J. Cent. South Univ. (2017) 24: 782-788
DOI: 10.1007/s11771-017-3480-2

Precipitation and decomposition behaviors of carbides in AISI M2 high-speed steel with nitrogen and mischmetal
LIU Bao-long(刘宝龙)1, 3, L Zhi-qing(吕知清)1, 2, FENG Wei-wei(冯唯伟)3,
Zhi-qing(吕知清)1, 2, FENG Wei-wei(冯唯伟)3,
REN Ting-zhi(任廷志)1, FU Wan-tang(傅万堂)2
1. College of Mechanical Engineering, Yanshan University, Qinhuangdao 066004, China;
2. State Key Laboratory of Metastable Material Science and Technology, Yanshan University,
Qinhuangdao 066004, China;
3. China First Heavy Industries, Qiqihar 161042, China
 Central South University Press and Springer-Verlag Berlin Heidelberg 2017
Central South University Press and Springer-Verlag Berlin Heidelberg 2017
Abstract:
The behaviors of the precipitation and decomposition of carbides in AISI M2 high-speed steel modified by nitrogen and mischmetal were investigated using DSC, XRD, SEM and TEM. The as-cast microstructure of the experimental steel consists of dendrites of iron matrix, networks of eutectic carbides and secondary carbides. The average distance between networks is about 34 μm. The carbides mainly include M2C, M(C,N) and M6C, and their relative contents are 58.5%, 30.3% and 11.2%, respectively. The average spacing between the M2C fibers is 1.5 μm. The decomposition of M2C occurs from 897.2 to 1221.5 °C (heating rate of 200 °C/h). Some precipitated carbide particles occur in the M2C matrix after holding for 15 min at 1100 °C. With increasing holding time, the carbide fibers neck down more and more obviously until they are broken down. The spectral peaks of M2C almost disappear after holding for 60 min. The spectral peaks of M6C gradually strengthen with the holding time, and the relative content of M6C increases to 79.8% after holding for 60 min. After holding for 180 min, the carbide fibers disappear, and the decomposition products consist of fine carbide particles (about 300 nm) and short rod-like carbides (about 3.5 μm).
Key words:
high-speed steel; metastable carbides; decomposition; phase transformation refinement;
1 Introduction
High-speed steels (HSSs) are high-carbon and high- alloy steels and are widely used in the manufacture of cutting tools, molds, and rollers due to the special features of these steels, such as high hardness, strength, red hardness, and good wear resistance, combined with a correspondingly high toughness. These excellent properties result from a well-defined balance of the alloying elements including carbon, chromium, tungsten, molybdenum, vanadium, and cobalt [1-4]. Carbides also play an important role in HSSs, which can obviously enhance the mechanical and wear-resistance properties of materials [2-6]. The variations of carbide types and distribution in steels result in differences in the properties of materials. Different kinds of eutectic carbides are generated by the decomposition of liquid, mainly including M2C, M6C and MC, depending on the chemical compositions and solidification conditions of HSSs [7-10]. As for the most common M2 high speed steel (HSS), the M2C type eutectic carbides with hexagonal close-packed structure are the predominant type in as-cast microstructure [8, 9]. During the solidification of HSSs, M2C eutectic carbides generally form coarse networks, which are harmful to the properties of steels [3-6, 8]. Different methods have been tried to improve the distribution and homogeneity of carbides in HSSs, such as modification, heat treatment, and forging [3-6, 11]. As important modifiers, mischmetal and nitrogen have been extensively used, which can increase the hot plasticity and refine the networks of eutectic carbides as well as promoting the spheroidization of carbides [3, 11, 12]. During heat treatment, M2C carbides are metastable and are decomposed into M6C and MC, which improve the microstructural homogeneity of the steels [1, 4, 5, 13- 15]. The transformation process has been described as M2C+γ(Fe)→M6C+MC, and the morphology of carbides has also been studied in M2 HSSs in previous studies; however the transformation mechanism and decomposition behaviors of carbides in modified HSSs with mischmetal and nitrogen are little known and have rarely been reported so far.The main objective of this work is to investigate the behaviors of the precipitation and decomposition of carbides in M2 HSS with nitrogen and mischmetal. The types and relative contents of carbides in the steel are analyzed. The effects of nitrogen and mischmetal on carbides and the refinement behaviors of eutectic carbides are also discussed.
