
Effects of synthesis conditions on layered Li[Ni1/3Co1/3Mn1/3]O2 positive-electrode via hydroxide co-precipitation method for lithium-ion batteries
HU Chuan-yue1, 2, GUO Jun2, DU Yong1, XU Hong-hui1, HE Yue-hui1
1. State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China;
2. Department of Chemistry and Materials Science, Hunan Institute of Humanities, Science and Technology,
Loudi 417000, China
Received 6 Januray 2010; accepted 10 May 2010
Abstract:
Layered Li[Ni1/3Co1/3Mn1/3]O2 was synthesized with complex metal hydroxide precursors that were prepared by a co-precipitation method. The influence of coordination between ammonia and transition-metal cations on the structural and electrochemical properties of the Li[Ni1/3Co1/3Mn1/3]O2 materials was studied. It is found that when the molar ratio of ammonia to total transition-metal cations is 2.7:1, uniform particle size distribution of the complex metal hydroxide is observed via scanning electron microscopy. The average particle size of Li[Ni1/3Co1/3Mn1/3]O2 materials was measured to be about 500 nm, and the tap-density was measured to be approximately 2.37 g/cm3, which is comparable with that of commercialized LiCoO2. XRD analysis indicates that the presently synthesized Li[Ni1/3Co1/3Mn1/3]O2 has a hexagonal layered-structure. The initial discharge capacity of the Li[Ni1/3Co1/3Mn1/3]O2 positive-electrode material is determined to be 181.5 mA?h/g using a Li/Li[Ni1/3Co1/3Mn1/3]O2 cell operated at 0.1C in the voltage range of 2.8-4.5 V. The discharge capacity at the 50th cycle at 0.5C is 170.6 mA?h/g.
Key words:
layered structure; coordination effect; hydroxide co-precipitation; cathode material; lithium ion batteries;
1 Introduction
In recent years, LiNi1/3Co1/3Mn1/3O2 used as the cathode material in lithium-ion batteries has been extensively investigated because of its high capacity, good thermal stability and relatively low cost compared with LiCoO2[1-2]. The rechargeable capacity of Li[Ni1/3Co1/3Mn1/3]O2 was determined to be 160 mA?h/g with the cell operated in the voltage range of 2.5-4.4 V and to be more than 200 mA?h/g in the voltage range of 2.8-4.6 V [3-4]. It is well known that the layered LiNi1-x-yCoxMnyO2 can be synthesized by various methods, such as hydroxide co-precipitation route[1], carbonate co-precipitation route[5-6], oxalate co- precipitation route[7-8], and solid state method[9]. The structure stability and cycling stability of LiNi1-x-yCoxMnyO2 are greatly related to its precursors used in the synthesis process, and can be further improved by using dopants, such as Cr[10], F[8, 11] and Al[11-12]. For example, Li[(Ni1/3Col/3Mn1/3)0.96Al0.02- B0.02]O1.98F0.02, which was synthesized by hydroxide co-precipitation method, delivered a reversible discharge capacity of 190 mA?h/g in the voltage range of 3.0-4.7 V[13]. However, the reversible capacities of the no-doped Li[Ni1/3Co1/3Mn1/3]O2 composites, which were synthesized by the same method, were reported to be 177 mA?h/g in the voltage rang of 2.8-4.5 V[14], 169 mA?h/g in the voltage rang of 3.0-4.3 V[15], and 162.1 mA?h/g in the voltage rang of 3.0-4.3 V[1], respectively. It is obvious that the latter three values of reversible capacity are lower than the former value reported by YE et al[13].
The hydroxide co-precipitation method is commonly employed for the preparation of new materials via a liquid-solution transition. However, some of the precipitated transition-metal hydroxides are readily oxidized in the aqueous solution. For instance, Mn(OH)2 is gradually oxidized in air to form MnOOH or MnO2, which is regarded as the impurity of the final product. It was demonstrated[14] that how to control the valence state of Mn in aqueous solution is critical to obtain homogeneous Li[Ni1/3Co1/3Mn1/3]O2 materials. Although some works have been done to solve this problem, the homogeneity and morphology of Li[Ni1/3Co1/3Mn1/3]O2 materials are influenced by the process of hydroxide co-precipitation. In this work, the effect of the coordination of transition-metal cations with ammonia on the structure and electrochemical performance of Li[Ni1/3Co1/3Mn1/3]O2 was investigated.
