μm3/s at 586 °C) and magnetohydrodynamic (MHD) stirring (1 043 μm3/s at 580 °C) [6, 18-19]. All of these values are much higher than our current value of 269 μm3/s and the theory calculation according to Eq.(4) (229 μm3/s at 589 °C and 249 μm3/s at 580 °C).
Particle coarsening in semi-solid state is a two-phase coarsening process. The particle growth mechanisms proposed for the coarsening in the semi-solid state contain coalescence and Ostwald ripening[5-7]. Investigations[3, 5-7, 20] showed that

Fig.7 Cubic coarsening rate constant as function of temperature for Al-Si alloy in this work and other studies
the coalescence plays an important role in the evolution of non-dendritic microstructures produced by MHD stirring and SIMA in semi-solid state. The higher K value in partial re-melting can be attributed to the higher potential of special feedstock materials for particle coalescence due to two points: (1) Growth by coalescence is dominant in a short time after liquid is formed during partial re-melting at high solid fraction [3, 5-7], because the thin liquid film at the early stages of re-melting leads to rapid particle growth; (2) A less random orientation of neighboring particles or preferred orientation (texture) relationship of neighboring particles in the special feedstock is produced by direct casting (DC), magnetohydrodynamic stirring (MHD), ultrasonic sound treatment (UST) and strain induced melt activated process (SIMA)[3,6,19], which provides a high proportion of low angle boundaries[20]. These boundaries with low energy enhance the coalescence by solid-solid contact[3, 22]. Thus, the effect of coalescence is greater, resulting in a higher coarsening rate constant.
However, Ostwald ripening is considered to be dominant in a long time and at a high liquid fraction[5-7]. According to the assumptions [13], LSW theory is more suitable for the coarsening of isolated globular particles than for dendritic or rosette particles. Thus, the AO-treated semi-solid slurry is most likely to adopt the slow Ostwald repining as the main growth mechanism which is learned from the fine and isolated globular particles with no entrapped liquid in them (which is an indication of grain agglomeration), as shown in Fig.3. The slow growth of the primary α(Al) particles in the AO-treated semi-solid slurry is an advantage that reveals the feasibility and competence of AO as a potential technique to produce semi-solid slurry for rheocasting process.
Ostwald ripening mechanism is driven by the presence of variable curvature of different particles. On one hand, it is easily understood that the smaller primary α(Al) particles with size below the average diameter dissolved gradually but the larger primary α(Al) particles with size above the average diameter grew, and the particle density decreased, as shown in Figs.3 and 4. On the other hand, such ripening is in favor of the spheroidizing of solid particles because of the reduction of surface energy and overlapped solution fields of neighboring particles. According to previous investigation[23], the solid-liquid interface may be instable when the particle size is large enough, resulting in the decrease of shape factor, as shown in Figs.3 and 5.
4 Conclusions
1) The coarse and dendritic α(Al) phase in the A356 alloy transforms into a fine and globular one by using AO technique. Particle coarsening during subsequent isothermal holding obeys Ostwald repining and can be described by LSW theory. The coarsening rate constant is 269 μm3/s. This provides an effective way to estimate the particle size in semi-solid slurry for industrial applications.
2) Particles exhibit a globular shape and do not entrap liquid throughout the coarsening process, but a longer holding time causes a slight reduction of spheroidization.
References
[1] FLEMINGS M C. Behavior of metal alloys in the semi-solid state [J]. Metallurgical Transactions A, 1991, 22 (4): 957-981.
[2] ATKINSON H V. Modelling the semisolid processing of metallic alloys [J]. Progress in Materials Science, 2005, 50(3): 293-412.
[3] LUO S, CHEN Q, ZHAO Z. An investigation of microstructure evolution of RAP processed ZK60 magnesium alloy [J]. Materials Science and Engineering A, 2009, 501(1/2): 146-152.
[4] ANAVARAPU S, DOHERTY R D. Inhibited coarsening of solid-liquid microstructures in spray casting at high volume fractions of solid [J]. Acta Metall Mater, 1995, 43(8): 3207-3230.
