
Bioactivity of mica/apatite glass ceramics
FENG Yun-Zhi(冯云枝)1, WANG Ya-chong(王压冲)1, TAN Yan-ni(谭燕妮)2,
LIU Yong(刘 咏)2, XIAN Qi-jun(向其军)2, SHENG Xiao-xian(盛小娴)2
1. Department of Stomatology, Xiangya Second Hospital, Central South University, Changsha 410011, China;
2. State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China
Received 21 March 2007; accepted 31 May 2007
Abstract:
The bioactivity of mica/apatite glass ceramic composites, including the in vitro behavior in simulated body fluid and the histological appearance of the interface between the mica/apatite glass ceramics and the rabbit mandible defect in vivo under a dynamic condition. The results show that biological apatite layer forms on the surface of the mica/apatite glass ceramics after 1 d of immersion in the simulated body fluid, and becomes dense after 14 d. In vivo tests indicate that bone formation occurs after implantation for 14 d, and strong bonding of bone to the implant occurs after 42 d. No aseptic loosening occurs during 42 d of implantation. The finding shows that mica/apatite glass ceramics have good bioactivity and osteoconductivity for constructing bone graft, and can be promising for biomedical application.
Key words:
mica/apatite glass ceramics; bioactivity; osteoconductivity;
1 Introduction
Bone defects are widespread diseases found in clinical research, and nowadays, there is no satisfactory method to treat segment bone defect. Although the autograft was used to solve this problem, there are limitations in the quantity of bone available. To overcome the limited availability of natural bone graft materials for hard tissue repair and replacement, various synthetic biomaterials have been developed[1-2]. There are several basic requirements for bone substitutes: biocompatibility, mechanical integrity and osteoconductivity. Bioactive ceramic is used in clinics because it possesses excellent biocompatibility and bioactivity[3-4]. However, the brittleness limits its clinical applications. To solve the problem, the authors had developed several new types of mica/apatite glass ceramic composites. Our previous work has shown these materials have good mechanical strength and proper modulus[5]. This study aimed to investigate their biocompatibility and bioactivity.
2 Experimental
The chemical reagents of CaCO3, CaHPO4, CaF2, SiO2, Al2O3, MgO and NaF were mixed and the nominal chemical compositions were calculated according to the mass ratio of stoichiometric fluoroapatite to stoichiometric mica. The nominal compositions of the mica/apatite glass ceramics specimens are mica 80%, apatite 20% (mass fraction).
The glass-ceramics were prepared by two-stepped melting[6]. At first, the raw powders were mixed for 6 h, and then the mixtures were melted at 1 500 ℃ for 1.5 h and quenched in water. The quenched glass was milled and re-melted at 1 500 ℃ for 2 h. The melt was poured into a graphite mold, which was heated to 900 ℃. The as-cast glass-ceramics were immediately put into a furnace heated to 650 ℃ for 1 h, in order to release the thermal stress. The glass ceramics were then heat treated at 925 ℃ for different time, because the differential scanning calorimeter analysis showed that the peak temperature of crystallization of mica phase was 916 ℃.
The materials were shaped into columns with a size of d 1 mm×3 mm. In vitro experiments were carried out in simulated body fluid at constant temperature of 37 ℃ for 14 d. The ion compositions of the simulated body fluid are listed in Table 1.
Table 1 Ion concentration in blood plasma and simulated body fluid
For in vivo study, ten New Zealand mature rabbits (age 6-8 years; mass 0.98-1.25 kg) were used under an experimental protocol approved board. Anesthesia was induced and maintained by Pentobarbital (30 mg/kg) throughout the operation.
All the mica/apatite glass ceramic composites were sonicated for 30 min. The tools used in the surgery were sterilized. Every rabbit underwent surgery to form columnar bone defect in both sides of mandible molar region, which was load-borne, by drilling d 1 mm holes, and then different composites were implanted in the defects. During the procedure, 5% sodium solution was used to cool the drill, avoiding the injury of osteoblast. After that, 5% sodium solution was also used to wash the surgical region. The next step was to cover the region with periosteum and the layer muscles. The next three days, all the rabbits received 800000? Penicilin injections twice a day. Two rabbits were killed at 14, 28, 42 d respectively and the mandible was removed. Then the mandible segments were fixed with 20% Formaldehyde immediately. Radiographic scans were performed. After all the specimens were fixed in 20% Formaldehyde for about one month, they were routinely embedded and the operative segments were subjected to undecalcified processing. Histological evaluation was performed using scanning electron microscope(SEM) and energy dispersive spectrum(EDS) analysis.
3 Results
Fig.1 shows the microstructure of the mica/apatite glass ceramics. The fluoroaptites crystals are needle-like with diameters smaller than 1 mm and the matrix is mica glass ceramics. The morphology of the apatites is very similar to that in human bones.