2 Experimental
In order to prepare a homogenous (Ni1/3Co1/3Mn1/3)(OH)2 precursor, the hydroxide co-precipitation method was employed, as reported by LEE et al[14]. CoSO4?7H2O (AR), NiSO4?6H2O (AR), MnSO4?H2O (AR), NaOH (AR), NH4OH (AR) and LiOH (AR) were used as starting materials. Three aqueous solutions, viz. 2 mol/L sodium hydroxide solution, 2 mol/L transition-metal salt solution and 0.36 mol/L ammonia solution, were prepared. A complex solution was obtained by simultaneously dropping the mixed transition-metal sulfate solution (n(Ni):n(Co): n(Mn)=1:1:1) and ammonia solution into a hot reaction bath (60 °C) in an inert atmosphere. Then, a pink (Ni1/3Co1/3Mn1/3)(OH)2 precipitate was obtained by adjusting pH value of the complex solution to 11 with sodium hydroxide solution. After strongly stirring overnight in an inert atmosphere, the pink (Ni1/3Co1/3Mn1/3)(OH)2 particles were filtered, washed several times with NH4OH solution, and dried.
The transition-metal hydroxide powder was mixed with excessive 8% LiOH·H2O (excess amount of Li salts was used to compensate for possible Li loss during the calcinations). After ball milling for 10 h, the mixture was heated to 480 °C and held for 5 h, and then calcined at 950 °C for 10 h in air atmosphere to form the Li(Ni1/3Co1/3Mn1/3)O2 solid solution. The powder X-ray diffraction (XRD) analysis was performed using a Cu Kα radiation of Rigaku D/max 2550 diffractometer. The morphology and particle size of the powders were examined by using scanning electron microscope (SEM).
A R2025 cell, which consisted of a cathode and a lithium foil anode separated by a Celgard 2400 porous polypropylene film, was assembled in a glove box filled with dried argon gas. The cathode contained a mixture of the accurately weighed Li(Ni1/3Co1/3Mn1/3)O2 powder, carbon black and PVDF in a mass ratio of 90.5:3.5:6.0. The electrolyte was 1 mol/L LiPF6 in ethylene carbonate (EC)-dimethylene carbonate (DMC)-ethylmethyl carbonate (EMC) (1:1:1 in mass ratio). The charge- discharge tests were performed using the coin-type R2025 cells operated at the current density of 16 mA/g in
the voltage range of 2.8-4.5 V at 25 °C (160 mA/g was assumed to be the 1C ratio capacity.
3 Results and discussion
3.1 Coordination effect of ammonia with transition- metal cation during co-precipitation
It is well known that NH3 plays an important role in facilitating the formation of dense sphere-like hydroxide according to the following reactions:
Ni2+(aq)+Co2+(aq)+Mn2+(aq)+(x+y+z)NH3·H2O(aq)→[Ni(NH3)x2++Co(NH3)y2++Mn(NH3)z2+](aq)+ (x+y+z)H2O (1)
[Ni(NH3)x2++Co(NH3)y2++Mn(NH3)z2+](aq)+6OH-+gH2O→3(Ni1/3Co1/3Mn1/3)(OH)2(s)+ gNH4OH(aq)+(x+y+z-g)NH3(g) (2)
The co-precipitation reaction occurs when the transition-metal salts solution and the NH3·H2O solution are mixed together. The individual hydroxides Ni(OH)2, Co(OH)2 and Mn(OH)2, which are regarded as the impurity phases in (Ni1/3Co1/3Mn1/3)(OH)2 product, are prone to separately form at different pH values corresponding to the different solubility product constants of these hydroxides, respectively, if certain ligand is not employed. The ligand NH3·H2O, which can considerably decrease the concentrations of free ion Ni2+, Co2+ and Mn2+ due to formation of the complex groups, facilitates their co-precipitation, resulting in the formation of a homogeneous (Ni1/3Co1/3Mn1/3)(OH)2 phase.
