DOI: 10.11817/j.issn.1672-7207.2020.12.013
熊去氧胆酸小檗红碱酯的制备
龙硕1,刘珍宝2,彭东明3,刘晓钦1,蒋婷1,刘艳飞1
(1. 中南大学 化学化工学院,湖南 长沙,410083;
2. 中南大学 湘雅药学院,湖南 长沙,410013;
3. 湖南中医药大学 药学院,湖南 长沙,410208)
摘 要:
,对小檗碱进行熊去氧胆酸酯化修饰,合成新型化合物熊去氧胆酸小檗红碱酯,以提高小檗碱的降脂活性。首先以熊去氧胆酸为原料,1,2-二溴乙烷为桥连试剂,合成熊去氧胆酸-β-溴乙酯,再将其与小檗碱高温裂解后的产物小檗红碱反应,合成目标产物;探究反应温度、反应时间、投料比和反应溶剂等参数;对化合物进行核磁共振谱和质谱表征分析。研究结果表明:酯化反应最优条件是温度为25 ℃,以二甲基甲酰胺(DMF)为溶剂、K2CO3为缚酸剂;在醚化反应中,当温度为60 ℃,以DMF为溶剂、KI为催化剂时,收率最大,总收率达53.0%。
关键词:
中图分类号:R914.4 文献标志码:A
文章编号:1672-7207(2020)12-3389-07
Preparation of ursodeoxycholic acid berberrubine ester
LONG Shuo1, LIU Zhenbao2, PENG Dongming3, LIU Xiaoqin1, JIANG Ting1, LIU Yanfei1
(1. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. Xiangya School of Pharmaceutical Sciences, Central South University, Changsha 410013, China;
3. School of Pharmacy, Hunan University of Chinese Medicine, Changsha 410208, China)
Abstract: To improve the lipid-lowering activity of berberine, a novel compounds berberrubine ursodeoxycholate was synthesized by bridged esterification method to modify the 9-O berberine with ursodeoxycholic acid. Firstly, ursodeoxycholic acid was used as the raw material and 1, 2-dibromoethane was used as the bridging reagent to synthesize ursodeoxycholic acid-β-bromoethyl ester, and then it reacted with the high temperature pyrolytic product of berberine to synthesize the target product. The conditions such as temperature, time, feeding ratio and solvent were studied. The compound structure was confirmed by nuclear magnetic resonance(NMR) spectroscopy and mass spectrometry. The results show that the optimal conditions of the ester formation reaction are DMF used as solvent and K2CO3 as acid binding agent at 25 ℃. In the ether forming reaction, the yield is the highest when DMF is used as solvent and KI as catalyst at 60 ℃, and the total yield is 53.0%.