2 Experimental procedure
The parent material was an ingot of industrial M2 HSS that was remelted in a 25 kg vacuum induction melting furnace. Mischmetal (Ce-La, RE) and nitrided ferrochrome were added when the molten steel was ready. The ingot with riser was produced by casting the molten steel into a cast iron mold. The basic chemical composition of the remelted HSS was as follows (mass fraction, %): 0.87C, 6.06W, 4.79Mo, 4.09Cr, 1.89V, 0.42Si, 0.39Mn, 0.22Ni, 0.12Cu, 0.022P, 0.007S, 0.08RE and 0.06N, while the balance was Fe. The specimens used in the following procedures were taken from the center of the ingots at about one third of the height.
The pseudo-binary equilibrium phase diagram of M2 HSS was calculated using Thermo-Calc software (TCFE3 database). The phase transformation points were determined by STA-449C differential scanning calorimetry (DSC). The sample was heated to 1450 °C at a rate of 200 °C/h, held for 2 min, and then cooled to room temperature at the same rate. The heating and cooling rates of the test setting are close to equilibrium to allow comparison with the phase diagram. Transmission electron microscopy (TEM) and X-ray diffraction (XRD) were used to study the structural transition of eutectic carbides with the addition of mischmetal and nitrogen. The crystallographic structures of carbides were determined by selected area electron diffraction (SAED). The specimens for TEM were thin foils prepared by mechanical polishing and dual ion milling and then examined by JEM 2010 TEM with an acceleration voltage of 200 kV. The samples for XRD were examined using a Rigaku D/MAX-2500PC diffractometer. The tube voltage and current were 40 kV and 40 mA, respectively. The tube anode was Cu Kα1 (λ=0.15406 nm), and the width of the receiving slit was 2 mm. Using the Rietveld method [16], the volume fraction of carbides was calculated. Morphological and composition analyses were conducted on a Hitachi S-4800 field emission scanning electron microscope (SEM) with energy dispersive spectrometry (EDS) at an acceleration voltage of 15 kV.
3 Results
3.1 Solidification and phase transformation
Figure 1 shows the pseudo-binary equilibrium phase diagram of the steel, where the vertical dashed-dotted line corresponds to the experimental M2 HSS. The critical points of the phase transformation are numbered and the critical temperatures are listed. At equilibrium, the liquidus, solidus, Acm and A1 temperatures of the M2 HSS are 1412, 1252, 821 and 795 °C, respectively. The solidification process of the experimental M2 HSS mainly includes four stages from high temperature to low temperature, which are the precipitation of δ, the precipitation of MN, the peritectic transformation (L+δ→γ), and the eutectic reaction (L+δ→γ+MxC). The balanced phase contents at room temperature are composed of α+M6C+M(CN)+M23C6. According to the phase diagram, the temperature range and phase contents of each phase field are listed in Table 1.