The n value of complex M(NH3)n (M=Ni2+, Co2+, Mn2+) can be from 1 to 6 when the molar ratio of ammonia to total transition-metal ions in Eq.(1) changes. The stability constants of complex compounds change with the coordination numbers of metal ions. Thus, only under the condition that the three complex ions of Ni(NH3)x2+, Co(NH3)y2+ and Mn(NH3)z2+ have the approximate stability constants (forming the appropriate coordination effect of ammonia with transition-metal ion), the transition-metal ions of Ni2+, Co2+ and Mn2+ would be precipitated at the same time. For the preparation of a homogeneous (Ni1/3Co1/3Mn1/3)(OH)2 phase, it is rather critical to obtain complex ion of Ni(NH3)x2+, Co(NH3)y2+, and Mn(NH3)z2+, of which the stability constants are approximate.
In the present work, (Ni1/3Co1/3Mn1/3)(OH)2, the precursor for the preparation of Li[Ni1/3Co1/3Mn1/3]O2, was prepared in an inert atmosphere. The molar ratios of ammonia to total transition-metal cations were 2.1:1, 2.4:1, 2.7:1, and 3.0:1, which are denoted as Sa, Sb, Sc, and Sd in the following sections, respectively.
The co-precipitated (Ni1/3Co1/3Mn1/3)(OH)2 powders were dried at 80 °C in vacuum to remove the adsorbed
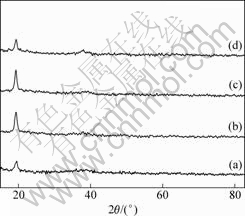
Fig.1 XRD patterns of (Ni1/3Co1/3Mn1/3)(OH)2 powders prepared at various molar ratios of ammonia to transition-metal ions: (a) Sa, 2.1:1; (b) Sb, 2.4:1; (c) Sc, 2.7:1; (d) Sd, 3.0:1
water because the divalent Co and Mn are readily oxidized at 110 °C in air atmosphere. The atomic absorption spectroscopy (AAS) measurement was performed to determine the chemical compositions of the co-precipitation powders. The molar ratio of Ni/Co/Mn was measured to be approximately 1:1:1, which is almost the same as the nominal one.
Fig.1 shows XRD patterns of (Ni1/3Co1/3Mn1/3)- (OH)2 powders prepared at different molar ratios of ammonia to Ni+Co+Mn. As shown in Fig.1, a broad line and a intense peak at around 2θ=19° were observed in the XRD patterns of the four samples, which is consistent with the typical fingerprint of NixCoyMnz(OH)2 structure[14]. The absence of impurity
phases suggests that Ni, Co, and Mn should be homogeneously crystallized in the hydroxide of (Ni1/3Co1/3Mn1/3)(OH)2.
Fig.2 shows SEM images of the (Ni1/3Co1/3Mn1/3)(OH)2 powders. The particle shape of Sample Sc is much more spherical than Sample Sa, Sb, or Sd. Most of the particles have the size of 4-10 μm and there is no particle agglomeration. Hence, the coordination effect corresponding to the molar ratio 2.7:1 of ammonia to total transition-metal ions is appropriate and gives rise to narrow particle size distribution without particles agglomeration.
