
Relationship and effect of redox potential, jarosites and extracellular polymeric substances in bioleaching chalcopyrite by acidithiobacillus ferrooxidans
YU Run-lan1, 2, ZHONG Dai-li1, 2, MIAO Lei1, 2, WU Fa-deng1,2, QIU Guan-zhou1, 2, GU Guo-hua1, 2
1. School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China;
2. Key Laboratory of Biometallurgy of Ministry of Education, Central South University, Changsha 410083, China
Received 25 August 2010; accepted 17 November 2010
Abstract:
The changes of pH, redox potential, concentrations of soluble iron ions and Cu2+ with the time of bioleaching chalcopyrite concentrates by acidithiobacillus ferrooxidans were investigated under the different conditions of initial total-iron amount as well as mole ratio of Fe(III) to Fe(II) in the solutions containing synthetic extracellular polymeric substances (EPS). When the solution potential is lower than 650 mV (vs SHE), the inhibition of jarosites to bioleaching chalcopyrite is not vital as EPS produced by bacteria can retard the contamination through flocculating jarosites even if concentration of Fe(III) ions is up to 20 g/L but increases with increasing the concentration of Fe(III) ions; jarosites formed by bio-oxidized Fe3+ ions are more easy to adhere to outside surface of EPS space on chalcopyrite; the EPS layer with jarosites acts as a weak diffusion barrier to further rapidly create a high redox potential of more than 650 mV by bio-oxidizing Fe2+ ions inside and outside EPS space into Fe3+ ions, resulting in a rapid deterioration of ion diffusion performance of the EPS layer to inhibit bioleaching chalcopyrite severely and irreversibly.
Key words:
extracellular polymeric substances; chalcopyrite; bioleaching; jarosites; redox potential;
1 Introduction
Chalcopyrite, CuFeS2, is the most abundant copper mineral in nature counting for about 70% of copper reserves in world, and is also the most recalcitrant to hydrometallurgical processes. But biohydrometallurgy as an alternative instead of pyrometallurgy presents important advantages such as the possibility of treating low grade ores and easier control of waste, with the attendant benefits to the environment [1]. A lot of works have been focused on bioleaching chalcopyrite so far [2-5]. However, the leaching oxidation kinetics and copper extraction yields with mesophilic bacteria are still unsatisfactory at room temperature due to a suitable potential range and passivation appearance in bioleaching chalcopyrite, which results in the limited use in commercial applications [6]. Many investigators thought that ferric ions are responsible for the oxidation of chalcopyrite but also promote its passivation [7-8]. However, the mechanism of bioleaching chalcopyrite is still in debate and has not yet been elucidated [1-3, 6-8].
Now, non-contact and contact mechanisms based on extracellular polymeric substances (EPS) for bioleaching sulfides have been accepted extensively [9-11]. The EPS facilitates the attachment of microorganisms to the mineral surface. The EPS layer constitutes a special reaction space, attacking mineral through an electrochemical dissolution. However, the behaviors of bioleaching chalcopyrite in the EPS space have not been clearly understood so far.
The objective of this work is to investigate the interactions among EPS, redox potential and jarosites in EPS space and their effects on bioleaching chalcopyrite by acidithiobacillus ferrooxidans through the changes of initial concentrations of Fe(II) and Fe(III) ions as well as total-iron amount in synthetic EPS solutions and in no synthetic EPS solutions.
2 Materials and methods
2.1 Chalcopyrite
A flotation chalcopyrite concentrate obtained from Dexing Mine (Jiangxi, China) was washed with 1 mol/L HCl, 2 mol/L H2SO4 in turn three times, then washed with acetone to remove the flotation reagent. The chalcopyrite concentrate was put in air for 4 h, then dried at 100 °C for 24 h; finally, the mineral was sterilized in an asepsis room for 24 h with UV. The chemical and mineralogical analyses of chalcopyrite concentrate by X-ray diffraction show that the sample contains 33.15% Cu, 29.86% Fe, 33.22% S, and minor amounts of quartz, pyrite and covellite as accessory minerals.
2.2 Leaching solution
The leaching solution contained 3.00 g/L (NH4)2SO4, 0.10 g/L KCl, 0.50 g/L K2HPO4, 0.50 g/L MgSO4·7H2O and 0.01 g/L Ca(NO3)2. A synthetic EPS solution was prepared according to the EPS components studied by previous researchers [10-11]. Its main components are as follows (mass fraction): rhamnose 10.8%, fucose 17.1%, mannose 0.7%, glucose 15.2%, fatty acid 21.6%, pH 2.0 adjusted with sulfuric acid. The medium was sterilized in steam at 121 °C for 15 min before use.