Key words: berberrubine; ursodeoxycholic acid; esterification; nucleophilic substitution
小檗碱(berberine,BBR)是天然药物黄连中的主要有效成分,故又称为黄连素。小檗碱化学结构是一类含喹嗪的生物季铵碱[1-2]。研究表明小檗碱作为一类天然产物,毒副作用小,具有降血脂[3]、降血糖[4]、抗菌[5]、抗肿瘤[6]等药理作用。小檗碱由于其溶解性和渗透性差,在体内不容易被吸收,生物利用度低,因此,对小檗碱进行结构修饰成为了研究热点[7]。目前,对小檗碱的结构修饰主要集中在其异喹啉环的N(7),C(8),C(9),C(12)和C(13)这5个位置单位点或者双位点改造。BIAN等[8]将小檗碱芳香族季氮还原为叔氮,然后烷基化生成N-烷基衍生物,再氧化生成N-氧化物,并探究了体外的降糖活性。YANG等[9]以小檗碱为原料,以环小檗碱为先导化合物合成了一系列新的8-取代环小檗碱衍生物,研究发现其对革兰氏阳性病菌的抗菌活性比小檗碱的强。LIU等[10-11]合成了一系列新的9-O取代小檗碱衍生物,并对其抗炎活性进行了评价,与小檗碱相比,布洛芬和萘丙嗪修饰的小檗碱衍生物具有增强抗炎活性的作用。ENKHTAIVAN等[12]在小檗红碱C(12)上连接哌嗪类分子,合成了哌嗪类小檗碱衍生物,并发现小檗碱衍生物的抗流感活性较小檗碱的强,其中,对氟苯基取代的哌嗪小檗碱抗流感活性较对照药物奥司他韦强。PARK等[13]通过在小檗碱和小檗红碱的13-C中引入不同的芳香基团,合成了一系列13-取代苄基的小檗碱和小檗红碱衍生物,并对其抗真菌活性进行了研究,研究发现合成的化合物具有比小檗碱和小檗红碱更强的抗真菌活性。鉴于此,基于小檗碱药物功能需求,各类不同药理活性的修饰基团或化合物被设计并连接于小檗碱各个位点,为小檗碱衍生物合成提供了实验基础。熊去氧胆酸(ursodeoxycholic acid,UDCA)最初由北极熊胆内提取出来,作为一种次级胆酸[14],其结构中包含甾体母体及羟基、羧基双官能团。传统中药中熊胆曾被用来清除毒素、治疗肝疾和眼疾[15]。现阶段研究报道UDCA具有溶解胆固醇结石[16],有利于治疗胆汁淤积性肝功能疾病,并显著降低胆汁中胆固醇饱和度的优良药物性能[17]。在药理学方面,因为它具有很好的疗效且没有毒副作用,被认为是很好的治疗胆结石的药物[18]。因此,UDCA在临床上主要用于治疗高血脂和肝胆疾病[19]。本文作者拟以熊去氧胆酸修饰9-O小檗碱,期望2种具有降脂功能的药物能在体内发挥出协同作用,并通过引入熊去氧胆酸,改善小檗碱酯油水分配系数以提高生物利用度,增强降脂活性。通过直接偶联实验发现,小檗红碱修饰位点酚羟基酯化活性较低,且空间位阻大,难以与熊去氧胆酸直接酯化连接,故采用桥连[20]的方法修饰小檗碱。
1 实验
1.1 样品与试剂
样品有小檗碱(湖南湘泉制药有限公司生产)和熊去氧胆酸(湖南云港生物科技有限公司生产),其他试剂和溶剂均为分析纯。
1.2 小檗红碱的制备
称取10.0 g(0.028 mol)小檗碱,在温度为190 ℃、压力为2.67 kPa真空干燥箱中反应0.5 h,小檗碱黄色粉末完全变为暗红色固体。将小檗碱黄色粉末冷却至室温,将其用中性氧化铝柱(流动相用乙醇与二氯甲烷体积比(V(乙醇):V(二氯甲烷))为1:9)进行分离,浓缩收集液,于35 ℃旋转蒸发,得到暗红色纯品化合物Ⅰ小檗红碱,产率为94.6%。
1.3 直接酯化法制备熊去氧胆酸小檗红碱酯
称取0.50 g (1.56 mmol)小檗红碱置于50 mL圆底烧瓶中,加入20 mL除水CH3CN,超声使其充分溶解,然后在体系中加入0.095 g (0.78 mmol) 4-二甲氨基吡啶(DMAP)和0.61 g (1.56 mmol)熊去氧胆酸,在恒温磁力搅拌器上搅拌5 min后加入0.15 g (0.78 mmol) 1-乙基-(3-二甲基氨基丙基碳二亚胺盐酸盐)(EDCI),在25 ℃下搅拌反应12 h,利用薄层色谱(TLC)(V(乙醇):V(二氯甲烷)=1:10为流动相)监测反应。