Fig. 1 Pseudo-binary equilibrium phase diagram of steel
Table 1 Temperature range and phase contents of phase fields
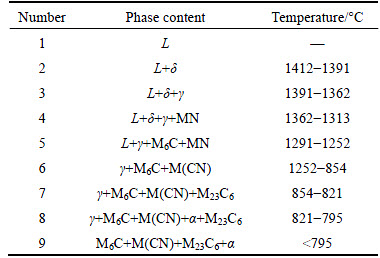
Figure 2 shows the DSC curves of the experimental M2 HSS during the cooling process from 1450 °C. During the solidification, there are three significant exothermic peaks and the corresponding temperatures are 1413, 1275 and 1233 °C, respectively. Combined with Table 1 and Fig. 1, the corresponding phase transformation is marked at each exothermic peak in Fig. 2. In the DSC curve, the peak of 1413 °C corresponds to the beginning of δ precipitation from liquid; subsequently, the peritectic reaction (L+δ→γ) occurs and γ-austenite forms continuously. The exothermic peak of 1275 °C is very sharp, which indicates that the phase transformation at this temperature is severe and corresponds to the eutectic transformation (L→γ+M6C). The liquid disappears at the peak of 1233 °C. The experimental values are close to the calculated ones (1413/1412, 1275/1291 and 1233/ 1252), which are also similar to the measured values of M2 HSS [3]. In the DSC curve, there are no obvious peaks below 1150 °C, and the exothermic peak of MN precipitation is not found. This mainly results from the relatively small amount of MN and the overlap of the exothermic peaks.
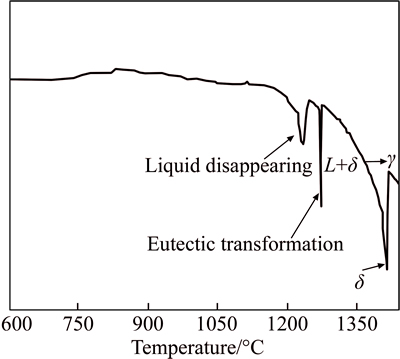
Fig. 2 DSC curve of experimental M2 HSS during cooling
3.2 Phase composition and microstructure
From the analyses of the phase diagram and the DSC curve, it can be found that a large number of carbides occur during the cooling of the experimental M2 HSS. Figure 3 shows the XRD spectra of as-cast M2 HSS. From Fig. 3(a), it can be seen that the as-cast microstructure mainly consists of α-Fe, M2C and M(C,N), and the diffraction peaks of M6C are not obvious due to the strong diffraction peaks of α-Fe. The carbide powders were extracted from the ingot and the iron matrix was corroded off; then the carbide powders were examined by XRD. The XRD spectra of the powders are shown in Fig. 3(b), and it can be seen that the carbides mainly consist of M2C, M(C,N) and M6C. The volume fractions of carbides are 58.5%, 30.1% and 11.4%, respectively. The peaks of M23C6 are not found in the XRD spectra. The main reason is that the quantity of M23C6 is too small to be seen in the diffraction spectra.
Figure 4 shows the morphology of carbides in the experimental M2 HHS steel and the alloy compositions of M2C. From Fig. 4, it can be seen that the as-cast structure consists of dendrites of iron matrix, networks of eutectic carbides, and secondary carbides. The averagedistance between networks is 34 μm and the particles of secondary carbides are homogeneously distributed in the iron matrix. Generally, the morphologies of M2C carbides have two types: a plate-like shape and a fibrous one [4, 8, 14]. In this steel, M2C shows the fibrous feature in Fig. 4(b); the fibers bend locally and the average spacing between them is 1.5 μm. The EDS analysis shows the alloy contents of M2C in Fig. 4(d). M2C mainly consists of W, Mo, V, Cr and Fe.

Fig. 3 XRD spectra of experimental steel:
3.3 Decomposition of carbides
Figure 5 shows the DSC curve of the experimental steel during heating to liquid at a speed of 200 °C/h. The phase transformation corresponding to each endothermic peak is marked in Fig. 5. The larger endothermic peak from 897.2 to 1221.5 °C corresponds to the decomposition reaction of M2C. M2C decomposes slowly below 1050 °C, and its decomposition speed reaches a peak value at about 1120 °C. When heating to 1221.5 °C, M2C completely decomposes into M6C and M(C,N). With the rise in temperature, M6C and M(C,N) are dissolved into liquid at different temperatures. Finally, solid is completely transformed into liquid at about 1420 °C.
