DOI: 10.11817/j.ysxb.1004.0609.2020-39705
酸性溶液中铼的电沉积行为
洪 标1, 2,王友彬1, 2,李 锋2,韦悦周1, 2,Toyohisa FUJITA1, 2
(1. 广西大学 广西有色金属及特色材料加工重点实验室,南宁 530004;
2. 广西大学 资源环境与材料学院,南宁 530004)
摘 要:
采用电化学循环伏安法、恒电位沉积法和电化学石英微晶天平(EQCM)研究铼在酸性溶液中的电沉积行为。结果表明:当溶液接近中性(pH=6)时,金属铼难以被还原出来;当溶液酸度较低(pH=3)时,电沉积产物含有铼的氧化物(ReO3);当溶液酸度较高时(pH=1),电沉积产物为金属铼,所得产物的微观形貌中存在明显的裂纹;且随着溶液中 浓度的增加,铼的电沉积得到促进。在1.0 mmol/L
浓度的增加,铼的电沉积得到促进。在1.0 mmol/L (pH=1)溶液中铼的沉积电位为-0.68 V。通过恒电位沉积得出不同条件下的电解效率,最高可达5.62%。
(pH=1)溶液中铼的沉积电位为-0.68 V。通过恒电位沉积得出不同条件下的电解效率,最高可达5.62%。
关键词:
文章编号:1004-0609(2021)-03-0748-08 中图分类号:TQ151.8 文献标志码:A
引文格式:洪 标, 王友彬, 李 锋, 等. 酸性溶液中铼的电沉积行为[J]. 中国有色金属学报, 2021, 31(3): 748-755. DOI: 10.11817/j.ysxb.1004.0609.2020-39705
HONG Biao, WANG You-bin, LI Feng, et al. Electrodeposition of rhenium from acidic solution[J]. The Chinese Journal of Nonferrous Metals, 2021, 31(3): 748-755. DOI: 10.11817/j.ysxb.1004.0609.2020-39705
铼是一种稀贵金属,具有高硬度、耐高温、抗蠕变等性能,因此在高温合金及涂层[1-3]、催化[4-5]、能源[6]、生物医学[7-8]等领域具有重要的应用,是一种重要的战略资源[9-10]。目前,工业上常用氢气还原高铼酸铵制备金属铼[11-12],电化学还原沉积的方法较少[13]。与氢还原法相比,电沉积法具有过程简单、易操作、安全性高的特点,是一种简便的制备金属铼的方法[13-15]。然而,铼在酸性溶液中的最高价态为+7价,在还原过程中很容易生成中间价态铼的化合物,如ReO2和ReO3,因此得出确切的还原机理较为困难;另一方面,由于高价态的铼电沉积过程一般存在多步骤的电子还原,形成中间价态,造成电流空耗。
国内外的许多研究者已经在铼的电沉积行为研究方面取得了一些进展。在酸性电解液中,铼的电沉积产物会随着酸度的不同而发生改变[16-17]。在碱性电解液中,铼的电沉积产物包含金属铼和铼的氧化物[18]。然而,对于铼在酸性溶液中的电沉积行为,其最佳的沉积条件和阴极还原过程的理论分析至今还没有达成一个共识[13]。因此,铼在酸性溶液中的电沉积行为有着很重要的研究价值。
不仅如此,在铼的电沉积过程中,其副反应析氢反应剧烈发生,铼还原电流被析氢电流所掩盖,难以确定其电化学反应过程。电化学石英微晶天平技术(EQCM)可以实时监测电沉积和溶解过程中的电极的质量变化和电流变化,这为研究电极表面复杂的电化学行为提供了有利的工具[19-21]。
为了进一步理解铼在酸性溶液中的电沉积行为,在本工作中,通过联合电化学工作站和微晶天平进行研究。采用扫描电子显微镜(SEM),X射线能谱分析(EDS)和X射线衍射分析(XRD)分析电沉积产物的形貌、成分和结构,通过恒电位沉积法确定其电流效率。
1 实验
实验所用溶液都是用分析级试剂和超纯水(18 MΩ·cm)制备。高铼酸铵购于上海麦克林生化科技有限公司,硫酸购于国药集团化学试剂有限公司,用以配制不同酸度和 浓度的溶液。为了防止水溶液中的氧气对电化学反应造成的干扰,在实验前,对溶液通入氮气20 min,然后在氮气氛围下进行后续实验。
浓度的溶液。为了防止水溶液中的氧气对电化学反应造成的干扰,在实验前,对溶液通入氮气20 min,然后在氮气氛围下进行后续实验。
采用美国Ametek公司PARSTAT 4000A型电化学工作站进行电化学实验。循环伏安实验和恒电位沉积实验采用三电极系统:工作电极和对电极均为铂片,参比电极为饱和甘汞电极(SCE)。工作电极和对电极在每次使用前,首先用0.05 μm的氧化铝悬浊液进行机械抛光,接着在硝酸溶液(体积比为1:1)中超声清洗20 min,然后再在无水乙醇和超纯水中分别超声处理20 min,最后用超纯水清洗。所有实验在室温下进行。