1.4 熊去氧胆酸-β-溴乙酯的制备
称取1.0 g(2.55 mmol)熊去氧胆酸白色粉末置于50 mL圆底烧瓶中,将其溶于10 mL二甲基甲酰胺(DMF)溶液并在体系中滴加3 mL 1,2-二溴乙烷,在不断搅拌下加入1.4 g K2CO3。对反应体系在常温(25 ℃)下搅拌,利用TLC(V(乙酸乙酯):V(石油醚)=2:1,为流动相)监测,4 h反应完全。过滤除去反应液中K2CO3固体,加入20 mL氯仿萃取3次,收集合并萃取液,先后用50 mL饱和NaHCO3溶液、50 mL饱和NaCl溶液洗涤,洗涤后加入适量无水MgSO4干燥24 h。于30 ℃旋蒸浓缩反应液,以硅胶为固定相,V(乙酸乙酯):V(石油醚)=1:1为流动相进行柱层析分离,收集合并分离液,蒸干溶剂得白色粉末状固体,得目标化合物Ⅱ,产率为73.1%。
1.5 熊去氧胆酸-(9-O-乙基)-小檗红碱酯的合成
称取0.50 g(1.4 mmol)化合物Ⅰ,0.75 g (1.5 mmol)化合物Ⅱ以及0.025 g KI (0.15 mmol)于50 mL圆底烧瓶,加入10 mL DMF使其充分溶解,体系在60 ℃下搅拌反应10 h,利用TLC监测,反应完全。冷却至室温,滴加乙醚至析出晶体,将其抽滤,滤饼于35 ℃真空干燥12 h,得到粗品黄色固体。粗产品以中性氧化铝柱为固定相,V(乙醇):V(二氯甲烷)=1:10为流动相进行柱层析分离得暗黄色最终产物目标化合物,产率为72.5%。
2 实验结果与讨论
2.1 熊去氧胆酸小檗红碱酯合成
熊去氧胆酸小檗红碱酯的直接偶联法合成路线见图1。
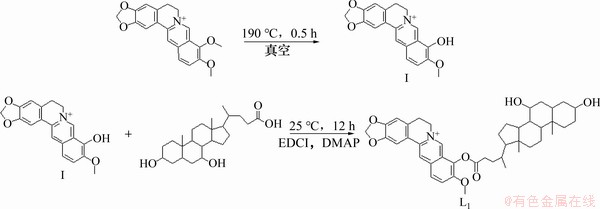
图1 化合物L1的合成路线
Fig. 1 Synthesis route of compound L1
通过对反应条件的多次探究,发现小檗红碱与熊去氧胆酸难以直接酯化,分析其原因可能是:一方面,熊去氧胆酸是一类天然有机弱酸,作为酯化反应中心的羧基表现出较低的反应活性;另一方面,小檗红碱中羟基为酚羟基,受苯环共轭效应与临位甲氧基空间位阻的影响,难以同熊去氧胆酸酯化缩合。而桥连法能有效解决分子间因空间位阻较大而难以偶联的难点,因此,将熊去氧胆酸小檗红碱酯的合成策略改变为桥连合成法。
熊去氧胆酸小檗红碱酯的桥连法合成路线见图2。
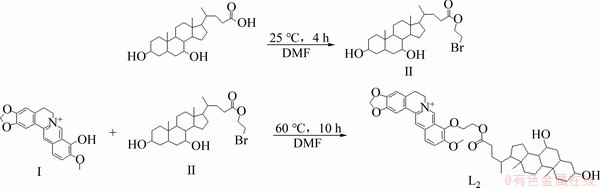
图2 化合物L2的合成路线
Fig. 2 Synthesis route of compound L2
对所合成的化合物使用Bruker Avance III400 MHz核磁仪进行氢谱和碳谱表征,使用Bruker compact Q-TOF质谱仪进行质谱表征。
中间体熊去氧胆酸-β-溴乙酯的核磁共振氢谱图如图3所示。其1H NMR(400 MHz, CDCl3)表征数据为:化学位移δ 4.38(t, J=6.1 Hz, 2H),3.58 (dd,J=10.3, 5.5 Hz, 2H),3.51 (t, J=6.1 Hz,2H),2.40 (ddd, J=15.1, 9.9, 5.1 Hz, 2H),2.34~2.20 (m, 2H),0.95 (s, 6H), 0.68 (s, 3H)(其中,s指单峰,d指双重峰,t指三重峰,m指多重峰,dd指双二重峰,ddd指对称的2个dd峰)。由图3可以看出:在接入溴乙基之后,在化学位移4.38和3.58处出现2组三重峰,为溴乙基上乙基的峰,表示小檗红碱酯成功与熊去氧胆酸相连接,而由于溴乙基的影响,熊去氧胆酸环上面氢的化学位移都产生微小变化。
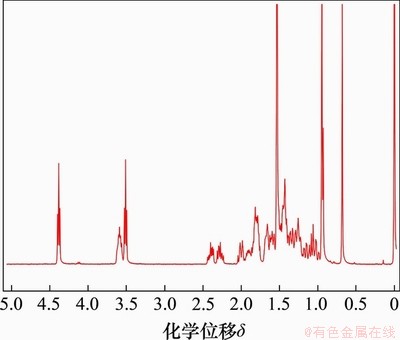
图3 熊去氧胆酸-β-溴乙酯的核磁共振氢谱图
Fig. 3 1H NMR spectrum of ursodeoxycholic acid-β-bromoethyl ester