2.3 Bacteria
Acidithiobacillus ferrooxidans used in this study were initially adapted to grow with chalcopyrite in several subcultures in liquid medium through successive replacement of Fe2+ with chalcopyrite. The supernatant solution from the culture was used as inoculum.
2.4 Bioleaching
Leaching experiments were performed in 250 mL- shake flasks containing 200 mL leaching solution and 1% chalcopyrite concentrate, 1% (volume) EPS solution mentioned above. Fe3+ and Fe2+ ions were added in forms of Fe2(SO4)3 and FeSO4, respectively, according to Table 1 and Table 2. Flasks were placed on an orbital shaker and incubated at 30 °C, shaking at the rate of 180 r/min.
Table 1 Initial mole ratios of Fe3+ to Fe2+

Table 2 Initial concentrations of Fe3+ and Fe2+

2.5 Analytical procedures
The pH was measured at the interval of two days with PHS-3C pH meter, the solution potential (φ) was measured with Pt and Ag-Ag chloride double electrodes during bioleaching. All the solution potentials (φ) have been converted vs. standard hydrogen electrode (SHE). Soluble copper ions were determined with atomic absorption spectrophotometer (AAS), and ferrous ion was determined by titration with potassium dichromate (K2Cr2O7), after centrifugation to separate the solid material and bacteria. Finally, the residues of leached samples were dried in vacuum desiccator, and characterized using X-ray diffractometry (XRD).
3 Results and discussion
3.1 Influence of initial amount of total iron ions on chalcopyrite bioleaching
The velocity of bioleaching copper is the quotient of the change of the concentration of Cu2+ dividing into the change of leaching time, namely, the slope of leaching curve. If the concentration of leached Cu2+ does not change with leaching time, it is known as the passivation of bioleaching chalcopyrite. Now, the relationship among the velocity of bioleaching copper, EPS, jarosites and redox potential is discussed according to our experimental results shown in Fig. 1.
In the system of no synthetic EPS solutions, the velocities of bioleaching copper are very rapid before the third day, and rapidly decrease from the third to the sixth day; after leaching for 6 d, the concentration of leached Cu2+ increase very slowly with the leaching time even when initial concentrations of iron ions are 0.5 g/L and 1.0 g/L, does not increase and obvious passivation phenomena occurs when initial concentrations of iron ions are more than 5.0 g/L, as shown in Fig. 1(b). But in the system of synthetic EPS solutions, the concentrations of copper ions continually increase before the 12th day, from the twelfth day, the passivation appearance seems to occur and is obvious when initial concentration of iron ions is 20 g/L, as seen from Fig. 1(a). Obviously, the synthetic EPS has a very important effect on bioleaching chalcopyrite.
We found that the forming of passivation phenomenon is consanguineously relative with solution redox potential rather than jarosites. Those passivation phenomena in synthetic EPS solutions (Fig. 1(a)) do not occur before the 12th day of bioleaching because solution redox potential does not reach the maximal value, only about 650 mV seen from Fig. 1(g), but the obvious passivation phenomena occur in no synthetic EPS solutions after bioleaching for 6 d (see Fig. 1(b)) because all solution potentials have rapidly reached the maximal values (see Fig. 1(h)). Jarosites have a negative effect on bioleaching but do not matter to inhibit the bio-oxidation severely even if concentration of Fe(III) ions is up to 20 g/L when solution potential is lower than 650 mV (vs SHE) according to our experimental results; however, the inhibition action increases with icreasing the concentration of Fe(III) ions. The passivation appearance still occurs at high solution redox potential even when initial concentrations of iron are 0.5 g/L and 1.0 g/L although jarosites or Fe3+ depositions are very poor here. This indicates that the passivation appearance is consanguineously relative with solution redox potential rather than with jarosites. KAWABE et al [12] thought the smallest value at which the oxidation is completely inhibited is 16.75 g/L Fe(III). The result of MOOUSAVI et al [13] was above the value. But we think that jarosites or Fe3+ depositions are not vital for inhibiting the oxidation completely even if concentration of Fe(III) ions is very high, such as 20 g/L, as long as solution potential is lower than 650 mV (vs SHE).