循环伏安的扫描速率是10 mV/s。本实验中恒电位沉积实验时,沉积电位均为-0.80 V,沉积时间为5000 s。电沉积结束后,快速取出工作电极并用去离子水冲洗电极表面残余的电解液,快速吹干,后置于电干燥箱内在 75 ℃下干燥。采用荷兰飞纳Phenom ProX型分析产物的形貌和元素组成,测试电压为15 kV。采用日本理学Rigaku SMARTLAB3KW型X射线衍射仪(XRD)表征电沉积产物的物相,测试电压为40 kV,电流密度 30 mA,扫描速率为8 (°)/min。
采用式(1)计算电沉积电流效率:
 (1)
(1)
式中:η为电流效率,%;m为电沉积前后工作电极的质量变化,g;n为转移的电子数,n=7;F为法拉第常数,96485 C/mol;Q为电沉积消耗的总电荷,C;M为铼的相对原子质量,MRe=186.21 g/mol。
电化学石英微晶天平实验(EQCM)的工作电极是AT-cut石英晶体铂晶振片电极(基准频率f0=9.0 MHz,有效表面面积A=0.198 cm2),对电极是铂片,参比电极是饱和甘汞电极。电极频率变化(△f)与质量变化(△m)之间的关系用Sauerbrey方程来描述[22]
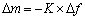 (2)
(2)
式中:△f为石英晶体晶振片的频率变化;△m为石英晶体晶振片的质量变化;K为石英晶体晶振片的灵敏度系数;对于QCM922A 9 MHz晶体,1 Hz的质量变化为1.068 ng。
根据法拉第定律
 (3)
(3)
式中:m为沉积的摩尔质量,g/mol;△q为反应消耗的总电荷,C;z为参与反应的电子量。对其进行适当变换,可以写成:
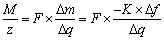 (4)
(4)
M/z即电极表面反应时每1 mol电子转移时引起的质量变化量。可以根据实验的质量变化和电荷变化得出。实验M/z值可以推测出电极表面沉积物种和沉积物的摩尔质量。
2 结果与分析
图1所示为在不同酸度条件下5.0 mmol/L  溶液中的循环伏安曲线。在5.0 mmol/L
溶液中的循环伏安曲线。在5.0 mmol/L  (pH=3)溶液中的负向扫描过程中,在不同电位下出现了还原峰,分别标记为C1、C2、C3、C4。其中,C1处相对应的电化学反应方程式为式(5),C2处相对应的电化学反应方程式为式(6),C3处相对应的电化学为析氢反应,C4处相对应的电化学反应方程式为式(7)[13]。由于析氢反应电流峰C3电位高于铼的还原对应的C4电位,所以在铼的电沉积过程析氢反应持续存在。在正向扫描过程中,在电位-0.32 V处出现的氧化峰对应的峰值电流密度为0.26 mA/cm2,其电量为14.80 μC。
(pH=3)溶液中的负向扫描过程中,在不同电位下出现了还原峰,分别标记为C1、C2、C3、C4。其中,C1处相对应的电化学反应方程式为式(5),C2处相对应的电化学反应方程式为式(6),C3处相对应的电化学为析氢反应,C4处相对应的电化学反应方程式为式(7)[13]。由于析氢反应电流峰C3电位高于铼的还原对应的C4电位,所以在铼的电沉积过程析氢反应持续存在。在正向扫描过程中,在电位-0.32 V处出现的氧化峰对应的峰值电流密度为0.26 mA/cm2,其电量为14.80 μC。
 (aq)+2H++e→ReO3(s)+H2O, φ0=0.77 V (5)
(aq)+2H++e→ReO3(s)+H2O, φ0=0.77 V (5)
ReO3(s)+2H++2e→ReO2(s)+H2O, φ0=0.4 V (6)
ReO2(s)+4H++4e→Re(s)+H2O, φ0=0.251 V (7)
 (aq)+8H++7e→Re(s)+4H2O, φ0=0.363 V (8)
(aq)+8H++7e→Re(s)+4H2O, φ0=0.363 V (8)
在5.0 mmol/L  (pH=6)溶液中的在正向扫描过程中,在电位-0.54 V处出现了氧化峰,对应的峰值电流密度为0.09 mA/cm2,其电量为4.93 μC。在5.0 mmol/L