目标化合物熊去氧胆酸小檗红碱酯的核磁共振氢谱如图4所示。其1H NMR(400 MHz, DMSO-d6)表征数据为:化学位移δ 9.85 (s, 1H),8.99(s, 1H),8.22 (d, J=9.1 Hz, 1H),8.02 (d, J=9.1 Hz, 1H),7.81 (s, 1H),7.10 (s, 1H),6.18 (s, 2H),4.95 (t, J=5.9 Hz, 2H),4.54 (d, J=4.2 Hz, 2H),4.47(t, J=3.4 Hz, 2H),4.07 (s, 3H),0.86 (s, 6H),0.54 (s, 3H)。由图4可知:在化学位移6~8之间出现多组峰;对应的是小檗碱母体苯环上氢的峰;化学位移4.95和4.47对应的是溴乙基的峰;化学位移0.5~2.5之间对应的是熊去氧胆酸母体的峰。
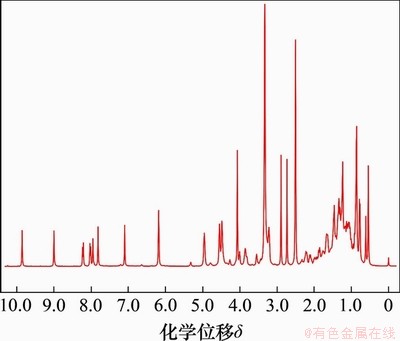
图4 化合物L2的核磁共振氢谱图
Fig. 4 1H NMR spectrum of compound L2
目标化合物熊去氧胆酸小檗红碱酯的核磁共振碳谱如图5所示。其13C NMR(101 MHz,DMSO-d6)表征数据为:化学位移173.7,150.6,150.4,148.3,145.8,143.0,138.0,133.5,131.1,127.2,124.1,122.1,120.9,120.8,108.9,105.9,102.6,72.3,70.2,70.0,64.0,57.6,56.2,55.1,43.6,43.5,42.6,39.1,38.2,37.7,36.3,35.3,34.2,31.3,31.1,30.7,28.6,27.1,26.9,23.8,21.3,18.7,12.5,12.4。碳谱表征结果与化合物小檗红碱熊去氧胆酸酯分子式的结构特征相符。
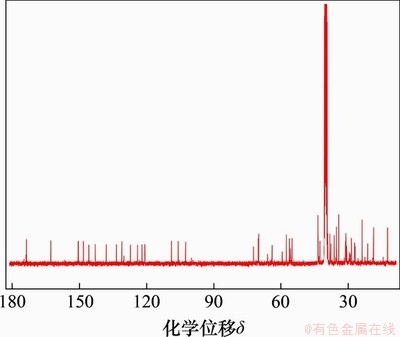
图5 化合物L2的核磁共振碳谱图
Fig. 5 13C NMR spectrum of compound L2
熊去氧胆酸小檗红碱酯的质谱如图6所示。经数据分析可知:其分子离子峰质荷比(m/z)为740.451 1,这与[C45H58NO8+]的质荷比预测值740.42相吻合,确定为目标化合物。
2.2 反应条件优化
在酯化反应中,将熊去氧胆酸与二溴乙烷反应制备出熊去氧胆酸-β-溴乙酯,该步反应所优化条件与产率见表1。
表1 酯化反应合成条件
Table 1 Synthesis conditions of ester formation reaction

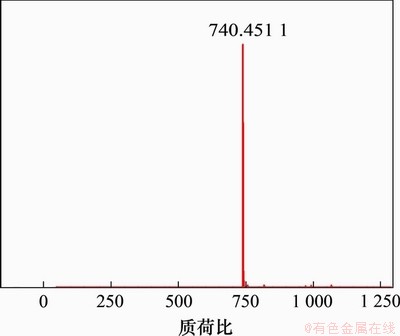
图6 化合物L2的质谱图
Fig. 6 Mass spectrum of compound L2
首先对反应温度进行考察。由表1可知:在温度为20~40 ℃时,温度与产率的关系呈现出近似抛物线关系;25 ℃时,产率最高,达73.1%。造成这种趋势的原因在于在碱性环境中,较低温度无法提供反应所需达到能垒的能量,从而使得产率稍低;随着温度提升,卤代烃发生消除反应的趋势增大,故产率又有所下降,但因为缚酸剂K2CO3作为固相与整个液相体系分离,碱性较低使得下降程度并不十分明显。而TLC显示,反应从1 h开始便有产物点生成,4 h后反应结束,延长反应时间对产率无明显提升。
选择DMF、乙酸乙酯(EA)和乙醇(EtOH)为溶剂,发现熊去氧胆酸能很好地溶于DMF与EtOH,在EA中溶解度较低,以DMF为溶剂时产率最高,达73.1%,而在EA中反应产率稍有降低,这可能是反应底物在该溶剂中的溶解度下降所致。虽然底物能很好地溶于EtOH,但产率骤减至17.2%,其原因可能是:作为溶剂的乙醇同时也是该步亲核取代反应的竞争物质,从而导致反应不充分,副产物主要为乙基-β-溴乙基醚。考虑到该步反应将产生游离HBr,需要加入适量缚酸剂以促进反应正向进行,根据已有研究,首先选择最为常见的K2CO3作为缚酸剂;随后,考虑到K2CO3的加入使得反应体系为非均相反应,又选择常用的有机缚酸剂吡啶进行研究。结果表明:作为缚酸剂,吡啶的效果略微优于K2CO3的效果,产率达到74.7%,虽然以K2CO3为缚酸剂的产率(73.1%)略低于吡啶为缚酸剂的产率,但考虑到后续处理简便以及试剂的毒性,选择K2CO3为最优缚酸剂。