In order to investigate the decomposition behavior of M2C further, the experimental steel was heated to 1100 °C and held for different time. Figure 6 shows the SEM morphologies of M2C carbides after holding for different time at 1100 °C. From Fig. 6, it can be seen that some precipitated carbide particles are found in the M2C matrix after holding for 15 min. After holding for 30 min, the fibers of M2C bend and can be seen necking down, as shown in Fig. 6(b). The amount of decomposed products increases gradually with the increase of the holding time. The carbide fiber necks down more and more obviously until the fibers are broken with increasing holding time (see Figs. 6(c) and (d)). After holding for 180 min, the carbide fibers disappear and the decomposition products consist of fine carbide particles (about 300 nm) and short rod-like carbides (about 3.5 μm).
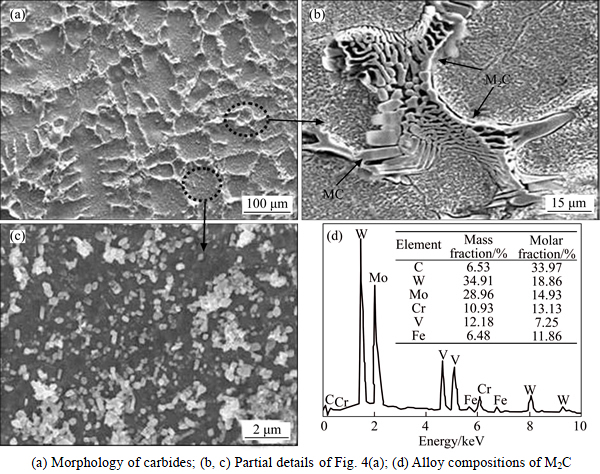
Fig. 4 As-cast microstructure of experimental steel and alloy compositions of M2C:
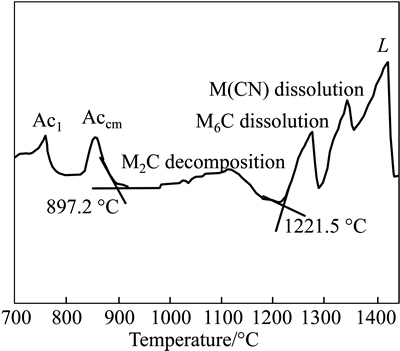
Fig. 5 DSC curve of experimental steel during heating
The carbide powders were extracted from the samples after holding for different times at 1100 °C, and then the carbide powders were examined by XRD. Figure 7 shows the XRD spectra of each sample. From Fig. 7, it can be seen that the peaks of M2C become gradually weaker with increasing holding time, which indicates the decomposition of M2C. Using the Rietveld full-spectrum fitting method, the contents of different carbides were calculated and are shown in Fig. 8. From Fig. 8, it can be seen that the relative content of M2C decreases with increasing holding time, while the relative content of M6C increases with increasing holding time. The decrease of M2C and the increase of M6C are due to the decomposition of M2C and the formation of M6C. Although MC is also the decomposition product of M2C, the relative content of MC decreases with increasing holding time. During the decomposition of M2C, plenty of Fe atoms from austenite participate in the formation of M6C, which results in the formation of a much larger quantity of M6C compared to MC. The strongest spectral peak of M2C 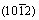 almost disappears after holding for 60 min and the relative content is below 5%. The spectral peaks of M6C gradually increase with holding time, and the relative content of M6C increases to 79.8% (from 11.4%, as-cast sample) after holding for 60 min. The relative contents of carbides clearly change during the initial stage of the holding temperature and then gradually become stable with increasing holding time, and finally the decomposition of M2C is completed.
almost disappears after holding for 60 min and the relative content is below 5%. The spectral peaks of M6C gradually increase with holding time, and the relative content of M6C increases to 79.8% (from 11.4%, as-cast sample) after holding for 60 min. The relative contents of carbides clearly change during the initial stage of the holding temperature and then gradually become stable with increasing holding time, and finally the decomposition of M2C is completed.