(pH=6)溶液中的在正向扫描过程中,在电位-0.54 V处出现了氧化峰,对应的峰值电流密度为0.09 mA/cm2,其电量为4.93 μC。在5.0 mmol/L  (pH=1)溶液中进行循环伏安扫描时,当电位从终止电位向正方向扫描时,阴极电流下降较为缓慢,使反向扫描曲线与正向扫描曲线相交,形成了典型的成核环,这是电结晶成核过程的特点[23]。这说明了在此条件下铼在电极上电结晶,其铼的多步骤还原过程合并为式(8)。在正向扫描过程中,在0.73 V处出现的氧化峰,其峰值电流密度为65.30 mA/cm2,其电量为4.13 mC。从3种条件下的氧化峰电流和电量可以知道,在5.0 mmol/L
(pH=1)溶液中进行循环伏安扫描时,当电位从终止电位向正方向扫描时,阴极电流下降较为缓慢,使反向扫描曲线与正向扫描曲线相交,形成了典型的成核环,这是电结晶成核过程的特点[23]。这说明了在此条件下铼在电极上电结晶,其铼的多步骤还原过程合并为式(8)。在正向扫描过程中,在0.73 V处出现的氧化峰,其峰值电流密度为65.30 mA/cm2,其电量为4.13 mC。从3种条件下的氧化峰电流和电量可以知道,在5.0 mmol/L  (pH=1)的溶液中,循环伏安扫描过程中的氧化峰电流和电量远远大于另外两种条件下的。这说明在酸度较高的条件下,在还原反应过程中产生的铼较多,金属铼的电沉积较为容易,这与先前的研究相一致[24-25]。
(pH=1)的溶液中,循环伏安扫描过程中的氧化峰电流和电量远远大于另外两种条件下的。这说明在酸度较高的条件下,在还原反应过程中产生的铼较多,金属铼的电沉积较为容易,这与先前的研究相一致[24-25]。
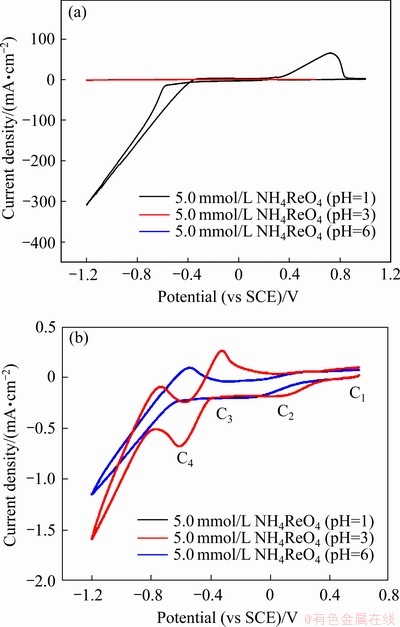
图1 不同酸度条件下5.0 mmol/L  溶液中的循环伏安曲线及5.0 mmol/L
溶液中的循环伏安曲线及5.0 mmol/L  (pH=3)和5.0 mmol/L
(pH=3)和5.0 mmol/L  (pH=6)溶液中循环伏安曲线的放大图
(pH=6)溶液中循环伏安曲线的放大图
Fig. 1 CV curves in solutions containing 5 mmol/L  with different pH (a) and enlarged figures (b) of cyclic voltammetry curves of 5.0mmol /L
with different pH (a) and enlarged figures (b) of cyclic voltammetry curves of 5.0mmol /L  (pH=3) and 5.0 mmol /L
(pH=3) and 5.0 mmol /L  (pH=6) in solution as shown in Fig. 1(a) (Scanning rate of 10 mV/s, room temperature)
(pH=6) in solution as shown in Fig. 1(a) (Scanning rate of 10 mV/s, room temperature)
图2所示为相同酸度(pH=1)条件下不同 浓度的循环伏安曲线。由于剧烈的析氢反应,无法观察到铼的还原峰。但可以清楚地看到,在3种条件下的循环伏安过程中,正向扫描过程中出现的氧化峰,其峰值电流密度分别为65.30、40.67和21.83 mA/cm2,其电量分别为4.13、2.00和1.29 mC。更大的氧化峰电流表明着在还原过程中沉积了更多的金属铼。这说明提高
浓度的循环伏安曲线。由于剧烈的析氢反应,无法观察到铼的还原峰。但可以清楚地看到,在3种条件下的循环伏安过程中,正向扫描过程中出现的氧化峰,其峰值电流密度分别为65.30、40.67和21.83 mA/cm2,其电量分别为4.13、2.00和1.29 mC。更大的氧化峰电流表明着在还原过程中沉积了更多的金属铼。这说明提高 浓度有利于铼还原反应的进行,进而提高了铼的电沉积速率。
浓度有利于铼还原反应的进行,进而提高了铼的电沉积速率。
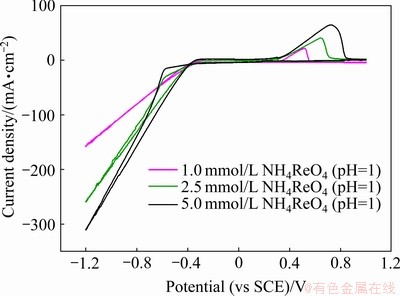
图2 在相同酸度(pH=1)条件下不同 浓度溶液中的循环伏安曲线
浓度溶液中的循环伏安曲线
Fig. 2 CV curves in solutions with different concentrations of  (pH=1) (Scanning rate of 10 mV/s, room temperature)
(pH=1) (Scanning rate of 10 mV/s, room temperature)
由上可知,提高酸度和 浓度均可以促进铼的电沉积。在5.0 mmol/L