3.2 Phase structure of synthesized Li[Ni1/3Co1/3Mn1/3]- O2 powder
Fig.3 shows the XRD pattern of the Li[Ni1/3Co1/3Mn1/3]O2 powders synthesized with different metal hydroxide precursors. No secondary phases as impurities are observed. All these powders are characterized as a layered oxide structure based on a hexagonal α-NaFeO2 structure (space group R-3m). Generally, the Li ions occupy the 3a sites, while the M ions (M=Co, Ni, Mn) and O ions occupy the 3b and the 6c sites, respectively. Due to the ionic radius of Ni2+ (0.69 ?) close to that of the Li+ (0.76 ?), the Li+ and Ni2+ ions are expected to partially substitute for each other to form ionic solid solution in the sublattices corresponding to the 3b and 3a sites, respectively, which would give rise to disordering in structure called ‘cation mixing’. It has been revealed that the cation mixing deteriorates the electrochemical performance of the layered compounds previously mentioned. The integrated
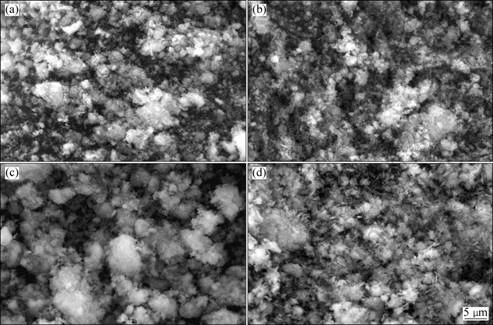
Fig.2 SEM images of (Ni1/3Co1/3Mn1/3)(OH)2 powders prepared at various molar ratios of ammonia to transition-metal cations: (a) Sa, 2.1:1; (b) Sb, 2.4:1; (c) Sc, 2.7:1; (d) Sd, 3.0:1
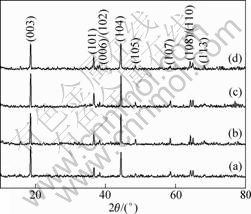
Fig.3 Powder XRD patterns of Li[Ni1/3Co1/3Mn1/3]O2 powders prepared with different (Ni1/3Co1/3Mn1/3)(OH)2 precursors: (a) Sa; (b) Sb; (c) Sc; (d) Sd
intensity ratio of plane (003) to plane (104) in the XRD patterns was regarded as a measurement of the cation mixing and had a direct impact on the electrochemical properties of the system[16]. Generally, when I(003)/I(104)>1.2, the positive-electrode material has a good layered structure due to the small cation mixing. The oxygen sublattice in the α-NaFeO2 type structure is distorted from the fcc array in the direction of hexagonal c-axis. This distortion gives rise to a splitting of the lines assigned to the Miller indices (006)/(102) and (108)/(110) in the XRD patterns, which is the characteristic of the layered structure. The hexagonal lattice parameters, a and c as well as the c/a and I(003)/I(104) and the R-factor which is defined as (I(006)+(102))/I(101), are presented in Table 1. These parameters were obtained by Rietveld
refinement with the software MDI Jade 5.0. The a parameter is equal to the intralayer metal-metal distance, while c parameter is equal to three times the interslab distance. The ratio of the intensity of the (003) peak to that of (104) peak is larger than 1.2 except for the sample Sd, which matches well with above the values for the compounds such as LiNi1-xCoxO2 and LiNiO2. The present result suggests that no undesirable cation mixing occurs and the presently synthesized Li[Ni1/3Co1/3Mn1/3]O2 powders with precursors of Sa, Sb and Sc deliver good electrochemical performance. Clear splitting (006)/(102) and (108)/(110) peak pairs in the XRD pattern and the large c/a above the required one for distortion of oxygen lattice indicate that the layered structure is formed[17]. In addition, the R-factor, which was defined by DAHN et al[18] as (I(006)+(012))/I(101), is considered to be a indication of hexagonal ordering. The lower the R-factor, the higher the hexagonal ordering. In the present work, the R-factor of the sample Li[Ni1/3Co1/3Mn1/3]O2 with Sc is the minimum, indicating that this sample exhibits the maximum hexagonal ordering.