Figure 9 shows the TEM morphologies of carbides in the experimental steel after holding for 60 min at 1100 °C. The crystallographic structures of the decomposition products (MC and M6C) are determined by SAED. From Fig. 9, it can be seen that MC (face centered cubic, FCC) precipitates from the matrix of M2C and the remaining part of M2C combined with Fe transforms into M6C. M6C type carbides with FCC structure, mainly consisting of Fe, W, and Mo, have high phase stability [17].
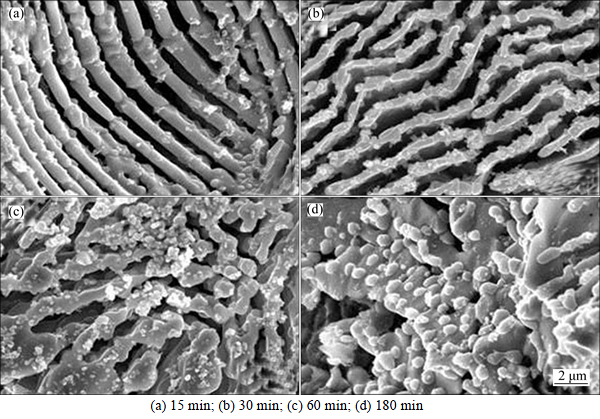
Fig. 6 SEM morphologies of M2C after holding for different time at 1100 °C:

Fig. 7 XRD patterns of carbide powders extracted from samples after holding for different times at 1100 °C

Fig. 8 Carbide contents of each sample with different holding time

Fig. 9 TEM image of experimental steel after holding for 60 min at 1100 °C
4 Discussion
It is believed that the MN-type nitrides (main phases, VN) are precipitated in M2 HSS at high temperature after the addition of nitrogen, which will reduce the segregation of V during the eutectic transformation. To a certain extent, this can also promote the formation of M2C. The metastable M2C will be decomposed into M6C and MC during the subsequent heat treatment, which refines the carbides. Previous studies indicate that the morphologies of M2C carbides have two types: a plate-like shape and a fibrous one, depending on the cooling conditions and chemical composition [4, 8, 14]. Figure 10 shows the morphologies of the as-cast microstructures of M2 HSS before and after the addition of RE + N. From Figs. 10(a) and (b), it can be seen that the morphology of M2C is plate-like in as-cast M2 HSS and there are sharp corners at the edges of the carbides. These sharp corners will cause a concentration of stress during deformation and lead to the initiation of cracks. After the addition of RE and nitrogen, in Figs. 10(c) and (d), the morphology of M2C becomes fiber-like, the lamellae of M2C are irregular and bent, the spacing between fibers decreases, and the interfaces between carbides and primary austenite are very smooth. In AISI M2 HSS, the complete decomposition of M2C to M6C and MC requires holding for 4 h at 1100 °C [5], while the decomposition process requires only 60 min in this work. The greater the degree of irregularity of the carbide, the lower its thermal stability is. The decomposition of irregular M2C is easier than that of regular M2C. The addition of nitrogen and mischmetal increases the irregularity of M2C.
The coarse network carbides in the as-cast microstructure reduce the mechanical properties of HSS, and the pressing work, such as forging and rolling, can break down the coarse carbides and give them a uniform distribution in the steels. M2C carbides are metastable and decompose into M6C and MC, and the transformation reaction is described as M2C+γ(Fe)→ M6C+MC [4, 5]. The formation and separation of M6C and MC lead to a certain degree of refinement of the network carbides. The morphology and distribution of carbides can be improved through the decomposition of M2C during heat treatment. The formation of new carbides and the disappearance of the original carbides enhance the degree of refinement of the carbides, increase the effectiveness of the carbide spheroidization process, and reduce the difficulty of deformation refinement. Deformation refinement combined with the decomposition of eutectic carbides greatly increases the efficiency of hot working, which will further improve the distribution of carbides and enhance the mechanical properties of HSS.