Fig. 1 Relationship among velocity of bioleaching copper, EPS, pH, redox potential and total iron ions in bioleaching chalcopyrite
HIROYOSHI et al [5], OKAMOTO et al [14] and PETERSEN and DIXON [15] thought that the redox potential determined by the concentration ratio of Fe3+ to Fe2+ in solution is an important factor affecting CuFeS2, the leaching rate increases with increasing redox potential and reaches a maximum at an optimum redox potential, after which it decreases at higher potentials, and at a very high potential the rate becomes less dependent on the potential. We agree these viewpoints and the optimum redox potential is about 650 mV according to our experimental results shown in Fig. 1.
KINZLER et al [10] thought that EPS can concentrate ferric ions and H+ to help bioleaching. We do agree the viewpoint that EPS can concentrate ferric ions but do not think that it helps bioleaching, because the viewpoint can not explain our experimental results that the velocities of bioleaching copper are lower in synthetic EPS solutions than in no synthetic EPS solutions before the sixth day of leaching, also can not explain the severe passivation phenomenon in no synthetic EPS solutions when solution redox potential is over 650 mV.
C?RDOBA et al [16] thought that precipitation and nucleation of jarosites on chalcopyrite particles are the principal cause of chalcopyrite passivation in ferric sulfate medium, the formation of jarosites depends on the redox potential of the leaching solution and speeds up when the potential is above a critical value, near 450 mV (vs Ag/AgCl). This can not explain our experimental results clearly and completely. Because even if precipitation and nucleation of jarosites on chalcopyrite particles speed up at high redox potential, the amount of jarosites still is very poor when concentrations of iron ions are very low, such as 0.5 g/L and 1.0 g/L, the passivation phenomena should not be obvious in no synthetic EPS solutions. Obviously, the passivation phenomenon is not consanguineously relative with solution redox potential but also with the mechanism of forming EPS layer according to our experimental results.
We think that the EPS film layer produced gradually by bacterial with iron depositions, such as jarosites, can adhere on chalcopyrite and act as a weak diffusion barrier against transferring of ferric ions and then further rapidly creates a high redox potential of more than 650 mV by bio-oxidizing Fe2+ inside and outside EPS space; on the contrary, the high redox potential further speeds up jarosites deposition to EPS space on the surface of chalcopyrite seriously and irreversibly to form ion diffusion barrier layer. As bacteria are live, they can leave the diffusion barrier of EPS layer with iron depositions to look for available energies [17], e.g. to oxidize Fe2+ in the solution or fresh chalcopyrite surface. Such processes were carried out repeatedly, consequently, the diffusion barrier may gradually recover whole surface of chalcopyrite and the passivation phenomenon of bioleaching chalcopyrite obviously occurs.
The mechanism mentioned above can better explain our experimental results. All known, the synthetic EPS can chelate Fe3+. At the same time, the synthetic EPS can flocculate these depositions of Fe3+, such as jarosites or Fe(OH)3, because the concentrations of soluble irons (containing soluble Fe3+ and Fe2+ ions) in the solutions decrease more rapidly in synthetic EPS solutions than in no synthetic EPS solutions with leaching time according to Figs. 1(c) and (d). As the synthetic EPS is only a little amount compared with the concentrations of added Fe3+ ions, it is not enough to chelate them completely, but most of added irons have deposited in synthetic EPS solutions according to Fig. 1(c). It is understood that the synthetic EPS is of promoting and flocculating the precipitations of Fe3+ ions, so that chemical leaching by Fe3+ ions is reduced in synthetic EPS solutions before the sixth day of leaching. It can better explain the fact that the velocities of bioleaching copper are lower in synthetic EPS solutions than in no synthetic EPS solutions before the sixth day of leaching. The synthetic EPS has a negative effect on bioleaching when the concentration of Fe3+ is low to reduce the chemical leaching and has a positive effect when the concentration of Fe3+ is high through promoting and flocculating the precipitations of Fe3+ as long as solution redox potential is under 650 mV.