Fig.4 shows the SEM image of Li[Ni1/3Co1/3Mn1/3]O2
Table 1 Lattice parameter a, c, c/a, R-factor and intensity ratios of (003) to (104) (I(003)/I(104)) of Li(Ni1/3Co1/3Mn1/3)O2 synthesized with different (Ni1/3Co1/3Mn1/3)(OH)2 precursors
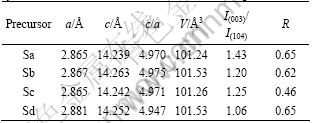
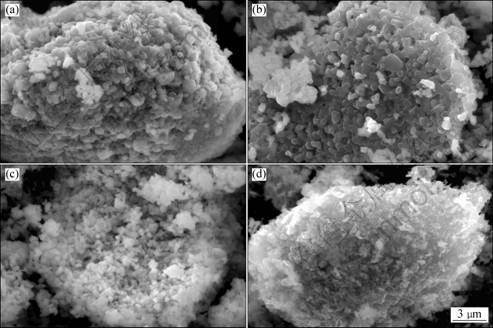
Fig.4 SEM images of Li[Ni1/3Co1/3Mn1/3]O2 samples prepared with (Ni1/3Co1/3Mn1/3)(OH)2 precursors: (a) Sa; (b) Sb; (c) Sc; (d) Sd
materials synthesized with different (Ni1/3Co1/3Mn1/3)- (OH)2 precursors at 950 °C for 10 h in air atmosphere. It could be seen that the Li[Ni1/3Co1/3Mn1/3]O2 materials prepared with precursor Sc are well crystallized and their average particle size is the smallest among 4 samples of Li[Ni1/3Co1/3Mn1/3]O2 materials. Generally, the Li[Ni1/3Co1/3Mn1/3]O2 materials with small particle (high surface area) size show excellent rate capability but their tap density would be low. High tap density is one of the important parameters for the positive-electrode materials because it influences the volumetric capacity of the commercial lithium ion batteries. The aggregated particles of primary particles can be found in Fig.4(c). The square shapes of their particles (about 500 nm) lightly protrude toward the outside of the aggregated particles. The tap density was measured to be 2.37 g/cm3 close to that of commercialized LiCoO2. Hence, one can achieve both high rate capability and high tap density from the aggregated particles of primary particles, as shown in Fig.4(c). The small and uniform particles are the important reasons of Li[Ni1/3Co1/3Mn1/3]O2 material with good reversible capacity and cycling performance. This indicates that the phase structure and particle morphology of Li[Ni1/3Co1/3Mn1/3]O2 powder should be attributed to the appropriate coordination effect of ammonia with transition-metal cations during the hydroxide co-precipitation process.
3.3 Electrochemical characteristic of synthesized Li[Ni1/3Co1/3Mn1/3]O2 powders
The results on the charge/discharge performance and cycling behaviors of Li[Ni1/3Co1/3Mn1/3]O2 are illustrated in Fig.5. The tests were carried out at room temperature using the cell voltages of 2.8-4.5 V and the current density of 16 mA/g. The initial discharge capacities and charge-discharge efficiency are summarized in Table 2. The charging process was not ended until the cell voltage rose rapidly to 4.5 V, which
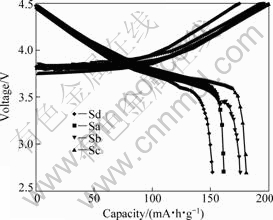
Fig.5 Effect of (Ni1/3Co1/3Mn1/3)(OH)2 precursors on initial charge-discharge curves of Li/Li[Ni1/3Co1/3Mn1/3]O2 cells
Table 2 Electrochemical performance (for the first cycle) of Li[Ni1/3Co1/3Mn1/3]O2 samples tested at constant current density of 16 mA/g
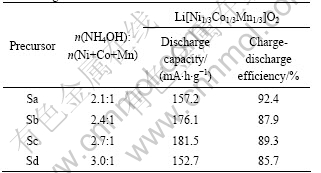
can be attributed to the Ni2+/Ni4+ redox reaction. As shown in Fig.5, each sample shows a distinct plateau for the cell voltage in the charging process. The sample Sc has the best electrochemical properties in the first cycle. The initial discharge capacity was measured to be 181.5 mA?h/g and the initial charge-discharge efficiency is 89.3%, indicating that the appropriate coordination effect of ammonia with transition-metal cations can improve the charge transfer efficiency and reduce the irreversible capacity.