浓度均可以促进铼的电沉积。在5.0 mmol/L  (pH=1)溶液的循环伏安过程中,由于铼的还原沉积反应发生在析氢反应的电位区域内,析氢反应非常剧烈,将铼的还原峰掩盖住,故导致无法观察其还原峰位置,难以确定铼的电沉积电位。由此,进行了电化学石英微晶天平实验,用来进一步研究在1.0 mmol/L
(pH=1)溶液的循环伏安过程中,由于铼的还原沉积反应发生在析氢反应的电位区域内,析氢反应非常剧烈,将铼的还原峰掩盖住,故导致无法观察其还原峰位置,难以确定铼的电沉积电位。由此,进行了电化学石英微晶天平实验,用来进一步研究在1.0 mmol/L  (pH=1)溶液中的循环伏安过程。
(pH=1)溶液中的循环伏安过程。
图3所示为在1.0 mmol/L  (pH=1)溶液中的循环伏安曲线及相应的质量变化。在-0.36 V处出现还原电流时,质量并无任何变化,说明这是析氢反应起始电位[26]。在-0.68 V处质量增加,同时,从电流上并没有观察到还原峰,说明这是铼的还原电位,且其还原峰被剧烈的析氢反应所掩盖。在正向扫描过程中,在0.29 V左右出现氧化电流,随之质量也发现剧烈变化,陡然下降,到最后电流和质量都回归于开始的位置。这说明在1.0 mmol/L
(pH=1)溶液中的循环伏安曲线及相应的质量变化。在-0.36 V处出现还原电流时,质量并无任何变化,说明这是析氢反应起始电位[26]。在-0.68 V处质量增加,同时,从电流上并没有观察到还原峰,说明这是铼的还原电位,且其还原峰被剧烈的析氢反应所掩盖。在正向扫描过程中,在0.29 V左右出现氧化电流,随之质量也发现剧烈变化,陡然下降,到最后电流和质量都回归于开始的位置。这说明在1.0 mmol/L  (pH=1)溶液中金属铼的氧化电位是0.29 V,且铼的氧化溶解较为迅速且彻底。
(pH=1)溶液中金属铼的氧化电位是0.29 V,且铼的氧化溶解较为迅速且彻底。
当过电位较高时,铼的多步骤还原过程如式(8)所示。铼发生还原反应时,M (MRe)为186.21g/mol,z为7,理论值M/z为26.57 g/mol。在铼的还原阶段,在较高过电位下时,根据实验得出的质量变化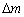 为15.62 μg,电量变化
为15.62 μg,电量变化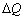 为2.74 C。根据式(9)可得,实验值M/z为0.55 g/mol。这说明在铼还原反应过程中,析氢反应占据了主导地位,析氢反应消耗了大量的电子,导致电量变化
为2.74 C。根据式(9)可得,实验值M/z为0.55 g/mol。这说明在铼还原反应过程中,析氢反应占据了主导地位,析氢反应消耗了大量的电子,导致电量变化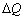 变大,进而使得实验值M/z比理论值M/z小得多,这也表现了铼的电沉积电流效率非常低。
变大,进而使得实验值M/z比理论值M/z小得多,这也表现了铼的电沉积电流效率非常低。
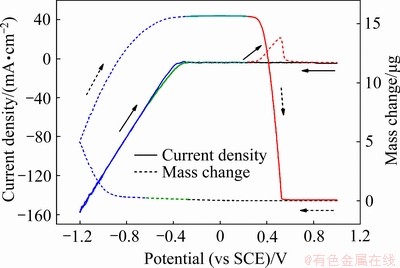
图3 在1 mmol/L  (pH=1) 溶液中的循环伏安曲线及相应的电极质量变化
(pH=1) 溶液中的循环伏安曲线及相应的电极质量变化
Fig. 3 CV curve and corresponding mass change in solutions containing 1 mmol/L  (pH=1) (Scanning rate of 10 mV/s, room temperature)
(pH=1) (Scanning rate of 10 mV/s, room temperature)
为了进一步探究溶液组成对铼的电沉积影响,利用电化学工作站进行了恒电位沉积实验。图4所示为在不同酸度条件的5.0 mmol/L  溶液中于-0.80 V下沉积5000 s的铼的恒电位沉积曲线。所有的恒电位沉积曲线都有着相同的趋势:一开始电流减小,出现一个峰值电流,随后电流逐渐增大,最后趋于稳定,这是较为典型的电结晶的特征。表1所示为在不同酸度条件下5.0 mmol/L
溶液中于-0.80 V下沉积5000 s的铼的恒电位沉积曲线。所有的恒电位沉积曲线都有着相同的趋势:一开始电流减小,出现一个峰值电流,随后电流逐渐增大,最后趋于稳定,这是较为典型的电结晶的特征。表1所示为在不同酸度条件下5.0 mmol/L  溶液中铼的电沉积电流效率。由表1可知,随着酸度的增加,电沉积产物质量增加,电流效率也增加。由此可知,铼的还原反应是一个消耗8个H+的多步骤电化学反应,在反应的开始阶段,H+的供应是足够的,所以电沉积初始阶段的电流较大。但随着反应时间的延长,H+被大量消耗,局部酸度下降,这就使得还原反应的进行变得困难,还原电流也变小,使得电沉积速率变得缓慢。这说明酸度对铼的还原反应有着较为重要的影响。