Why can the EPS produced gradually by bacteria themselves not effectively prevent the rapid increases of solution redox potentials in no synthetic EPS solutions? FU et al [18] indicated that forming an integrated EPS film layer of A.f bacteria needs 2-3 weeks, but their results lack unambiguous information of redox potential and jarosites. All know that EPS forming is a passive respond of bacteria for disadvantageous environment. After 12 d of bioleaching, as synthetic EPS solutions have combined and flocculated absolute most of Fe3+ (seen from Fig. 1(c)), it results in that solution redox potential cannot rapidly increase, at the same time, the EPS layer produced by bacteria themselves makes the second adaptive periods about 725 mV in the synthetic EPS solutions, but there still are a lot of soluble iron ions in no synthetic EPS solutions, adhered bacteria and their EPS produced gradually and passively by bacteria themselves will combine and flocculate the depositions of Fe3+ in the no synthetic solutions on the surface of chalcopyrite to form the inhibition layer according to the mechanism mentioned above, so their passivation appearances are more obviously [19].
3.2 Influence of initial mole ratio of Fe3+ to Fe2+ on chalcopyrite bioleaching
The effects of initial mole ratios of Fe3+ to Fe2+ in synthetic EPS and no synthetic EPS solutions on bioleaching chalcopyrite are shown in Fig. 2.
The bioleaching recoveries in no synthetic EPS solutions are A2>A3>A4>A5>A1>A0. Interestingly, the highest bioleaching recovery occurs when the mole ratio of Fe3+ to Fe2+ is closer to 1 and it is the minimal in the case of a lot of ferrous ions as energy resource. But the maximal value of solution redox potential for A5 is not as high as other experiments (see Fig. 2(d)). This experimental result further reveals that initially high redox potential of solution harms bacterial activity severely. The leaching rate of experiment A0 is the lowest and its maximal redox potential is the highest. The results also further approve that suggested mechanism by us is right. This means that bacteria preferably use Fe2+ as energy and produce a lot of Fe3+ ions in the solutions, then EPS layer is passively formed by bacteria to stand against the disadvantageous environment, the adhered EPS layer on the surface of chalcopyrite with iron depositions such as jarosites gradually acts as a bioleaching barrier to create a high redox potential rapidly through bio-oxidizing Fe2+ ions in solution and EPS space.
The effect of EPS on bioleaching is evident in synthetic EPS solutions seen from Fig. 2(a). The bioleaching recoveries are A4>A5>A3>A2>A1≈A0. The velocities of bioleaching copper are lower in synthetic EPS solutions than in no synthetic EPS solutions before the 4th day of leaching due to reducing chemical leaching by combination of synthetic EPS and flocculation. At the same time, as the synthetic EPS weakens the harm of high redox potential of the solutions with high mole ratios of Fe3+ to Fe2+ to bacteria, leaching results of experiments A4 and A5 are better. Interestingly, the redox potential in the experiment A5 (here mole ratio of Fe2+ to Fe3+=0:10) in synthetic solution(see Fig. 2(b)) can be up to the maximal value but cannot in no synthetic EPS solution (see Fig. 2(d)). The results of redox potentials in the experiment A0 (here mole ratio of Fe3+ to Fe2+=0:10) is just reverse (see Figs. 2(b) and (d)). These results further demonstrate that Fe3+ deposits such as jarosites produced by bacterial oxidation are easy to adhere on the surface of EPS layer to form a bioleaching barrier layer, resulting in the inhibition of bioleaching chalcopyrite severely and irreversibly.
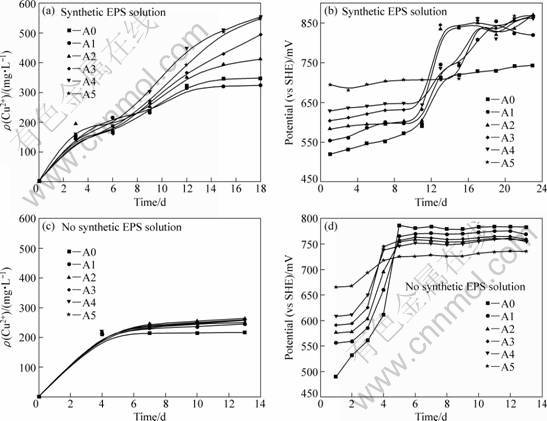
Fig. 2 Effect of initial mole ratio of Fe3+ to Fe2+ on bioleaching chalcopyrite
The analysis of bioleaching residues in the system of EPS solution by XRD confirms jarosites in Fig. 3.