Fig.6 shows the cycling performances of the Li[Ni1/3Co1/3Mn1/3]O2 materials with the cell operated at 0.5C ratio in the voltage range of 2.8-4.5 V. The sample Sc material exhibits the least capacity fade ratio (about 6.0%) after 50 cycles. Thus, the appropriate coordination effect is obtained during the hydroxide co-precipitation process when the molar ratio of ammonia to transition-metal ions is 2.7:1, i.e. Sc sample. The appropriate coordination effect is effective to stabilize the layered structure not only by enhancing structural stability during cycling, but also by reducing cation mixing to facilitate good capacity retention. The
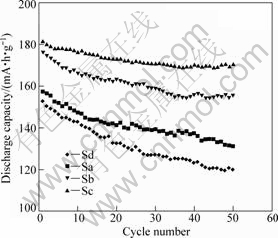
Fig.6 Effect of (Ni1/3Co1/3Mn1/3)(OH)2 precursors of Li[Ni1/3Co1/3Mn1/3]O2 materials on cycling profiles of Li/Li[Ni1/3Co1/3Mn1/3]O2 cells
improvement of charge transfer efficiency and cycle stability can be attributed to the appropriate coordination effect.
Fig.7 shows the AC impedance spectra of Li[Ni1/3Co1/3Mn1/3]O2 position-electrodes after the first cycle. The impedance spectra of samples Sb and Sc include two semicircles: a semicircle at the high-to-medium frequencies reflects the resistance to Li+ ion migration through the surface film and film capacitance; the another semicircle at lower frequencies reflects charge-transfer resistance and interfacial capacitance between the electrodes and electrolyte. The impedance spectra of samples Sa and Sd only include a single semicircle, i.e., the two semicircles at high-to-medium frequencies and lower frequencies combine into one semicircle. The diameter summation values of semicircles of Li[Ni1/3Co1/3Mn1/3]O2 electrodes are 180 W for Sa, 166 W for Sb, 117 W for Sc, and 286 W for Sd, respectively. That is, the samples Sb and Sc deliver the lower electrochemical reaction impedance, due to the larger c/a value and the smaller R-factor. Furthermore, the diameter of second semicircle of the sample Sc is only 18 W, especially, indicating the lower charge-transfer impedance of positive-electrode. The lowest impedance of sample Sc is consistent with the highest initial discharge and the best cycling performance.
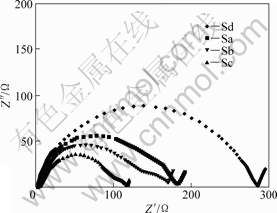
Fig.7 AC impedance spectra of Li(Ni1/3Co1/3Mn1/3)O2 positive-electrodes prepared with (Ni1/3Co1/3Mn1/3)(OH)2 precursors
4 Conclusions
1) Layered Li[Ni1/3Co1/3Mn1/3]O2 positive-electrode material for lithium-ion batteries was synthesized by using a hydroxide co-precipitation method. The influence of coordination effect of ammonia with transition-metal ions on the structural and electrochemical properties of Li[Ni1/3Co1/3Mn1/3]O2 was studied.
2) The XRD analysis demonstrates that the layered-structure Li[Ni1/3Co1/3Mn1/3]O2 material is obtained when the metal hydroxide, (Ni1/3Co1/3Mn1/3)- (OH)2, is prepared with the molar ratio 2.7:1 of ammonia to total transition-metal ions. It has a larger I(003)/I(104)= 1.25 and a lower R=I(006+102)/I(101)=0.46.
3) The charge-discharge tests results show that the Li[Ni1/3Co1/3Mn1/3]O2 positive-electrode material delivers a high initial discharge capacity (181.5 mA?h/g) and stable cycling performance (capacity fade ratio of 94.0% after 50 cycles at 0.5C ratio). The good electrochemical performance is attributed to its good layered structure and the uniform particles with size of 700 nm.
References
[1] LUO Xu-fang, WANG Xian-you, LIAO Li, WANG Xi-min, SERGIO G, SEBASTIAN P J. Effects of synthesis conditions on the structural and electrochemical properties of layered Li[Ni1/3Co1/3Mn1/3]O2 cathode material via the hydroxide co-precipitation method Lib Scitech [J]. J Power Sources, 2006, 161: 601-605.