溶液中铼的电沉积电流效率。由表1可知,随着酸度的增加,电沉积产物质量增加,电流效率也增加。由此可知,铼的还原反应是一个消耗8个H+的多步骤电化学反应,在反应的开始阶段,H+的供应是足够的,所以电沉积初始阶段的电流较大。但随着反应时间的延长,H+被大量消耗,局部酸度下降,这就使得还原反应的进行变得困难,还原电流也变小,使得电沉积速率变得缓慢。这说明酸度对铼的还原反应有着较为重要的影响。
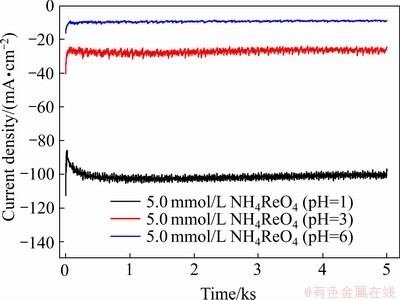
图4 在不同酸度条件下5.0 mmol/L  溶液中铼的恒电位沉积曲线
溶液中铼的恒电位沉积曲线
Fig. 4 Potentiostatic electrodeposition curves of Re in solutions containing with 5.0 mmol/L  with different pH (Electrodeposition potential of -0.80 V, electro- deposition time of 5000 s, room temperature)
with different pH (Electrodeposition potential of -0.80 V, electro- deposition time of 5000 s, room temperature)
表1 在不同酸度条件下5.0 mmol/L  溶液中铼的电解效率
溶液中铼的电解效率
Table 1 Current efficiency of Re in solutions containing with 5.0 mmol/L with different pH
with different pH
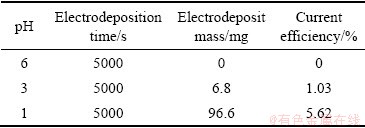
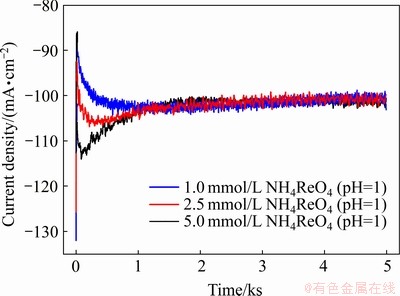
图5 在相同酸度(pH=1)条件下不同 浓度下溶液中铼的恒电位沉积曲线
浓度下溶液中铼的恒电位沉积曲线
Fig. 5 Potentiostatic electrodeposition curves of Re in solutions containing with different concentrations of  (pH=1) (Electrodeposition potential of -0.80 V, electro- deposition time of 5000 s, room temperature)
(pH=1) (Electrodeposition potential of -0.80 V, electro- deposition time of 5000 s, room temperature)
图5所示为在相同酸度(pH=1)条件下,不同 浓度溶液中在-0.80 V下的恒电位沉积曲线。表2所示为在不同
浓度溶液中在-0.80 V下的恒电位沉积曲线。表2所示为在不同 浓度下溶液(pH=1)中的电流效率,随着
浓度下溶液(pH=1)中的电流效率,随着 浓度的增加,电沉积产物质量也随之增加,电流效率也增加。这说明提高
浓度的增加,电沉积产物质量也随之增加,电流效率也增加。这说明提高 浓度是有利于铼的还原沉积。
浓度是有利于铼的还原沉积。
表2 在相同酸度(pH=1)条件下不同 浓度下溶液中铼的电解效率
浓度下溶液中铼的电解效率
Table 2 Current efficiency of Re in solutions containing with different concentrations of  (pH=1)
(pH=1)
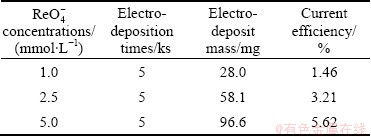
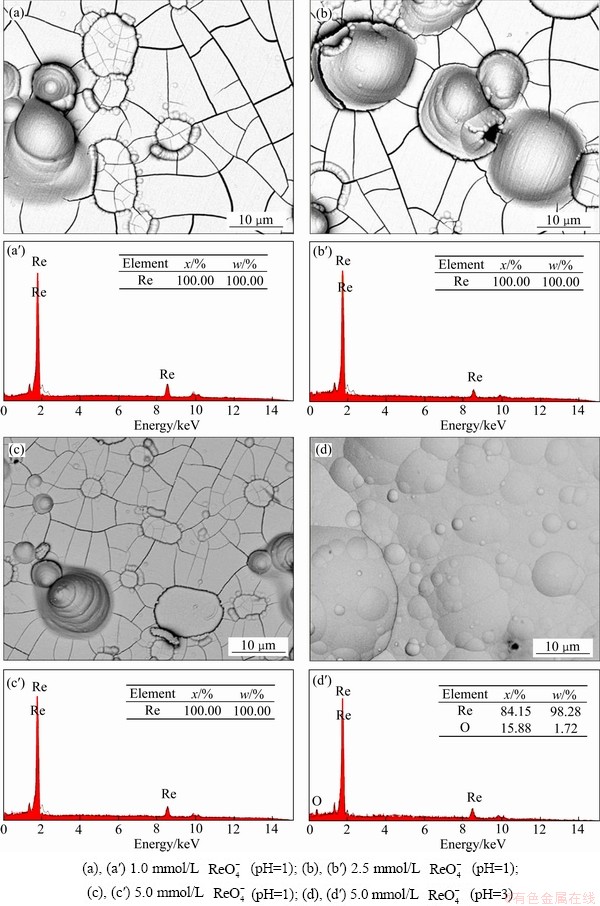
图6 在不同条件下获得的电沉积产物的微观形貌和EDS能谱
Fig. 6 SEM images((a), (b), (c), (d)) and EDS data((a′), (b′), (c′), (d′)) of electrodeposition products obtained from different composition solutions