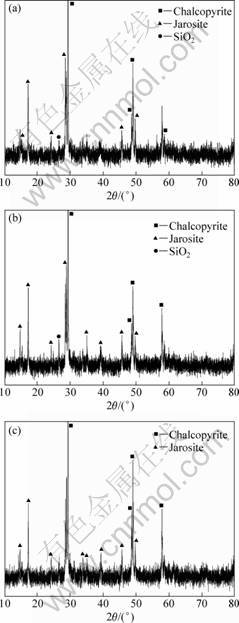
Fig. 3 X-ray patterns of bioleaching residues: (a) A1, n(Fe3+)/n(Fe2+)=2:8; (b) A2, n(Fe3+)/n(Fe2+)=4:6; (c) A4, n(Fe3+)/n(Fe2+)=8:2
Jarosite is a little comparatively when initial mole ratio of Fe3+ to Fe2+ is closer to 1. Ferric ions from bacteria oxidizing Fe2+ ions are easy to form jarosites on chalcopyrite and EPS reduces the crystallization of jarosites because EPS adsorbed on chalcopyrite can flocculate a lot of iron depositions to play a role in preventing iron ions to move between EPS space and solution.
4 Conclusions
1) When solution potential is lower than 650 mV (vs SHE), the inhibition of jarosites for bioleaching chalcopyrite is not vital as EPS produced by bacteria can retard the contamination through flocculating action even if concentration of Fe(III) ions is up to 20g/L but the inhibition increases with increasing the concentrations of Fe(III) ions.
2) EPS is a passive response of bacteria for disadvantageous environment, jarosites formed by bio-oxidized Fe3+ ions are more easy to adhere to the EPS space. The EPS layer with jarosites acts as a weak diffusion barrier to further rapidly create a high solution redox potential of more than 650 mV by bio-oxidizing Fe2+ into Fe3+ inside and outside EPS space, resulting in a rapid deterioration of ion diffusion performance of the EPS layer to inhibit bioleaching chalcopyrite severely and irreversibly.
References
[1] C?RDOBA E M, MU?OZ J A, BL?ZQUEZ M L, GONZ?LEZ F, BALLESTER A. Leaching of chalcopyrite with ferric ion. Part I: General aspects [J]. Hydrometallurgy, 2008, 93: 81-87.
[2] WATLING H R. The bioleaching of sulfide minerals with emphasis on copper sulfides: A review [J]. Hydrometallurgy, 2006, 84: 81-108.
[3] KLAUBER C. A critical review of the surface chemistry of acidic ferric sulphate dissolution of chalcopyrite with regards to hindered dissolution [J]. Int J Miner Process, 2008, 86: 1-17.
[4] VILC?EZ J, YAMADA R, INOUE C. Effect of pH reduction and ferric ion addition on the leaching of chalcopyrite at thermophilic temperatures [J]. Hydrometallurgy, 2009, 96: 62-71.
[5] HIROYOSHI N, KUROIWA S, MIKI H, TSUNEKAWA M, HIRAJIMA T. Effects of coexisting metal ions on the redox potential dependence of chalcopyrite leaching in sulfuric acid solutions [J]. Hydrometallurgy, 2007, 87: 1-10.
[6] PRADHAN N, NATHSARMA K C, SRINIVASA RAO K, SUKLA L B, MISHRA B K. Heap bioleaching of chalcopyrite: A review [J]. Minerals Engineering, 2008, 21: 355-365.
[7] C?RDOBA E M, MU?OZ J A, BL?ZQUEZ M L, GONZ?LEZ F, BALLESTER A. Leaching of chalcopyrite with ferric ion. Part II: Effect of redox potential [J]. Hydrometallurgy, 2008, 93: 88-96.
[8] DAOUD J, KARAMANEV D. Formation of jarosite during Fe2+ oxidation by acidithiobacillus ferrooxidans [J]. Minerals Engineering, 2006, 19: 960-967.
[9] SAND W, GEHRKE H. Extracellular polymeric substances mediate bioleaching/ biocorrosion via interfacical processes involving iorn(Ⅲ) ions and acidophilic bacteria [J]. Research in Microbiology, 2006, 157: 49-56.
[10] KINZLER K, GEHRKEA T, TELEGDIB J, SAND W. Bioleaching—A result of interfacial processes caused by extracellular polymeric substances (EPS) [J]. Hydrometallurgy, 2003, 71: 83-88.
[11] HARNEIT K, G?KSEL A, KOCK D, KLOCK J H, GEHRKE T, SAND W. Adhesion to metal sulfide surfaces by cells of Acidithiobacillus ferrooxidans, Acidithiobacillus thiooxidans and Leptospirillum ferrooxidans [J]. Hydrometallurgy, 2006, 83: 245-254.
