
![]()

Trans. Nonferrous Met. Soc. China 22(2012) 831-836
Crystallization microstructure of Mg65Cu25Y10 bulk amorphous alloy
HUANG Kang, CHEN Gang, ZHAO Yu-tao, WANG Guo-lu, SHAO Yang
School of Materials Science and Engineering, Jiangsu University, Zhenjiang 212013, China
Received 22 April 2011; accepted 28 July 2011
Abstract:
Mg65Cu25Y10 bulk amorphous alloy specimens prepared by conventional copper mould method were heated at 200 ℃ for different time and the phase contents as well as microstructure were studied. The XRD results show that the crystallization of Mg65Cu25Y10 bulk amorphous alloy specimen becomes complete as the treating time increases and Mg2Cu, Mg24Y5 and HCP-Mg crystalline phases are found. Snowflake-like morphology is found in different specimens through SEM observation. The EDS patterns show that the composition of the snowflake-like structure is close to that of the as-cast alloy. Laminated structures are observed from the TEM images of the snowflake-like structure. From the electron diffraction patterns, it is seen that the snowflake-like structure is the combination of Mg24Y5 and amorphous matrix. The FCC-Mg phase in the matrix transforms into HCP-Mg during the heat-treating process.
Key words:
Mg-based bulk amorphous alloy; crystallization; snowflake-like structure; laminated structure;
1 Introduction
Magnesium has attracted wide attention in metallic material field due to its high specific stiffness and strength, good dimensional stability, damping capacity, machining property and recyclability [1,2]. Moreover, Mg-based amorphous alloys with specific stiffness and strength several times higher than those of the crystalline ones are considered green energy materials [3] with the highest potential of industrial application in the 21st century. It is well known that Mg-based amorphous alloy experiences the transition from unstable amorphous to stable crystalline state under suitable conditions. Some of the properties are changed during the crystallization process [4], and the loss of those advantages is proved to enlarge the limitation of the application of Mg-based amorphous alloys. However, the property change is directly bound up with the variation of internal structure [5,6]. When it is above the crystallization temperature (tx), or the holding time is long enough at a temperature above the glass transition temperature (tg), crystalline phases would generate and grow up in the amorphous matrix and the properties of the amorphous alloy will be changed [7-9]. Therefore, it is necessary to investigate the crystallization behavior of Mg-based amorphous alloys and the final phase contents.
Previous studies [10-13] showed that some of the crystalline phases grew from Mg65Cu25Y10 bulk amorphous alloy with preferred orientation above Tx. The aim of the present work is to study the phase contents of the Mg65Cu25Y10 bulk amorphous alloy after heat treatment at tx, and to determine the microstructure and composition of the crystalline phases.
2 Experimental
Mg-based alloy was produced by melting commercially pure magnesium (99.9%) and Cu-Y master alloy according to the nominal composition in an electrical resistance furnace in argon atmosphere. The Cu-Y master alloy was prepared by arc melting high purity copper (99.9%) and yttrium (99.99%) in argon atmosphere. Mg65Cu25Y10 bulk amorphous alloy with thickness of 3 mm was then fabricated by conventional copper mould method [14]. A D/max2500PC X-ray diffractometer (XRD) was employed to examine the alloy. Glass transition temperature (tg=150 ℃) and crystallization temperature (tx=200 ℃) were analysed by Netzsch DSC404 differential scanning calorimeter (DSC) under flowing purified argon atmosphere with a flow rate of 40 mL/min. Specimens of Mg65Cu25Y10 bulk amorphous alloy with size of 8 mm×8 mm×3 mm were prepared and reheated to 200 ℃ in the H201-500 methyl silicone oil bath [13,15] for 2, 7, 12 and 20 min, respectively. The flash point of methyl silicone oil is 300 ℃, and the temperature controlling error is ±1 ℃. The specimens heat-treated for different time were examined by XRD. After mechanical polishing, the microstructures of the specimens were then identified using Hitachi JSM-5800 scanning electron microscope (SEM). A JEM-2100HR transmission electron microscope (TEM) was also used to further observe the crystalline phases in the specimen heat-treated for 12 min. The electron diffraction patterns were analysed by Gatan Digital Micrograph 1.6.2 software.