图6所示为在不同条件下-0.80 V恒电位沉积获得的产物的微观形貌和相应的EDS元素分析。在接近中性(1.0 mmol/L  (pH=6))的溶液中,没有得到电沉积产物,故没有相应的扫描电镜图片。从电沉积产物的微观形貌来看(见图6(a)~(c)),在相同酸度(pH=1),不同
(pH=6))的溶液中,没有得到电沉积产物,故没有相应的扫描电镜图片。从电沉积产物的微观形貌来看(见图6(a)~(c)),在相同酸度(pH=1),不同 浓度的溶液中,得到的电沉积产物微观形貌相似,呈现较多局部凸起的沉积形貌,且沉积物表面布满裂纹,这和HUANG等[27]观察到的现象一致。从EDS成分分析结果可知,电沉积产物均为金属铼,纯度较高,无任何杂质;在5.0 mmol/L
浓度的溶液中,得到的电沉积产物微观形貌相似,呈现较多局部凸起的沉积形貌,且沉积物表面布满裂纹,这和HUANG等[27]观察到的现象一致。从EDS成分分析结果可知,电沉积产物均为金属铼,纯度较高,无任何杂质;在5.0 mmol/L  (pH=3)溶液中获得的电沉积产物的微观形貌(见图6(d)),也出现较多凸起的小山丘形状,但裂纹明显减少。从EDS数据来看,Re与O的摩尔比为1:0.19,电沉积产物基本是金属铼,但含有少量氧化物。另外,可能是电沉积速率较慢,所以裂纹较少。由此可知,在酸度较低(pH=3)的条件下,电沉积产物是金属铼和氧化物。
(pH=3)溶液中获得的电沉积产物的微观形貌(见图6(d)),也出现较多凸起的小山丘形状,但裂纹明显减少。从EDS数据来看,Re与O的摩尔比为1:0.19,电沉积产物基本是金属铼,但含有少量氧化物。另外,可能是电沉积速率较慢,所以裂纹较少。由此可知,在酸度较低(pH=3)的条件下,电沉积产物是金属铼和氧化物。
为了进一步确定在酸度不同的条件下沉积物的物相成分,对沉积产物进行了XRD测试,结果见图 7。在酸度较高(pH=1)的条件下,其沉积产物为金属铼,没有其他物相峰。然而,在酸度较低(pH=3)的条件下,出现了ReO3的特征峰。由此可以确定,在酸度较低的条件下,沉积产物中铼的氧化物是以ReO3的形式存在,致使其电沉积产物中存在ReO3与Re混合存在的现象。因此,酸度对电沉积产物的微观形貌和组成成分起着决定性作用,而 浓度对电沉积产物微观形貌和组成成分影响并不大。
浓度对电沉积产物微观形貌和组成成分影响并不大。
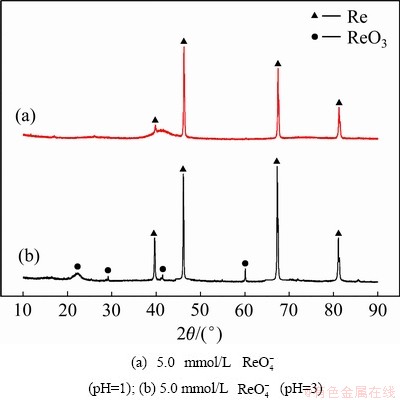
图7 在不同条件下获得的电沉积产物的XRD谱
Fig. 7 XRD patterns of electrodeposits obtained from different composition solutions
3 结论
1) 采用电化学循环伏安、恒电位电沉积和电化学石英微晶天平,对铼在酸性溶液的电沉积行为进行了探究。铼的电解还原受电解液的酸度和 的浓度影响,较高的酸度和
的浓度影响,较高的酸度和 浓度可以提高电流效率,促进铼的电沉积。在溶液接近中性(pH=6)时,金属铼难以被还原沉积出来;在酸度较低(pH=3)时,电沉积产物中含有少量铼的氧化物(ReO3);在酸度较高(pH=1)时,沉积产物的微观形貌都有裂纹,且电沉积产物都是金属铼。
浓度可以提高电流效率,促进铼的电沉积。在溶液接近中性(pH=6)时,金属铼难以被还原沉积出来;在酸度较低(pH=3)时,电沉积产物中含有少量铼的氧化物(ReO3);在酸度较高(pH=1)时,沉积产物的微观形貌都有裂纹,且电沉积产物都是金属铼。
2) 利用电化学石英微晶天平技术研究了1.0 mmol/L  (pH=1)溶液中的铼的氧化还原,确定了铼的析出电位为-0.68 V。通过恒电位沉积得出不同条件下铼的电解效率,最高可达5.62%。
(pH=1)溶液中的铼的氧化还原,确定了铼的析出电位为-0.68 V。通过恒电位沉积得出不同条件下铼的电解效率,最高可达5.62%。
REFERENCES
[1] BAIK S I, DUHIN A, PHILLIPS P J, et al. Atomic-scale structural and chemical study of columnar and multilayer Re-Ni electrodeposited thermal barrier coating[J]. Advanced Engineering Materials, 2016, 18(7): 1133-1144.