制备得到熊去氧胆酸-β-溴乙酯后,将其与小檗红碱进行亲核取代反应,并对该步反应中的反应温度、反应时间、溶剂种类、催化剂种类及用量进行条件优化,结果见表2。
表2 醚化反应合成条件
Table 2 Synthesis conditions of ether forming reaction
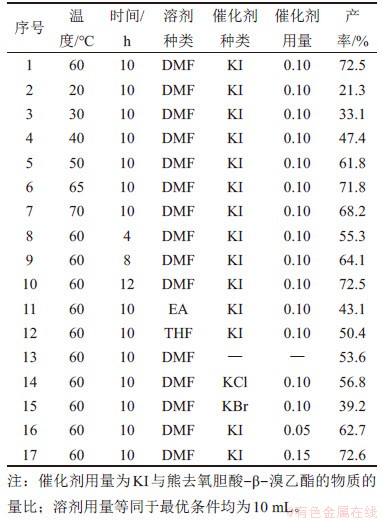
由表2可见:随着温度升高,醚化反应产率与温度呈抛物线关系,最优温度为60 ℃。当催化剂用量为0.10时,产率为72.5%;当催化剂用量为0.15时,产率为72.6%。经综合考虑,最优条件选用催化剂用量为0.10。结合酯化反应最高产率为73.1%,所得熊去氧胆酸小檗红碱酯的总收率为53.0%。
在反应过程中,酯化反应时间为4 h,而醚化反应需10 h完成,这也进一步说明在小檗红碱与熊去氧胆酸的缩合连接中,小檗红碱是整个反应的关键,需解决其羟基活性不足、所受空间位阻较大的问题。
在溶剂的选择上,已验证EtOH作为溶剂对反应体系的消极影响,改用非质子溶剂四氢呋喃(THF)。结果表明,DMF仍是最优的溶剂。最后,考虑到该步反应引入了连接点周围空间位阻较大的小檗碱,选择卤酸盐进行催化反应,并对催化剂的种类与用量进行筛选,发现当不使用任何催化剂时,该步反应产率仅有53.6%,而KI对该步反应有明显的催化效果,使得产率增加至72.5%。KCl对反应几乎没有催化作用,产率为56.8%。反常的是,KBr的加入使得反应产率较不添加催化剂时更低,产率仅为39.2%,其原因在于溴化钾的同离子效应使得熊去氧胆酸-β-溴乙酯中的溴元素去除难度增加,从而对反应产生了抑制作用。
3 结论
1) 以小檗碱和熊去氧胆酸为原料,通过小檗碱9-O位酯化,制备了新型化合物熊去氧胆酸-(9-O-乙基)-小檗红碱酯,并经氢谱、碳谱和质谱确证了化合物结构。
2) 以1,2-二溴乙烷为桥连试剂,克服了小檗碱和熊去氧胆酸连接位点空间位阻过大且反应活性较低等问题,成功得到目标化合物。此合成工艺简单,条件温和,得到的产物稳定,收率高达53.0%。
3) 基于药物的协同作用,该熊去氧胆酸修饰的小檗碱衍生物可望成为一种优良的降脂药物用于临床。
参考文献:
[1] CERNAKOVA M, KOST'ALOVA D, KETTMANN V, et al. Potential antimutagenic activity of berberine, a constituent of Mahonia aquifolium[J]. BMC Complementary and Alternative Medicine, 2002, 2: 2.
[2] ORFILA L, RODRIGUEZ M, COLMAN T, et al. Structural modification of berberine alkaloids in relation to cytotoxic activity in vitro[J]. Journal of Ethnopharmacology, 2000, 71(3): 449-456.
[3] CAO Shijie, YU Shengyang, CHENG Lina, et al. 9-O-benzoyl-substituted berberine exerts a triglyceride-lowering effect through AMPK signaling pathway in human hepatoma HepG2 cells[J]. Environmental Toxicology Pharmacology, 2018, 64: 11-17.