[2] LIU Zhao-lin, YU Ai-shui, LEE J Y. Synthesis and characterization of LiNi1-x-yCoxMnyO2 as the cathode materials of secondary lithium batteries [J]. J Power Sources, 1999, 81-82: 416-419.
[3] LI De-cheng, MUTA T, ZHANG Lian-qi, YOSHIO M, NOGUCHI H. Effect of synthesis method on the electrochemical performance of LiNi1/3Mn1/3Co1/3O2 [J]. J Power Sources, 2004, 132: 150-155.
[4] YABUUCHI N, OHZUKU T. Novel lithium insertion material of LiCo1/3Ni1/3Mn1/3O2 for advanced lithium-ion batteries [J]. J Power Sources, 2003, 119-121: 171-174.
[5] LEE D K, PARK S H, AMINE K, BANG H J, PARAKASH J, SUN Y K. High capacity Li[Li0.2Ni0.2Mn0.6]O2 cathode materials via a carbonate co-precipitation method [J]. J Power Sources, 2006, 162: 1346-1350.
[6] ZHANG Yao, CAO Hui, ZHANG Jian, XIA Bao-jia. Synthesis of LiNi0.6Co0.2Mn0.2O2 cathode material by a carbonate co-precipitation method and its electrochemical characterization [J]. Solid State Ionics, 2006, 177(37-38): 3303-3307.
[7] CHO T H, SHIOSAKI Y, NOGUCHI H. Preparation and characterization of layered LiMn1/3Ni1/3Co1/3O2 as a cathode material by an oxalate co-precipitation method [J]. J Power Sources, 2006, 159: 1322-1327.
[8] HE Yu-shi, LI Pei, LIAO Xiao-zhen, MA Zi-feng. Synthesis of Li[Ni1/3Co1/3Mn1/3]O2-zFz cathode material from oxalate precursors for lithium ion battery [J]. J Fluorine Chemistry, 2007, 128(2): 139-148.
[9] NAOAKI YABUUCHI, TSUTOMU OHZUKU. Electrochemical behaviors of LiCo1/3Ni1/3Mn1/3O2 in lithium batteries at elevated temperatures [J]. J Power Sources, 2005, 146: 636-639.
[10] SUN Yu-cheng, XIA Yong-gao, NOGUCHI H, The improved physical and electrochemical performance of LiNi0.35Co0.3-xCrxMn0.35O2 cathode materials by the Cr doping for lithium ion batteries [J]. J Power Sources, 2006, 159: 1377-1382.
[11] ZHOU Fu, ZHAO Xue-mei, LU Zhong-hua, JIANG Jun-wei, DAHN J R. The effect of Al substitution on the reactivity of delithiated LiNi1/3Mn1/3Co(1/3-z)AlzOz with non-aqueous electrolyte [J]. Electrochemistry Communications, 2008, 10: 1168-1171.
[12] LIU Dao-tan, WANG Zhao-xiang, CHEN Li-quan. Comparison of structure and electrochemistry of Al- and Fe-doped LiNi1/3Co1/3Mn1/3O2 [J]. Electrochimica Acta, 2006, 51(20): 4199-4203.
[13] YE Shang-yue, XIA Yong-yao, ZHANG Ping-wei, QIAO Zhi-yu. Al, B, and F doped Li[Ni1/3Co1/3Mn1/3]O2 as cathode material of lithium-ion batteries [J]. J Solid State Electrochem, 2007, 11: 805-810.
[14] LEE H, KANG Y J, MYUNG S T, SUN Y K. Synthetic optimization of Li[Ni1/3Co1/3Mn1/3]O2 via co-precipitation [J]. Eelectrochimica Acta, 2004, 50(4): 939-948.
[15] CHANG Zhao-rong, CHEN Zhong-jun, WU Feng, TANG Hong-wei, ZHU Zhi-hong, YUAN Xiao-zi, WANG Hai-jiang. Synthesis and characterization of high-density non-spherical Li(Ni1/3Co1/3Mn1/3)O2 cathode material for lithium ion batteries by two-step drying method [J]. Electrochimica Acta, 2008, 53: 5927-5933.
