
Debinding of stainless steel foam precursor with 3-D open-cell network structure
LI Chang-lin(李昌林), WANG Hui(王 辉), ZHOU Xiang-yang(周向阳),
LI Jie(李 劼), LIU Hong-zhuan(刘宏专)
School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
Received 13 July 2009; accepted 25 May 2010
Abstract:
The thermal debinding behavior of stainless steel foam precursor in vacuum was studied and compared with that in hydrogen. The formation cause of pore channel was analyzed. The experiment results show that the binder removal rate in vacuum is higher than that in hydrogen. In vacuum, the organic compounds can be removed effectively without change of pore size and the pore morphology for the sample. After pre-sintering, some sintering necks form and the sample has certain intensity. The initial surface pore forms with the temperature increasing at first, and then the internal melting binder is aspirated to form initial pore because of the capillary force and the metal powders re-arrange with the migration of binder at the same time.
Key words:
precursor; stainless steel foam; vacuum; thermal debinding; pore channel;
1 Introduction
The porous foam metal has many excellent characteristics, such as big aperture, high porosity and low density[1-2]. Moreover, its physical properties, such as filtration, antivibration, damping, sound absorption, sound insulation, radiation, absorption impactive energy and electromagnetic shield, make it possible to serve as the structural material and the functional material[3-5]. Therefore, the porous foam metal has obtained more and more widespread application[6-8]. Due to higher melting point and excellent anti-corrosive quality, porous metallic materials with 3-D open-cell network structure can be used as catalyst carriers in purifying automotive exhaust gases due to their high interface area between the catalysts and the gases to be reacted, or used as filters in cleaning solution or melt containing particles. Recently, ZHOU et al[9] focused on researching the stainless steel foams with 3-D open-cell network structure, and found many preparation approaches.
The debinding of precursor is the key in the preparation process. The inappropriate debinding approach usually results in the defects, such as distortions, collapses and cracks. So, how to remove the organic compounds completely with the precursor is very important. Some common debinding approaches, such as thermal debinding, catalyzed debinding and solvent debinding and the thermal debinding approach are most commonly used[10-11]. The debinding atmosphere and the conditions should be controlled strictly because the stainless steel powder is easy to oxidize. And the polyurethane sponge has the urethane structure to resist the corrosion of many kinds of acid and alkali and the organic solvent, therefore, the thermal debinding approach in vacuum is very effective. The thermal debinding behavior of precursor in vacuum was studied and compared with that in hydrogen.
2 Experimental
2.1 Experimental process
The stainless steel powders were the main material used in this study. Their chemical analysis results are shown in Table 1. Other materials were polyurethane sponge (0.8 pore/mm) and polyvinyl alcohol. The preparation process of stainless steel foam precursor with 3-D open-cell network structure could be seen in Ref.[9]. The thermal debinding approach in vacuum was used in this study, the temperature program was that the sample was heated up to 600 °C by 1 °C /min and kept warm for 60 min. In the process, the samples were separately kept warm at 250 °C and 400 °C for 30 min, and then they were pre-sintered at 800 °C to enable then certain intensity.
Table 1 Chemical composition of 316L stainless steel
2.2 Testing methods
Thermal analysis instrument (SDT Q600) was used to investigate the thermal decomposition process of polyvinyl alcohol, polyurethane sponge and precursor. In addition, the digital camera (SONY, DSC-P10) and scanning electron microscope (JEOL, JSM5600) were employed to observe the macro-shape and section morphology of samples, respectively.
3 Results and discussion
3.1 DSC-DTA testing of polyvinyl alcohol, polyurethane sponge and precursor
The DSC-DTA curves of polyvinyl alcohol, polyurethane sponge and precursor are schematically shown in Fig.1. It can be seen from Fig.1(a), there is an obvious mass loss rate of 56.14% between 205.59 °C and 280.97 °C, in which the polyvinyl alcohol decomposed; and the mass no longer changes after 501.24 °C. As can be seen from Fig.1(b) that the mass loss of polyurethane sponge mainly occurs at 263.37-317.85 °C and 317.85-419.03 °C. The first stage at 306.63 °C has an obvious heat absorption peak, and its mass loss rate is 28.27%; the second stage at 384.14 °C also has an obvious heat absorption peak, and its mass loss reaches 71.76%. Apparently, it can be easily seen from Fig.1(c), from the room temperature to 129.20 °C, the mass loss rate is about 0.3488%. The reason maybe is that there is a few free moistures in precursor. The mass loss rate is 13.971% between 223.00 °C and 503.34 °C due to the decomposition of polyvinyl alcohol and polyurethane sponge.

Fig.1 DSC-DTA curves of polyvinyl alcohol (a), polyurethane sponge (b) and precursor (c)
In decomposition process, the polyvinyl alcohol and polyurethane sponge will produce the massive gas which will bring about stress in the precursor. Therefore, the rising temperature velocity between 200 °C and 600 °C should be controlled. Otherwise, the defects, such as distortions, collapses and cracks, may generate.