Fig.1 Microstructure of mica/apatite glass ceramics
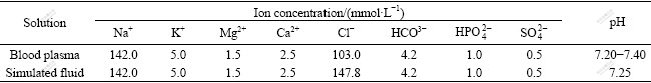
After immersion in simulated body fluids for 1 d, biological apatite can be found on the surface of the glass ceramics. With immersion time prolonging, the amount of biological apatite increases, and after immersion for 14 d, dense biological apatite layer forms, as shown in Fig.2.
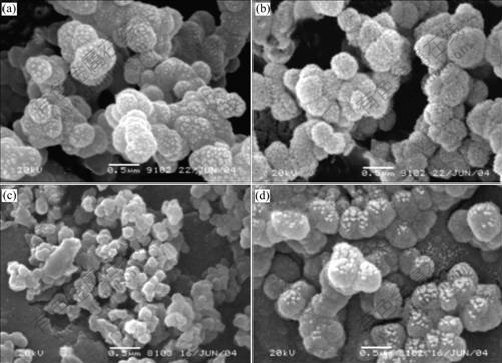
Fig.2 Biological apatite layer formed on surface of mica/apatite glass ceramics after immersion: (a) 1 d; (b) 3 d; (c) 7 d; (d) 14 d
During in vivo experiments, all the animals tolerate the surgery and remain alive throughout the observation periods with no evidence of severe pain or neurologic impairment. All the survival rabbits put on weight during this period.
At the second week, the glass ceramic segments adhere to chewing muscles, but are easily separated. Four weeks later, muscle fiber adhered to the segments is lined regularly as before and is hard to be removed from the surface of materials. At the sixth week, muscle fiber on the surface is almost the same to that two weeks ago. During the process, no aseptic loosing occurs within six weeks of observation even under a dynamic condition.
Postoperative radiographs show that no positional change occurs in any of the implanted prosthesis, and there is little evidence of loosing at the interface between the implant and the mandible. Two weeks later, bone formation at the interface in the radiograph is seen, while after four weeks, there is obvious bone formation at the interface (Fig.3). Six weeks later, bone-bonding at the whole interface occurs.
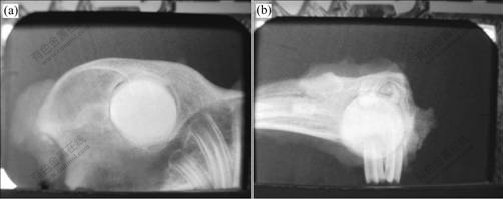
Fig.3 Postoperative radiographs showing bone formation between glass-ceramics and bone tissue: (a) 14 d; (b) 42 d
After two weeks, mineralized bone ingrowth continuous with mandibular bone is found at the interface, as shown in Fig.4. So the specimens at the fourth week are identified as positive for bone ingrowth. The depth of bone ingrowth is within a few layers from the surface, and ingrowth bone is interlocked with mandibular bone.
Fig.4 Interface between glass ceramics and bone tissue
4 Discussion
In the development of an artificial bone replacement, the prosthesis must be determined to possess certain indispensable characteristics associated with osteoinductive, osteoconductive, biocompatibility, and long-term durable fixation to the bone. Furthermore, the interface between biomaterial and its host tissue is critical in evaluating its suitability for bone replacement.
The bioactive glass ceramic is preferred as implant materials because of its convenience in adjustment of mechanical properties by controlling heat treatment processes. For implanting in human body, the machinability of glass ceramics is of great importance because implants of different dimension and shape are always required for the demand of different cases. Mica-based glass ceramics hence are studied extensively recently. There are many factors influencing their bioactivity, biocompatibility and machinability, including phase composition, structure defects and crystallinity. In our previous work[5-7], mica/apatite glass ceramics with different contents of fluoroapatite (Ca10(PO4)6F2) and fluorophlogopite (mica) (NaMg3(AlSi3O10)F2), were prepared. The transverse rupture strength and elastic modulus of mica/apatite glass ceramics obtained are in the range of those of human bones.
The in vitro and in vivo results in this work show that the mica/apatite glass ceramics have very good biocompatibility and bioactivity. OONISHI et al[8] and WILSON et al[9] reported that new bone formation occurred much faster and more completely in bony defects filled with bioglass implants than in those filled with hydroxyapatite. A similar study using a different preparation of bioglass revealed a higher amount of bone formation in the bioactive glass than in hydroxyapatite[10]. Bioglass implants seem to have osteoproductive qualities. This means that the material does not simply serve as a scaffold for bone formation but seems to stimulate osteoblast activity. It is postulated that the slow release of silicon produced as the graft particles is consumed and then promotes an autocrine response with enhancement of osteoblastic activity. Therefore, bioactive glass ceramics have been used clinically as a synthetic bone substitute in orthopedic, dental, oral and maxillofacial surgery[11-16].