[2] MAJCHROWICZ K, PAKIELA Z, BRYNK T, et al. Microstructure and mechanical properties of Ti-Re alloys manufactured by selective laser melting[J]. Materials Science and Engineering A, 2019, 765: 138290.
[3] MAJCHROWICZ K, PAKIELA Z, MOSZCZYNSKA D, et al. Hot corrosion of Ti-Re alloys fabricated by selective laser melting[J]. Oxidation of Metals, 2017, 90(1/2): 83-96.
[4] GARCIA-GARCIA R, ORTEGA-ZARZOSA G, RINCON M E, et al. The hydrogen evolution reaction on rhenium metallic electrodes: A selected review and new experimental evidence[J]. Electrocatalysis, 2014, 6(3): 263-273.
[5] VARGAS-USCATEGUI A, MOSQUERA E, CHORNIK B, et al. Electrocatalysis of the hydrogen evolution reaction by rhenium oxides electrodeposited by pulsed-current[J]. Electrochimica Acta, 2015, 178: 739-747.
[6] WU M G, YANG J L, NG D H L, et al. Rhenium diselenide anchored on reduced graphene oxide as anode with cyclic stability for potassium-ion battery[J]. Physica Status Solidi-Rapid Research Letters, 2019, 13(10): 1900329.
[7] LO K K W. Luminescent rhenium(Ⅰ) and iridium(Ⅲ) polypyridine complexes as biological probes, imaging reagents, and photocytotoxic agents[J]. Accounts of Chemical Research, 2015, 48(12): 2985-2995.
[8] LEONIDOVA A, GASSER G. Underestimated potential of organometallic rhenium complexes as anticancer agents[J]. ACS Chemical Biology, 2014, 9(10): 2180-2193.
[9] NAOR A, ELIAZ N, GILEADI E, et al. Properties and applications of rhenium and its alloys[J]. The AMMTIAC Quarterly, 2010, 5(1): 11-15.
[10] 李文静, 黄 龙, 唐和清, 等. 铼元素分离富集研究进 展[J]. 中国有色金属学报, 2020, 30(1): 214-226.
LI Wen-jing, HUANG Long, TANG He-Qing, et al. Researches progress on separation and enrichment of rhenium[J]. The Chinese Journal of Nonferrous Metals, 2020, 30(1): 214-226.
[11] BAI M, LIU Z H, ZHOU L J, et al. Preparation of ultrafine rhenium powders by CVD hydrogen reduction of volatile rhenium oxides[J]. Transactions of Nonferrous Metals Society of China, 2013, 23(2): 538-542.
[12] LUIDOLD S, ANTREKOWITSCH H. Hydrogen as a reducing agent: State-of-the-art science and technology[J]. JOM, 2007, 59(6): 20-26.
[13] ZHULIKOV V V, GAMBURG Y D. Electrodeposition of rhenium and its alloys[J]. Russian Journal of Electrochemistry, 2016, 52(9): 847-857.
[14] VARGAS-USCATEGUI A, MOSQUERA E, CIFUENTES L. Analysis of the electrodeposition process of rhenium and rhenium oxides in alkaline aqueous electrolyte[J]. Electrochimica Acta, 2013, 109: 283-290.
[15] WANG J, BAI S, YE Y, et al. Microstructure and mechanical properties of rhenium prepared by electroforming in NaCl-KCl-CsCl-K2ReCl 6 molten salts[J]. International Journal of Refractory Metals and Hard Materials, 2018, 72: 263-269.
[16] SZABO' S, BAKOS I. Electrodeposition of rhenium species onto a gold surface in sulfuric acid media[J]. Journal of Solid State Electrochemistry, 2004, 8(3): 190-194.
[17] CAO H Z, CHAI D G, WU L K, et al. Communication—A mechanistic study on electrodeposition of rhenium from acidic solution of ammonium perrhenate[J]. Journal of The Electrochemical Society, 2017, 164(13): 825-827.
[18] USCATEGUI A V, MOSQUERA E, CIFUENTES L. Transmission electron microscopy study of electrodeposited rhenium and rhenium oxides[J]. Materials Letters, 2013, 94: 44-46.
[19] AZPEITIA L A, GERVASI C A, BOLZAN A E. Electrochemical aspects of tin electrodeposition on copper in acid solutions[J]. Electrochimica Acta, 2019, 298: 400-412.