[5] MANSON-WHITTON E D, STONE I C, JONES J R, GRANT P S, CANTOR B. Isothermal grain coarsening of spray formed alloys in the semi-solid state [J]. Acta Materialia, 2002, 50(10): 2517-2535.
[6] LOUE W R, SUERY M. Microstructure evolution during partial remelting of Al-Si7Mg alloys [J]. Materials Science and Engineering A, 1995, 203(1/2): 1-13.
[7] KIM H S, STONE I C, CANTOR B. Microstructure evolution in semi-solid AA7034 [J]. Journal of Materials Science, 2008, 43(4): 1292-1304.
[8] KAUFMANN H, WABUSSEG H, UGGOWITZER P J. Metallurgical and processing aspects of the NRC semi-solid casting technology [J]. Aluminum, 2000, 76(1/2): 70-75.
[9] GUO H M, YANG X J, LUO X Q. Formation of grain refined and non-dendritic microstructure of an aluminum alloy under angular oscillation [J]. Journal of Alloys and Compounds, 2009, 482(1/2): 412-415.
[10] MARTINEZ R A, FLEMINGS M C. Evolution of particle morphology in semisolid processing [J]. Metallurgical and Materials Transactions A, 2005, 36(8): 2205-2210.
[11] LIFSHITS I M, SLYOZOV V V. Kinetics of precipitation from supersaturated solid solutions [J]. J Phys Chem Solids, 1961, 19: 35-50.
[12] WAGNER C. Theory of aging from precipitation by Ostwald ripening [J]. Z. Electrochem, 1961, 65(7/8): 581-586.
[13] BALDAN A. Review progress in Ostwald ripening theories and their applications to nickel-base superalloys (Part I): Ostwald ripening theories [J]. Journal of Materials Science, 2002, 37(12): 2171-2202.
[14] VOORHEES P W, GLICKSMAN M E. Ostwald ripening during liquid phase sintering—Effect of volume fraction on coarsening kinetics [J]. Metallurgical Transactions A, 1984, 15(6): 1081-1088.
[15] VOORHEES P W, HARDY S C. Ostwald ripening in a system with a high volume fraction of coarsening phase [J]. Metallurgical Transactions A, 1988, 19(11): 2713-2721.
[16] KURZ W, FISHER D J. Fundamentals of solidification [M]. Lausanne, Switzerland Trans Tech Publications LTD, 1992: 30-80.
[17] MURRAY J L, mc ALISTER A J. The Aluminum-Silicon System [J]. Bull Alloy Phase Diagrams, 1984, 5: 74-84.
[18] ZOQUI E J, SHEHATA M T, PAES M, KAO V, ES-SADIQI E. Morphological evolution of SSM A356 during partial remelting [J]. Materials Science and Engineering A, 2002, 325(1/2): 38-53.
[19] KHALIFA W, TSUNEKAWA Y, OKUMIYA M. Effect of reheating to the semisolid state on the microstructure of the A356 aluminum alloy produced by ultrasonic melt-treatment[J]. Solid State Phenomena, 2008, 141/142/143: 499-504.
[20] TZIMAS E, ZAVALIANGOS A. Evolution of near-equiaxed microstructure in the semisolid state [J]. Materials Science and Engineering A, 2000, 289(1/2): 228-240.
[21] ATKINSON H V, LIU D. Microstructural coarsening of semi-solid aluminium alloys [J]. Materials Science and Engineering A, 2008, 496(1/2): 439-446.
[22] FERRENTE M, DE FREITAS E R. Rheological behavior and deformation characteristics of a commercial and a laboratory cast Al-4%Cu alloy in the semi-solid state [J]. Acta Materialia, 2001, 49(18): 3839-3847.
[23] GUO H M, YANG X J. Morphology evolution of primary particles in LSPSF rheocasting process [J]. International Journal of Modern Physics B, 2009, 23(6/7): 881-887.
(Edited by FANG Jing-hua)
Foundation item: Project (50804023) supported by the National Natural Science Foundation of China; Project (205084) supported by the Key Project of Science and Technology Research of Ministry of Education of China
Corresponding author: GUO Hong-min; Tel: +86-791-3969611; E-mail: guohongmin@ncu.edu.cn
DOI: 10.1016/S1003-6326(09)60305-8