The present study shows that mica/apatite glass ceramics gain a strong ability to form biological bonding with host tissue via an apatite layer between the tissue and materials. The histologic findings of this study indicate that the implants can get synosteosis with mandible. There is no histologic evidence of a significant inflammatory reaction surrounding the implants material, which suggests good tissue compatibility. There is no obvious evidence of reduction of the overall size of the graft substance and newly formed bone, although longer study duration will be necessary to demonstrate no long-term resorption.
5 Conclusions
1) Both in vitro and in vivo experiments show that the mica/apatite glass ceramics have excellent biocompatibility and bioactivity. In vitro, biological apatites form on the surface of the mica/apatite glass ceramics after 1 d of immersion in simulated body fluid and dense layer can be found after immersion for 14 d. In vivo, bone formation can be visible after implantation for 14 d, and bone ingrowth occurs after 42 d.
2) The results of proper wound-healing, tissue compatibility, and new bone formation capability indicate that mica/apatite glass ceramics can be a valuable resource as graft materials for bone replacement.
References
[1] TESSIER P, KAWAMOTO H, MATTHEWS D, POSNICK J, RAULO Y, TULASNE J F, WOLFE S A. Autogenous bone graft and bone substitutes—tools and techniques (I): A 20,000-case experience in maxillofacial and craniofacial surgery [J]. Plastic and Reconstructive Surgery, 2005,116(5): 6-24.
[2] LIU Yong, LIU Fang, ZHOU Ke-chao, HUANG Bai-yun. HA/Ti composite for biomedical application by mechanical milling [J]. Trans Nonferrous Met Soc China, 2003, 13(1): 60-64.
[3] HENCH L L, WEST J K. Biological applications of bioactive glasses [J]. Life Chem Rep, 1996, 13: 187-241.
[4] HENCH L L. Bioceramics: Material characteristics versus in vivo behavior [J]. Ann NY Acad Sci, 1988, 523(11): 1-298.
[5] LIU Yong, SHENG Xiao-xian, DAN Xiao-hong, XIANG Qi-jun. Preparation of mica/apatite glass ceramics biomaterials [J]. Mater Sci Eng C, 2006, 26(8): 1390-1394
[6] XIANG Qi-jun, LIU Yong, SHENG Xiao-xian, DAN Xiao-hong. Preparation of mica-based glass-ceramics with needle-like fluoroaptite [J]. Dental Materials, 2007, 23(2): 251-258.
[7] LIU Yong, HUANG Su-ping, DAN Xiao-hong, ZHOU Ke-chao. Growth of hydroxyapatite crystal in the presence of organic film [J]. Journal of Materials Science and Technology, 2004, 20(2): 223-226.
[8] OONISHI H, KUSHITANI S, YASUKAWA E, IWAKI H. Particulate bioglass compared with hydroxyapatite as a bone graft substitute [J]. Clin Orthop, 1997, 364(4): 316-325.
[9] WILSON J, LOW S, FETNER A. Bioactive materials for periodontal treatment: A comparative study [J]. Biomaterials and Clinical Applications, 1987(8): 223-228.
[10] CANCIAN D C, HOCHULI-VIEIRA E, MARCANTONIO R A, MARCANTONIO E. Use of biogram and calcite in bone defects: Histologic study in monkeys (cebus apella) [J]. International Journal of Oral and Maxillofac Implants, 1999,14(6): 859-864.
[11] LOVELANCE T B, MELLONING S T, MEFFERT R M, JONES A A, NUMMIKOSKI P V, COCHRAN D L. Clinical evaluation of bioactive glass in treatment of periodontal osseous defects in human [J]. J Periodontol, 1998, 69(9): 1027-1035.
[12] LOW S B, KING C, KRIEGER J. An evaluation of perioglass for the treatment of infrabony osseous defects [J]. International Journal of Periodontics and Restorative, 1997, 17: 359-367.
[13] QUINONES C R, LOVELACE T B. Utilization of a bioactive synthetic particulate for periodontal therapy and bone augmentation techniques [J]. Pract Periodont Aesthet Dent, 1997, 9: 1-7.
[14] SHAPOFF C A, ALEXANDER D C, CLARK A E. Clinical use of a bioactive glass particulate in the treatment of human osseous defects [J]. Compendium Cont Educ Dent, 1997, 18(4): 352-354.
[15] PELTOLA M, SUONP?? J, AITASALO K, VARPULA M, YLI-URPO A, RISTO-PEKKA H. Obliteration of the frontal sinus cavity with bioactive glass [J]. Head and Neck, 1998, 20(4): 315-319.
[16] PELTOLA M J, SUONP?? J T K, ANDERSSON ? H, M??TT?NEN H S, AITASALO K M J, YLI-URPO A, LAIPPALA P J. In vitro model for frontal sinus obliteration with bioactive glass [J]. Journal of Biomedical Materials Research, 2000, 53(2): 161-166.
Foundation item: Project(2003BA310A31) supported by Ministry of Science and Technology, China
Corresponding author: FENG Yun-zhi; Tel: +86-731-5295055; E-mail: fyz660303@163.com