3.2 Thermal debinding of precursor in vacuum
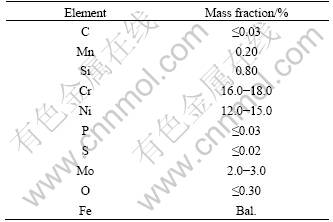
Fig.2 shows the macro-morphologies of precursor before and after debinding. It can be seen from Fig.2 that the organic compounds could be removed effectively without change pore size and morphologies for the sample. After pre-sintering, some sintering necks form and the sample has certain intensity (see Fig.3). The mass variation was surveyed in the debinding and the pre-sintering process. Fig.4 shows the mass variation of precursor in debinding and pre-sintering process. It can be seen from Fig.4 that when the temperature is between 600 °C and 800 °C, the curve is horizontal, which indicates that the organic compounds in precursor can decompose completely by the thermal debinding approach in vacuum.
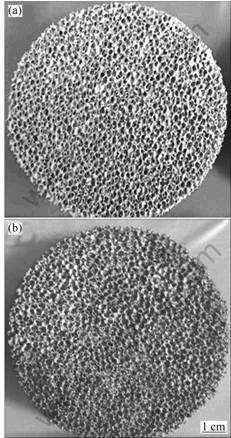
Fig.2 Macro-morphologies of precursor before (a) and after (b) debinding
3.3 Effects of atmosphere on debinding process
In order to understand the debinding process more clearly, the thermal debinding behavior of precursor in vacuum was compared with that in hydrogen. Fig.5 shows the mass variation in different atmosphere (the experimental condition was that sample was heated to different temperature at 1 °C/min and kept warm for 30 min).
Apparently, it can be seen from Fig.5 that in vacuum and the hydrogen atmosphere, the debinding tendencies are similar. With the temperature rising, the debinding quantity increases unceasingly, but the binder removal rate in vacuum is faster than that in hydrogen. The reason may be that, in vacuum condition, the environment pressure approaches zero throughout debinding process, which is much lower than the saturated steam pressure of binder, causing the binder to evaporate unceasingly to the external environment. And with the temperature rising, the saturated steam pressure increases, thus the evaporation velocity also correspondingly increases quickly, making the debinding quantity increase; but in hydrogen atmosphere, the difference between saturated steam pressure of binder and external environment pressure is relatively slight, so that the evaporation velocity in hydrogen atmosphere is smaller than that in vacuum.
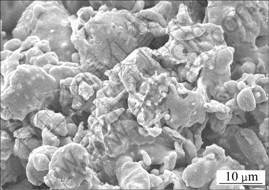
Fig.3 SEM image of cross section of precursor after debinding
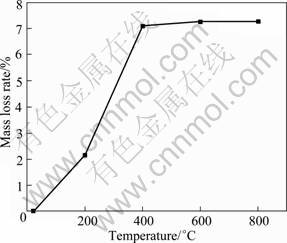
Fig.4 Mass variation of precursor in debinding and pre-sintering process with temperature
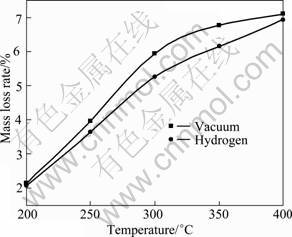
Fig.5 Mass variation of precursor with temperature in different atmosphere
3.4 Formation reason of pore channel
In the debinding process, the binder needs certain channel to remove. The rising temperate rate in the low temperature and the warm-keeping stages has great impact on the debinding velocity. At low temperature, the binder evaporates and the small pore channel forms through the diffusion, at this time, the evaporation process is the dynamical equilibrium process between molten mass and gaseous state of binder, the evaporation velocity in fact is the difference between the gasifying velocity and the congealment velocity, and then the evaporation velocity νw is defined as follows[12]:
![]() (1)
(1)
where ce is the saturated steam concentration of liquid binder; cw is the environment steam concentration; kw is the evaporation velocity constant of liquid binder; pe is the saturated steam pressure of liquid binder; pw is the environment steam pressure; R is the gas constant; T is the thermodynamic temperature; ν1 is the gasifying velocity; ν2 is the congealment velocity; νw is the evaporation velocity of binder. Therefore, the apparent evaporation velocity of binder is decided by the temperature as well as the difference between saturated steam pressure and the environment steam pressure. Only when the saturated steam pressure is higher than the environment steam pressure, the binder can be evaporated. From the microscopic analysis, in the low temperature debinding process, the molten binder flows with difficulty because of the function of the surface adsorption and surface tension in the debinding process at low temperature. At this time, the molten binder is evaporated depending on the increase of the temperature mainly, and afterwards, the pore channel forms in precursor. The main process may be comprise the formation of initial surface pore and the rearrangement of metal powders along the migration of binder in initial pore.
There are many metal powders with different particle sizes on the surface of precursor, which is not smooth from microcosmic view. Therefore, the crevice will form between the powders, and when the blinder filled in, the capillary vessel’s phenomenon produces[13]. With the debinding temperature rising, the binder starts to melt and transforms gaseous state to volatilize from the small crevice at certain temperature.