3 Results and discussion
Figure 1 shows the XRD patterns of different specimens. The specimens are the as-cast amorphous alloys aged at 200 ℃ for 2, 7, 12 and 20 min, respectively. From Fig. 1(a), the typical broad diffusion peak can be seen without crystalline peaks shown in Fig. 1(b), which are identified as Mg2Cu, Mg solid solution and Mg24Y5 crystal phase. It suggests that the incubation time of these three crystalline phases from the Mg65Cu25Y10 bulk amorphous alloy is very short at 200 ℃. As the holding time increases, more crystalline peaks can be seen from the XRD patterns in Figs. 1(c), (d) and (e). One more unknown peak appears in the pattern of sample aged for 7 min, as shown in Fig. 1(c), and two peaks identified as Cu2Y phase appear in the patterns of those aged for 12 and 20 min, as shown in Figs. 1(d) and (e). From the figures, it is clear that the Mg65Cu25Y10 alloy changes from absolute amorphous state to modified amorphous state, crystal phase and amorphous one, and then to absolute crystalline phases as the treating time increases. When the aging time reaches 12 min, there is no more new crystalline phase to be separated out from the matrix. It can also be seen that the pattern shown in Fig. 1(e) overlaps that in Fig. 1(d) well.
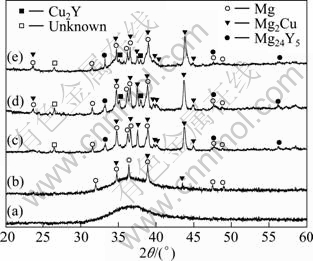
Fig. 1 XRD patterns of as-cast Mg65Cu25Y10 bulk amorphous alloy (a) and specimens heat-treated at 200 ℃ for 2 min (b), 7 min (c), 12 min (d) and 20 min (e)
Figure 2 shows the backscattering images of the specimens of Mg65Cu25Y10 bulk amorphous alloy aged at 200 ℃ for different time. The backscattering image of as-cast amorphous specimen is given in Fig. 2(a). It can be seen that there is gray amorphous matrix except several white oxide particles. It is shown from Figs. 2(b), (c), (d) and (e) that two types of particles are evenly embedded in the gray matrix of the specimens aged for different time, small round ones with diameter of several hundred nanometers and snowflake-like ones with a diameter of about 1 μm. EDS patterns show that the former is Mg2Cu phase, and the elemental composition of the latter is much close to that of the as-cast alloy. The snowflake-like particles look very different from the amorphous matrix. Therefore, it can be believed that they are a combination of the crystal phases shown in Fig. 1. From Fig. 2(b), it is shown that these snowflake-like phases could be separated out quickly from the amorphous matrix. As the aging time increases, the amount of snowflake-like phase increases.
Figure 3 shows the TEM images of Mg65Cu25Y10 bulk amorphous alloy specimen heat-treated at 200 ℃ for 12 min. Dendrites rather than particles and laminated structure appear in the images shown in Figs. 3(a) and (b). Therefore, despite of the disturbance of the matrix between the slices, the EDS pattern of the snowflake-like structure is much close to that of the as-cast alloy. Figs. 3(b), (c) and (d) show the TEM images of areas A, B and C in Fig. 3(a). The embedded images are the electron diffraction patterns of the areas in the corresponding boxes.
Figure 4 shows the calibration of electron diffraction pattern of the laminated structure in Fig. 3(b), which is composed of one relatively regular matrix and two somber concentric diffraction rings. By comparing the interplanar spacing di(i=1-5) of calibration with the powder diffraction file (PDF) of Mg-Cu-Y alloy system, it can be found that they agree well with that of Mg24Y5 phase. It suggests that the electron diffraction pattern in Fig. 4 authenticates the existence of Mg24Y5.