[4] HAO Min, LI Shuyuan, SUN Changkai, et al. Amelioration effects of berberine on diabetic microendothelial injury model by the combination of high glucose and advanced glycation end products in vitro[J]. European Journal of Pharmacology, 2011, 654(3): 320-325.
[5] YAN Dan, JIN Cheng, XIAO Xiaohe, et al. Antimicrobial properties of berberines alkaloids in Coptis chinensis Franch by micro calorimetry[J]. Journal of Biochemical and Biophysical Methods, 2008, 70(6): 845-849.
[6] JEON Y W, JUNG J W, KANG M, et al. NMR studies on antitumor drug candidates, berberine and berberrubine[J]. Bulletin of the Korean Chemical Society, 2002, 23(3): 391-394.
[7] 吴龙龙, 方方. 小檗碱的结构修饰及其构效关系研究进展[J]. 中国新药杂志, 2020, 29(11): 1257-1264.
WU Longlong, FANG Fang. Advances in structural modification and structure-activity relationship of berberine[J]. Chinese Journal of New Drugs, 2020, 29(11): 1257-1264.
[8] BIAN Xiaoli, HE Langchong, YANG Guangde. Synthesis and antihyperglycemic evaluation of various protoberberine derivatives[J]. Bioorganic & Medicinal Chemistry Letters, 2006, 16(5): 1380-1383.
[9] YANG Yuanshuai, LU Xi, ZENG Qingxuan, et al. Synthesis and biological evaluation of 7-substituted cycloberberine derivatives as potent antibacterial agents against MRSA[J]. European Journal of Medicinal Chemistry, 2019, 168: 283-292.
[10] LIU Zhenbao, WANG Xiaohong, ZHANG Hang, et al. Synthesis and anti-inflammatory effects of a series of novel 9-O-substituted berberine derivatives[J]. Medicinal Chemistry Research, 2017, 26(3): 672-679.
[11] ZHANG Shanshan, WANG Xiaohong, YIN Weicheng, et al. Synthesis and hypoglycemic activity of 9-O-(lipophilic group substituted) berberine derivatives[J]. Bioorganic & Medicinal Chemistry Letters, 2016, 26(19): 4799-4803.