After the initial surface pore forms, the internal melted binder will be aspirated as a result of capillary force. With the flowing of binder from the interior to the surface, the internal small metal powders flow to the surface. According to the formula of capillary force, the capillary force and radius of crevice are in reverse proportion, and the suction in small crevice place is intense, attracting the binder in big crevice to the small crevice.
![]() (2)
(2)
where рs is the additional pressure for capillary vessel; γ is the liquid surface tension; r is the radius of crevice.
According to the formation theory of small aperture, there maybe many tiny holes on the surface of sample, and as a result of the capillary force, the internal binder will be attracted to the surface to form the initial pore. At the same time, the internal small metal powders flow to the outside surface along with the following of binder, causing the quantity of small metal ball on the surface increase (see Fig.6). The volume of metal ball is less, its surface energy is higher, therefore, this mobile tendency of small metal ball is advantageous to the densification of sintering[14].

Fig.6 SEM image of surface of precursor near pore after debinding
After the initial pore forms, its internal surface will be similar to the surface of sample, in which the new pore will appear, forming the massive connective pore channel in the sample. At the end, the binder will remove outward unceasingly along these pore channels[15].
4 Conclusions
1) The organic compounds can be removed effectively without change of size and pore morphology for sample by the thermal debinding approach in vacuum. After pre-sintering, some sintering necks form and enable the sample to have certain intensity.
2) The thermal debinding of precursor in vacuum was compared with that in hydrogen, the binder removal rate in vacuum is faster than that in hydrogen.
3) In the debinding process, the binder needs certain channel for migration. At first, the initial surface pore forms on the surface of sample. As a result of capillary force, the internal melted binder is aspirated; at the same time, the metal powders rearrange with the migration of binder, which is advantageous to the densification of sintering.
References
[1] LEFEBVRE L P, BANTHART J, DAVID C D. Porous metals and metallic foams: Current status and recent developments [J]. Advanced Engineering Materials, 2008, 10(9): 775-787.
[2] TETSUJ M, MASAO I. Alporas aluminum foam: production process,properties and applications [J]. Advanced Engineering Materials, 2000, 2(4): 179-183.
[3] THOMAS K M. Hydrogen adsorption and storage on porous materials [J]. Catalysis Today, 2007, 120: 389-398.
[4] LIU Yu, GONG Xiao-lu. Compressive behavior and energy absorption of metal porous polymer composite with interpenetrating network structure [J]. Transactions of Nonferrous Metals Society of China, 2006, 16: 439-443.
[5] ZHANG Bo, CHEN Tian-ning. Calculation of sound absorption characteristics of porous sintered fiber metal [J]. Applied Acoustics, 2009, 70: 337-346.
[6] MAO Chun-sheng. Froth metal—— A new type of material used in cars [J]. World Nonferrous Metals, 2006 (4): 17-19. (in Chinese)
[7] LU Tian-jian, HE De-ping, CHEN Chang-qing, ZHAO Chang-ying, FANG Dai-ning, WANG Xiao-ling. The multi-functionality of ultra-light porous metals and their applications [J]. Advances in Mechanics, 2006, 4(36): 517-535. (in Chinese)
[8] BANTHART J. Manufacture, characterisation and application of cellular metals and metal foams [J]. Progress in Material Science, 2001, 46: 559-632.
[9] ZHOU Xiang-yang, LI Jie, DING Feng-qi, LIU Hong-zhuan, LONG Bo. The preparation means of the porous metal foam with 3-D open cells or portion cells connected with each other: CN 200510032174. 7[P]. 2007-03-28.
[10] GERMAN R M. Theory of thermal debinding [J] .Inter J Power Metallurgy, 1987, 23(4): 237-245.
[11] FINN C W. Vacuum binder removal and collection [J]. Inter J Power Metallurgy, 1991, 27(2): 127-132.
[12] LIU Shao-jun, QU Xuan-hui, LI Yi-min, HUANG Bai-yun. Thermal debinding processing of metal powder injection molding compacts in vacuum [J]. Materials Science and Engineering of Powder Metallurgy, 1999, 4(3): 179-184. (in Chinese)
[13] ZHAO Li-gang, LI Yi-min. Thermal debinding behavior of the initial stage in MIM [J]. Materials Science and Engineering of Powder Metallurgy, 2002, 7(3): 175-179. (in Chinese)
[14] HIROSHI O, KEIICHI M, KIMIHIRO N. Deformation behavior of metal injection molded compacts during sintering [M]. Princeton: Metal Power Industries Federation, 1992: 100-108.
[15] HANS H A, OMER O V D B. Low temperature debinding kinetics of two-component model systems [J]. Inter J Power Metallurgy, 1993, 29(3): 239-249.
(Edited by LI Yan-hong)
Foundation item: Project(50974136) supported by the National Natural Science Foundation of China; Project(CX2009B037) supported by the Graduate Degree Thesis Innovation Foundation of Central South University, China
Corresponding author: ZHOU Xiang-yang; Tel: +86-731-88836329; E-mail: zxy13908482918@163.com
DOI: 10.1016/S1003-6326(10)60652-8