Figure 5 shows the high-resolution photo and its Fourier transformation of the laminated structure. From Fig. 5(a), it is easily noticed that the laminated structure grows directionally. Figure 5(b) shows the Fourier transformation of Fig. 5(a). The interplanar spacing d=0.28 nm is obtained by measuring the sine transformation picture of the inverse Fourier transformation of two appropriate symmetric points on the same circle. By comparing d with the PDF of Mg-Cu-Y system alloy, it can be found that these two symmetric points are corresponding to the {400} crystal plane of Mg24Y5.
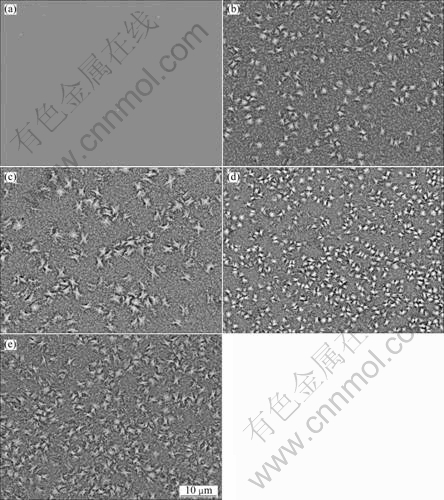
Fig. 2 Backscattering images of as-cast Mg65Cu25Y10 bulk amorphous alloy (a) and specimens heat-treated at 200 ℃ for 2 min (b), 7 min (c), 12 min (d) and 20 min (e)

Fig. 3 TEM image of specimen aged for 12 min (a) and high-resolution images and corresponding electron diffraction patterns of areas A (b), B (c) and C (d) in Fig. 3(a)

Fig. 4 Calibration of electron diffraction pattern of laminated structure of area A in Fig. 3(a)
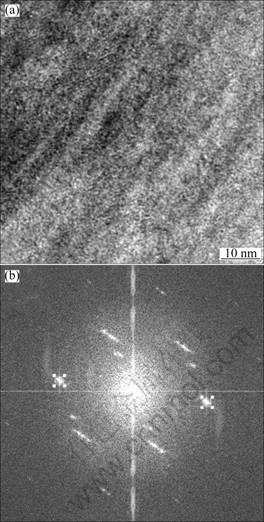
Fig. 5 High-resolution photo (a) and Fourier transformation picture (b) of laminated structure
Figure 6 shows the calibration of electron diffraction pattern in Fig. 3(c). Because of the mutual interference of several matrixes and two somber concentric diffraction rings, only one relatively distinct matrix is calibrated here. By comparing the interplanar spacing dj ( j=1-7) with the PDF of Mg-Cu-Y system alloy, it can be found that they agree well with that of Mg2Cu phase. It suggests that the electron diffraction pattern calibrated in Fig. 6 authenticates the existence of Mg2Cu.

Fig. 6 Calibration of electron diffraction pattern of of area B in Fig. 3(a)
Figure 7 shows the calibration of the electron diffraction pattern in Fig. 3(d). The matrix in Fig. 7 can not be distinguished distinctly, and the four diffraction rings are calibrated here. By comparing the interplanar spacing dk (k=1-7) with the PDF of Mg-Cu-Y system alloy, it can be found that they agree well with that of the FCC-Mg phase. It suggests that the diffraction rings calibrated in Fig. 7 authenticates the existence of FCC-Mg.

Fig. 7 Calibration of electron diffraction pattern of area C in Fig. 3(a)
Thermodynamically, the stacking fault energy of Mg and Cu are 125 and 40 mJ/m2, respectively. As the mass fraction of Cu in Mg65Cu25Y10 alloy is 39.15%, Cu seriously abates the stacking fault energy of Mg. Therefore, in the equilibrium cooling process, FCC-Mg probably remains, which can be seen from the relationship of free energy (G) of HCP-Mg, FCC-Mg and Mg2Cu phase to Cu content shown in Fig. 8 [16]. However, compared with HCP-Mg and Mg2Cu, FCC-Mg remains as a metastable state, and it transforms to HCP-Mg during the heat-treatment. As mentioned above, FCC-Mg exists in the matrix of the specimen aged at 200 ℃ for 12 min. It suggests that FCC-Mg in the specimen aged at 200 ℃ for 12 min has not transformed to HCP-Mg completely. Figure 9 shows the SEM image of the specimen aged at 200 ℃ for 7 min. By magnifying and sharpening the area in the box, the black spicules which are often referred as long-periodic acicular HCP-Mg can be easily found. Similarly, α-Mg was found in Mg-based amorphous alloy matrix composite materials by HUI et al [17], and HCP-Mg with nano-size was found in Mg85Cu5Zn5Y5 amorphous alloy by YUAN et al [18]. Furthermore, the content of Cu in the matrix reduces rapidly as the generation of Mg2Cu and Cu2Y, and the stable HCP-Mg phase is separated out.