[12] ENKHTAIVAN G, MUTHURAMAN P, KIM D H, et al. Discovery of berberine based derivatives as anti-influenza agent through blocking of neuraminidase[J]. Bioorganic & Medicinal Chemistry, 2017, 25(20): 5185-5193.
[13] PARK K D, LEE J H, KIM S H, et al. Synthesis of 13-(substituted benzyl) berberine and berberrubine derivatives as antifungal agents[J]. Bioorganic Medicinal Chemistry Letters, 2006, 16(15): 3913-3916.
[14] GOOSSENS J F, BAILLY C. Ursodeoxycholic acid and cancer: from chemoprevention to chemotherapy[J]. Pharmacology & Therapeutics, 2019, 203: 107396.
[15] 杨辛欣, 车燚, 李超英, 等. 熊胆缓释滴眼凝胶兔眼内药代动力学研究[J]. 时珍国医国药, 2017, 28(7): 1634-1636.
YANG Xinxin, CHE Yi, LI Chaoying, et al. Study on pharmacokinetics of bear gall sustained release ophthalmic gel in the rabbit eyes[J]. Lishizhen Medicine and Materia Medica Research, 2017, 28(7): 1634-1636.
[16] JANG S I, FANG S, KIM K P, et al. Combination treatment with n-3 polyunsaturated fatty acids and ursodeoxycholic acid dissolves cholesterol gallstones in mice[J]. Scientific Reports, 2019, 9: 12740.
[17] ZHANG Yunjing, ZHENG Xiaojie, HUANG Fengjie, et al. Ursodeoxycholic acid alters bile acid and fatty acid profiles in a mouse model of diet-induced obesity[J]. Front Pharmacol, 2019, 10: 842.
[18] TONIN F, ARENDS I. Latest development in the synthesis of ursodeoxycholic acid(UDCA): a critical review[J]. Beilstein Journal of Organic Chemistry, 2018, 14: 470-483.
[19] COSKUN B D O, YUCESOY M, GURSOY S, et al. Effects of ursodeoxycholic acid therapy on carotid intima media thickness, apolipoprotein A1, apolipoprotein B, and apolipoprotein B/A1 ratio in nonalcoholic steatohepatitis[J]. European Journal of Gastroenterology & Hepatology, 2015, 27(2): 142-149.
[20] HAO Mengjiao, LI Yan, LIU Lixian, et al. The design and synthesis of a novel compound of berberine and baicalein that inhibits the efficacy of lipid accumulation in 3T3-L1 adipocytes[J]. Bioorganic & Medicinal Chemistry, 2017, 25(20): 5506-5512.
(编辑 杨幼平)
收稿日期: 2020 -07 -21; 修回日期: 2020 -09 -11
基金项目(Foundation item):湖南省自然科学基金资助项目(2020JJ4680);湖南省教育厅科学重点研究项目(18A211);中南大学升华育英计划项目(CX20190242) (Project(2020JJ4680) supported by the Natural Science Foundation of Hunan Province; Project(18A211) supported by the Key Scientific Research of the Education Department of Hunan Province; Project(CX20190242)supported by the Sublimation Education of Central South University)
通信作者:刘艳飞,博士,副教授,从事药物化学与药物制剂研究;E mail: liuyf@csu.edu.cn
摘要:采用桥连酯化法,对小檗碱进行熊去氧胆酸酯化修饰,合成新型化合物熊去氧胆酸小檗红碱酯,以提高小檗碱的降脂活性。首先以熊去氧胆酸为原料,1,2-二溴乙烷为桥连试剂,合成熊去氧胆酸-β-溴乙酯,再将其与小檗碱高温裂解后的产物小檗红碱反应,合成目标产物;探究反应温度、反应时间、投料比和反应溶剂等参数;对化合物进行核磁共振谱和质谱表征分析。研究结果表明:酯化反应最优条件是温度为25 ℃,以二甲基甲酰胺(DMF)为溶剂、K2CO3为缚酸剂;在醚化反应中,当温度为60 ℃,以DMF为溶剂、KI为催化剂时,收率最大,总收率达53.0%。