[12] KAWABE Y, INOUE C, SUTO K, CHIDA T. Inhibitory effect of high concentrations of ferric ions on the activity of acidithiobacillus ferrooxidans [J]. Journal of Bioscience and Bioengineering, 2003, 96(4): 375-379.
[13] MOOUSAVI S M, YAGHMAEI S, VOSSOUGHI M, ROOSTAAZAD R, JAFARI A, EBRAHIMI M, HABIBOLLAHNIA CHABOK O, TURUNEN I. The effects of Fe(II) and Fe(III) concentration and initial pH on microbial leaching of low-grade sphalerite ore in a column reactor [J]. Bioresource Technology, 2008, 99: 2840-2845.
[14] OKAMOTO H, NAKAYAMA R, KUROIWA S, HIROYOSHI N, TSUNEKAWA M. Normalized redox potential used to assess chalcopyrite column leaching [J]. Journal of MMIJ, 2005, 121: 246-254.
[15] PETERSEN J, DIXON D G. Competitive bioleaching of pyrite and chalcopyrite [J]. Hydrometallurgy, 2006, 83: 40-49.
[16] C?RDOBA E M, MU?OZ J A, BL?ZQUEZ M L, GONZ?LEZ F, BALLESTER A. Leaching of chalcopyrite with ferric ion. Part IV: The role of redox potential in the presence of mesophilic and thermophilic bacteria [J]. Hydrometallurgy, 2008, 93: 106-115.
[17] YU Run-lan, TAN Jian-xi, YANG Peng, SUN Jing, OU Yang, XIONG Jing, DAI Yun-jie. EPS-contact-leaching mechanism of chalcopyrite concentrates by A. ferrooxidans [J]. Transactions of Nonferrous Metals Society of China, 2008, 18: 1427-1432.
[18] FU Jian-hua, QIU Guan-zhou, HU Yue-hua, XU Jing. The role of eps of thiobacillus ferrooxidans during bioleaching [J]. Acta Laser Biology Sinica, 2004, 13(1): 62-66.
[19] YU Run-lan, TAN Jian-xi, GU Gou-hua, HU Yue-hua, QIU Guan-zhou. Mechanism of bioleaching chalcopyrite by acidithiobacillus ferrooxidans in agar simulated extracelluar polymeric substances media [J]. Journal of Central south University of Technology, 2010, 17(1): 56-51.
嗜酸氧化亚铁硫杆菌浸出黄铜矿中
氧化还原电位、黄铁钾钒和胞外聚合物的关系和影响
余润兰1, 2,钟代立1, 2,苗 雷1, 2,吴发登1,2,邱冠周1, 2,顾国华1, 2
1. 中南大学 资源加工与生物工程学院,长沙 410083;
2. 中南大学 生物冶金教育部重点实验室,长沙 410083
摘 要:在合成的胞外聚合物(EPS)溶液中,研究不同起始总铁量、不同Fe(III)与Fe(II)摩尔比条件下嗜酸氧化亚铁硫杆菌浸出黄铜矿过程中pH、电位、可溶性铁离子和Cu2+浓度随浸出时间的变化。结果表明:当溶液电位低于650 mV (vs SHE)时,因细菌产生的EPS可通过絮凝黄铁钾钒延缓污染,即使铁离子浓度达到20 g/L,黄铁钾钒对细菌浸出黄铜矿的阻碍作用也不是致命的,但随着铁离子浓度的增加而增加; 细菌氧化的铁离子容易吸附在黄铜矿表面的EPS表层,有黄铁钾钒的EPS层是弱离子扩散壁垒,细菌通过把EPS空间内外的Fe2+氧化成Fe3+,进一步创造高于650 mV的电位,导致EPS层离子扩散性能的快速恶化,严重地和不可逆地阻碍生物浸出黄铜矿。
关键词:胞外聚合物;黄铜矿;生物浸出;黄铁钾钒;氧化还原电位
(Edited by YANG Hua)
Foundation item: Project(2010CB630904)supported by the National Basic Research Program of China; Project(50621063) supported by the Chinese Science Foundation for Distinguished Group
Corresponding author: YU Run-lan; Tel: +86-731-88836943; E-mail: yurunlan@mail.csu.edu.cn
DOI: 10.1016/S1003-6326(11)60907-2