Fig. 8 Relationship of free energy (G) of HCP-Mg, FCC-Mg and Mg2Cu phase to Cu content [16]
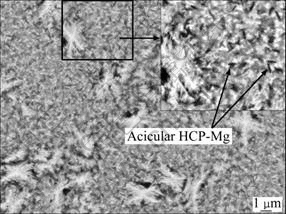
Fig. 9 Acicular HCP-Mg in Mg65Cu25Y10 bulk amorphous alloy after heat treatment
4 Conclusions
1) A snowflake-like structure with a diameter of about 1 μm can be separated out evenly from the Mg65Cu25Y10 bulk amorphous matrix during the crystallization process, and the number increases with increasing treating time.
2) The snowflake-like structure with laminated structures is the combination of Mg24Y5 and amorphous matrix.
3) The FCC-Mg phase in Mg65Cu25Y10 bulk amorphous alloy transforms into HCP-Mg in the heat-treating process.
References
[1] CHEN Gang, WANG Ning, ZHANG Wei. Study on aging of Mg65Cu25Y10 metallic glass [J]. Hot Working Technology, 2005(10): 13-17. (in Chinese)
[2] GAO R, HUI X, FANG H Z, LIU X J, CHEN G L, LIU Z K. Structural characterization of Mg65Cu25Y10 metallic glass from ab initio molecular dynamics [J]. Computational Materials Science, 2008, 44(2): 802-806.
[3] ZHANG Kou-shan, SI Nai-chao, CHEN Zhen-hua, ZHAO Wei, LIU Guang-lei. Preparation of bulky Mg amorphous alloy and its crystallization [J]. Special Casting and Nonferrous Alloys, 2007, 27(1): 21-23. (in Chinese)
[4] CHEN Gang, FERRY M. Crystallization of Mg-based bulk metallic glass [J]. Transactions of Nonferrous Metals Society of China, 2006, 16(4): 833-837.
[5] GUAN Le-ding, YAN Biao, YANG Sha. Micro and sub-micro morphology of Mg65Cu25Y10 bulk amorphous alloy containing primary crystalline phases [J]. Transactions of Nonferrous Metals Society of China, 2007, 17(s1): s1089-s1093.
[6] ELDRUP M, SCHR?DER, PEDERSEN A, OHNUMA M. Bulk amorphous alloys: Preparation and properties of (Mg0.98Al0.02)x(Cu0.75Y0.25)100-x [J]. Materials Science Forum, 2000, 343-346: 123-128.
[7] HUANG J C, CHU J P, JANG J S C. Recent progress in metallic glasses in Taiwan [J]. Intermetallics, 2009, 17(12): 973-987.
[8] JOHN A W, CHRISTIAN T, RUNE D J, MOGENS A. Forming of bulk metallic glass microcomponents [J]. Journal of Materials Processing Technology, 2009, 209(3): 1570-1579.
[9] JEAN-LOUIS S, SYLVAIN P. Phases formation during heating of Mg-Cu-Ag-Y bulk metallic glasses [J]. Journal of Alloys and Compounds, 2010, 495(2): 330-333.
[10] GYOO K S, INOUE A, MASUMOTO T. High mechanical strengths of Mg-Ni-Y and Mg-Cu-Y amorphous alloys with significant supercooled liquid region [J]. Materials Transactions, JIM, 1990, 31 (11): 929-934.