[20] ZELINSKY A G, NOVGORODTSEVA O N. EQCM study of the dissolution of gold in thiosulfate solutions[J]. Hydrometallurgy, 2013, 138: 79-83.
[21] JEE H W, PAENG K J, SON Y, et al. Electrosynthesis of cobalt telluride thin films by voltammetry combined with electrochemical quartz crystal microgravimetry[J]. Journal of The Electrochemical Society, 2018, 166(5): H3035-H3039.
[22] HONG B, WANG Y, WEI X, et al. EQCM study on the electrochemical redox behavior of gallium in alkaline solution[J]. Hydrometallurgy, 2020, 194: 105344.
[23] ZHANG J, AN M Z, CHEN Q, et al. Electrochemical study of the diffusion and nucleation of gallium(Ⅲ) in [Bmim] [TfO] ionic liquid [J]. Electrochimica Acta, 2016, 190: 1066-1077.
[24] MENDEZ E, CERDA M F, LUNA A M C, et al. Electrochemical behavior of aqueous acid perrhenate-containing solutions on noble metals: Critical review and new experimental evidence[J]. Journal of Colloid and Interface Science, 2003, 263(1): 119-132.
[25] KANAMURA S, MIZUGUCHI K, FUJITA R, et al. Electrodeposition of rhenium species at a stainless steel electrode from acidic, neutral, and alkaline solutions[J]. Journal of The Electrochemical Society, 2013, 161(3): D92-D96.
[26] SCHREBLER R, CURY P, SUAREZ C, et al. Study of the electrodeposition of rhenium thin films by electrochemical quartz microbalance and X-ray photoelectron spectroscopy[J]. Thin Solid Films, 2005, 483(1/2): 50-59.
[27] HUANG Q, LYONS T W. Electrodeposition of rhenium with suppressed hydrogen evolution from water-in-salt electrolyte[J]. Electrochemistry Communications, 2018, 93: 53-56.
Electrodeposition of rhenium from acidic solution
HONG Biao1, 2, WANG You-bin1, 2, LI Feng2, WEI Yue-zhou1, 2, Toyohisa FUJITA1, 2
(1. Guangxi Key Laboratory of Processing for Non-ferrous Metal and Featured Materials, Guangxi University, Nanning 530004, China;
2. School of Resources, Environment and Materials, Guangxi University, Nanning 530004, China)
Abstract: The electrodeposition of rhenium from acidic solution was studied by cyclic voltammetry, potentiostatic electrodeposition and electrochemical quartz microcrystal balance (EQCM). The results show that metal rhenium is difficult to be reduced from near neutral solutions (pH=6); the electrodeposition product contains rhenium oxide (ReO3) from solutions with low acidity (pH=3); In solutions with high acidity (pH=1), the electrodeposition product is metallic rhenium, increasing the concentration of  can promote the electrodeposition of rhenium, while there are obvious cracks in the morphology of metal rhenium. The electrodeposition potential of rhenium is determined to be -0.68 V in solutions containing 1.0 mmol/L
can promote the electrodeposition of rhenium, while there are obvious cracks in the morphology of metal rhenium. The electrodeposition potential of rhenium is determined to be -0.68 V in solutions containing 1.0 mmol/L  (pH=1). Current efficiency for different conditions is up to 5.62%, obtained by potentiostatic electrodeposition.
(pH=1). Current efficiency for different conditions is up to 5.62%, obtained by potentiostatic electrodeposition.
Key words: rhenium; electrodeposition; current efficiency; electrochemical quartz microcrystal balance
Foundation item: Project(11975082) supported by the National Natural Science Foundation of China; Project (AA17204100) supported by Guangxi Science and Technology Major Project, China
Received date: 2020-01-20; Accepted date: 2020-10-20
Corresponding author: WANG You-bin; Tel: +86-771-3224332; E-mail: wangyoubin@gxu.edu.cn
(编辑 龙怀中)
基金项目:国家自然科学基金资助项目(11975082);广西科技重大专项(AA17204100)
收稿日期:2020-01-20;修订日期:2020-10-20
通信作者:王友彬,讲师,博士;电话:0771-3224332;E-mail:wangyoubin@gxu.edu.cn
摘 要:采用电化学循环伏安法、恒电位沉积法和电化学石英微晶天平(EQCM)研究铼在酸性溶液中的电沉积行为。结果表明:当溶液接近中性(pH=6)时,金属铼难以被还原出来;当溶液酸度较低(pH=3)时,电沉积产物含有铼的氧化物(ReO3);当溶液酸度较高时(pH=1),电沉积产物为金属铼,所得产物的微观形貌中存在明显的裂纹;且随着溶液中 浓度的增加,铼的电沉积得到促进。在1.0 mmol/L
浓度的增加,铼的电沉积得到促进。在1.0 mmol/L (pH=1)溶液中铼的沉积电位为-0.68 V。通过恒电位沉积得出不同条件下的电解效率,最高可达5.62%。
(pH=1)溶液中铼的沉积电位为-0.68 V。通过恒电位沉积得出不同条件下的电解效率,最高可达5.62%。