[16] LI De-cheng, MUTA T, ZHANG Lian-qi, YOSHIO M, NOGUCHI H. Effect of synthesis method on the electrochemical performance of LiNi1/3Mn1/3Co1/3O2 [J]. J Power Sources, 2004, 132: 150-155.
[17] OHZUKU T, NAKURA K, AOKI T. Comparative study of solid-state redox reactions of LiCo1/4Ni3/4O2 and LiAl1/4Ni3/4O2 for lithium-ion batteries [J]. Eelectrochimica Acta, 1999, 45(1/2): 151-160.
[18] DAHN J R, SACKEN U, MICHAL C A. Structure and electrochemistry of Li±yNiO2 and a new Li2NiO2 phase with the Ni(OH)2 structure [J]. Solid State Ionics, 1990, 44(1-2): 87-97.
合成条件对氢氧化物共沉淀法制备锂离子电池层状正极材料Li[Ni1/3Co1/3Mn1/3]O2的影响
胡传跃1, 2,郭 军2,杜 勇1,徐洪辉1,贺跃辉1
1. 中南大学 粉末冶金国家重点实验室,长沙 410083;
2. 湖南人文科技学院 化学与材料科学系,娄底 417000
摘 要:以共沉淀法制备的过渡金属氢氧化物前驱体合成锂离子电池层状正极材料Li[Ni1/3Co1/3Mn1/3]O2。考察氨与过渡金属阳离子的配位效应对Li[Ni1/3Co1/3Mn1/3]O2材料的结构和电化学性能的影响。SEM分析结果表明,当NH3·H2O与过渡金属阳离子的总摩尔比为2.7:1时,获得了分布均一的颗粒为过渡金属氢氧化物共沉淀,合成的Li[Ni1/3Co1/3Mn1/3]O2材料的平均粒径约为500 nm,振实密度接近2.37 g/cm3,接近商品化的LiCoO2正极材料的振实密度。XRD分析结果表明,合成的Li[Ni1/3Co1/3Mn1/3]O2材料具有六角晶格层状结构。Li/Li[Ni1/3Co1/3Mn1/3]O2电池在2.8-4.5 V电压范围内的0.1C倍率测试结果表明,首次放电容量达181.5 mA?h/g,0.5C倍率循环50次后的放电容量为170.6 mA?h/g。
关键词:层状结构;配位效应;氢氧化物共沉淀;正极材料;锂离子电池
Foundation item: Project(50721003) supported by the National Natural Science Foundation of China; Project(07JJ6082) supported by the Natural Science Foundation of Hunan Province, China; Project supported by the Open Project of State Key Laboratory of Powder Metallurgy in Central South University, China
Corresponding author: HU Chuan-yue; Tel: +86-738-8325065; E-mail: huchuanyue@vip.sina.com
DOI: 10.1016/S1003-6326(11)60686-9
[Ni(NH3)x2++Co(NH3)y2++Mn(NH3)z2+](aq)+
[Ni(NH3)x2++Co(NH3)y2++Mn(NH3)z2+](aq)+
[1] LUO Xu-fang, WANG Xian-you, LIAO Li, WANG Xi-min, SERGIO G, SEBASTIAN P J.
[2] LIU Zhao-lin, YU Ai-shui, LEE J Y.
[3] LI De-cheng, MUTA T, ZHANG Lian-qi, YOSHIO M, NOGUCHI H.
[5] LEE D K, PARK S H, AMINE K, BANG H J, PARAKASH J, SUN Y K.
[7] CHO T H, SHIOSAKI Y, NOGUCHI H.
[9] NAOAKI YABUUCHI, TSUTOMU OHZUKU.
[10] SUN Yu-cheng, XIA Yong-gao, NOGUCHI H,
[16] LI De-cheng, MUTA T, ZHANG Lian-qi, YOSHIO M, NOGUCHI H.