[11] INOUE A, KATO A, ZHANG T. Mg-Cu-Y amorphous alloys with high mechanical strengths produced by a metallic mold casting method [J]. Materials Transactions, JIM, 1991, 32(7): 609-616.
[12] INOUE A, NAKAMURA T, NISHIYAMA N. Mg-Cu-Y bulk amorphous alloys with high tensile strength produced by a high-pressure die casting method [J]. Materials Transactions, JIM, 1992, 33 (10): 937-945.
[13] GEBERT A, WOLFF U, JOHN A, ECKERT J. Corrosion behaviour of Mg65Cu25Y10 metallic glass [J]. Scripta Materialia, 2000, 43(3): 279-283.
[14] INOUE A. Stabilization of metallic supercooled liquid and bulk amorphous alloys [J]. Acta Materialia, 2000, 48(1): 279-306.
[15] WU Shu-sen, LIU Wei, MAO You-wu, AN Ping. Effect of Nd on glass forming ability of bulk amorphous Mg-Cu-Y-Nd alloys [J]. The Chinese Journal of Nonferrous Metals, 2007, 17(6): 852-857. (in Chinese)
[16] SUN Guo-yuan, CHEN Guang, CHEN Gou-liang. Crystallization behavior of amorphous Mg-based alloys [J]. Nonferrous Metals, 2004, 56(4): 1-7. (in Chinese)
[17] HUI X, DONG W, CHEN L, YAO K F. Formation, microstructure and properties of long-period order structure reinforced Mg-based bulk metallic glass composites [J]. Acta Materialia, 2007, 55(3): 907-920.
[18] YUAN G Y, ZHANG T, INOUE A. Structure and mechanical properties of Mg85Cu5Zn5Y5 amorphous alloy containing nanoscale particles [J]. Materials Letters, 2004, 58(24): 3012-3016.
Mg65Cu25Y10块体非晶合金的晶化组织
黄 康,陈 刚,赵玉涛,王国路,邵 阳
江苏大学 材料科学与工程学院,镇江 212013
摘 要:将普通铜模浇铸法制得的Mg65Cu25Y10块体非晶合金试样置于200 ℃进行保温处理,对处理后的试样的相组成和显微组织进行研究。XRD分析结果表明,该块体非晶的晶化随保温时间的延长趋于完全,在此过程中有Mg2Cu、Mg24Y5和HCP-Mg等晶体相析出。通过SEM观察到雪花状组织,EDS结果表明该雪花状组织的成分接近于铸态合金的成分,TEM分析表明其为层片状结构。电子衍射花样分析表明,雪花状组织由Mg24Y5晶体和非晶相组成。在保温过程中,基体中的FCC-Mg会转变为HCP-Mg。
关键词:镁基块体非晶;晶化;雪花状组织;层片状结构
(Edited by FANG Jing-hua)
Foundation item: Project (2008-04) supported by the Top Talent Plan of Jiangsu University, China; Project (10KJA430008) supported by the Natural Science Foundation of Jiangsu Higher Education Institutions, China
Corresponding author: CHEN Gang, Tel: +86-511-88792033; E-mail: gchen@ujs.edu.cn
DOI: 10.1016/S1003-6326(11)61252-1
Abstract: Mg65Cu25Y10 bulk amorphous alloy specimens prepared by conventional copper mould method were heated at 200 ℃ for different time and the phase contents as well as microstructure were studied. The XRD results show that the crystallization of Mg65Cu25Y10 bulk amorphous alloy specimen becomes complete as the treating time increases and Mg2Cu, Mg24Y5 and HCP-Mg crystalline phases are found. Snowflake-like morphology is found in different specimens through SEM observation. The EDS patterns show that the composition of the snowflake-like structure is close to that of the as-cast alloy. Laminated structures are observed from the TEM images of the snowflake-like structure. From the electron diffraction patterns, it is seen that the snowflake-like structure is the combination of Mg24Y5 and amorphous matrix. The FCC-Mg phase in the matrix transforms into HCP-Mg during the heat-treating process.