Fig. 10 SEM morphologies of M2C in as-cast M2 HSS before and after addition of RE+N:
5 Conclusions
1) The as-cast microstructure of M2 HSS with nitrogen and rare earth consists of dendrites of iron matrix, networks of eutectic carbides, and secondary carbides. The average distance between networks is about 34 μm, and the particles of secondary carbides are homogeneously distributed in the iron matrix.
2) The carbides in as-cast ingots of M2 HSS with nitrogen and rare earth mainly include M2C, M(C,N), and M6C with relative contents of 58.5%, 30.3% and 11.2%, respectively. M2C-type carbides have the fibrous feature, the fibers bend locally, and the average spacing between the fibers is 1.5 μm. M2C-type carbides mainly consist of W, Mo, V, Cr and Fe.
3) From the DSC analysis (heating, 200 °C/h), the decomposition of M2C occurs from 897.2 to 1221.5 °C, M2C decomposes slowly below 1050 °C, and the decomposition speed reaches a peak value at about 1120 °C. On heating to 1221.5 °C, M2C completely decomposes into M6C and M(C,N). Some precipitated carbide particles occur in the M2C matrix after holding for 15 min at 1100 °C, and the fibers of M2C bend and neck down after holding for 30 min.
4) The amount of decomposed products increases gradually with increases in the holding time. The carbide fibers neck down more and more obviously until the fibers are broken down. After holding for 180 min, the carbide fibers disappear and the decomposition products consist of fine carbide particles (about 300 nm) and short rod-like carbides (about 3.5 μm). From the XRD analysis, the spectral peaks of M2C almost disappear after holding for 60 min and the decomposition of M2C is nearly complete. The spectral peaks of M6C increase gradually with the holding time, and the relative content of M6C increases to 79.8% (from 11.4% in the as-cast sample) after holding for 60 min.
References
[1] HWANG K C, LEE S, LEE H C. Effects of alloying elements on microstructure and fracture properties of cast high speed steel rolls [J]. Part I. Microstructural analysis, Material Science and Engineering A, 1998, 254: 282-295.
[2] WANG He-bin, HOU Long-gang, ZHANG Jin-xiang, LU Lin, CUI Hua, ZHANG Ji-shan. The secondary precipitates of niobium-alloyed M3:2 high speed steel prepared by spray deposition [J]. Materials Characterization, 2015, 106: 245-254.
[3] QV Ming-gui, WANG Zhen-hua, LI Hui, LV Zhi-qing, SUN Shu-hua, FU Wan-tang. Effects of mischmetal addition on phase transformation and as-cast microstructure characteristics of M2 high-speed steel [J]. Journal of Rare Earths, 2013, 31(6): 628-633.
[4] ZHOU X F, FANG F, JIANG J Q, ZHU W L, XU H X. Study on decomposition behaviour of M2C eutectic carbide in high speed steel [J]. Material Science and Technology, 2012, 28: 1499-1504.
[5] PAN Fu-sheng, WANG Wei-qing, TANG Ai-tao, WU Li-zhi, LIU Ting-ting, CHENG Ren-ju. Phase transformation refinement of coarse primary carbides in M2 high speed steel [J]. Progress in Natural Science: Material International 2011, 21: 180-186.
[6] ZHOU Bin, SHEN Yu, CHEN Jun, CUI Zhen-shan. Breakdown behavior of eutectic carbide in high speed steel during hot compression [J]. Journal of Iron and Steel Research, International, 2011, 18: 41-48.
[7] FU Han-guang, XING Jian-dong. Manufacturing technology of high speed steel rolls [M]. Beijing: Metallurgical Industry Press, 2007: 15. (in Chinese)
[8] ZHOU Xue-feng, FANG Feng, LI Gang, JIANG Jian-qing. Morphology and properties of M2C eutectic carbides in AISI M2 steel [J]. ISIJ International, 2010, 50: 1151-1157.
[9] FISCHMEISTER H F, RIDEL R, KARAGOZ S. Solidification of high-speed tool steels [J]. Metallurgical Transactions A, 1989, 20A: 2133-2148.
[10] VITRY V, NARDONE S, BREYER J P, SINNAEVE M, DELAUNOIS F. Microstructure of two centrifugal cast high speed steels for hot strip mills applications [J]. Materials and Design, 2012, 34: 372-378.
[11] ZHOU Xue-feng, YIN Xiao-yan, FANG Femg, JIANG Jian-qing, ZHU Wang-long. Influence of rare earths on eutectic carbides in AISI M2 high speed steel [J]. Journal of Rare Earths, 2012, 30: 1075-1078.
[12] HALFA H, MATTAR T, EISSA M. Precipitation behavior of modified as cast high nitrogen super hard high-speed tool steel [J]. Steel Research International, 2012, 83: 1071-1078.
[13] GHOMASHCHI M R. Quantitative microstructural analysis of M2 grade high speed steel during high temperature treatment [J]. Acta Materialia, 1998, 46: 5207-5220.
[14] BOCCALINI M, GOLDENSTEIN H. Solidification of high speed steels [J]. International Materials Reviews, 2001, 46: 92-115.
[15] LEE E S, PARK W J, JUNG J Y. Solidification microstructure and M2C carbide decomposition in a spray-formed high-speed steel [J]. Metallurgical and Materials Transactions A, 1998, 29: 1395-1404.
[16] YOUNG R A. The Rietveld Method [M]. Oxford: Oxford University Press, 1993: 101.
[17] LV Zhi-qing, ZHOU Ze-an, SUN Shu-hua, FU Wan-tang. Phase stability, electronic and elastic properties of Fe6-xWxC (x=0-6) from density functional theory [J]. Materials Chemistry and Physics, 2015, 164: 115-121.
(Edited by FANG Jing-hua)
Cite this article as:
LIU Bao-long, L Zhi-qing, FENG Wei-wei, REN Ting-zhi, FU Wan-tang. Precipitation and decomposition behaviors of carbides in AISI M2 high-speed steel with nitrogen and mischmetal [J]. Journal of Central South University, 2017, 24(4): 782-788.
Zhi-qing, FENG Wei-wei, REN Ting-zhi, FU Wan-tang. Precipitation and decomposition behaviors of carbides in AISI M2 high-speed steel with nitrogen and mischmetal [J]. Journal of Central South University, 2017, 24(4): 782-788.
Foundation item: Project(E2016203256) supported by the Natural Science Foundation of Hebei Province, China
Received date: 2016-07-20; Accepted date: 2016-11-13
Corresponding author: L Zhi-qing, Professor, PhD; Tel: +86-335-8052253; E-mail: zqlv@ysu.edu.cn
Zhi-qing, Professor, PhD; Tel: +86-335-8052253; E-mail: zqlv@ysu.edu.cn
Abstract: The behaviors of the precipitation and decomposition of carbides in AISI M2 high-speed steel modified by nitrogen and mischmetal were investigated using DSC, XRD, SEM and TEM. The as-cast microstructure of the experimental steel consists of dendrites of iron matrix, networks of eutectic carbides and secondary carbides. The average distance between networks is about 34 μm. The carbides mainly include M2C, M(C,N) and M6C, and their relative contents are 58.5%, 30.3% and 11.2%, respectively. The average spacing between the M2C fibers is 1.5 μm. The decomposition of M2C occurs from 897.2 to 1221.5 °C (heating rate of 200 °C/h). Some precipitated carbide particles occur in the M2C matrix after holding for 15 min at 1100 °C. With increasing holding time, the carbide fibers neck down more and more obviously until they are broken down. The spectral peaks of M2C almost disappear after holding for 60 min. The spectral peaks of M6C gradually strengthen with the holding time, and the relative content of M6C increases to 79.8% after holding for 60 min. After holding for 180 min, the carbide fibers disappear, and the decomposition products consist of fine carbide particles (about 300 nm) and short rod-like carbides (about 3.5 μm).


