Trans. Nonferrous Met. Soc. China 29(2019) 2371-2383
Effect of sodium lauryl sulphate on microstructure, corrosion resistance and microhardness of electrodeposition of Ni-Co3O4 composite coatings
K. O. NAYANA1, S. RANGANATHA2, H. N. SHUBHA3, M. PANDURANGAPPA1
1. Department of Chemistry, Bangalore University, Bengaluru 560001, India;
2. Department of Chemistry, School of Engineering, Presidency University, Bengaluru 560064, India;
3. Department of Mechanical Engineering, Indian Institute of Science, Bengaluru 560012, India
Received 7 February 2019; accepted 22 August 2019
Abstract:
Ni-Co3O4 composite coatings were electrodeposited on mild steel surface from a Watts-type bath in the presence of sodium lauryl sulfate (SLS). The dispersed Co3O4 particles in the presence of SLS have a greater tendency to move towards cathode and get incorporated in the coating. SLS modifies chemical composition, surface morphology and microstructure of the Ni-Co3O4 composite coating. The developed composite coating exhibits higher corrosion resistance and microhardness than the pure nickel coating. The loadings of bath solution with different concentrations of Co3O4 particles in the presence of SLS provide hydrophobic nature to the coating surface, which is much effective in enhancing the corrosion resistance of Ni-Co3O4 composite coating. The agglomeration of Co3O4 particles (>3 g/L) under high bath load condition develops defects and dislocation on the coating surface, which results in lower corrosion resistance of the deposit. The mechanical properties of the hydrophobic coatings were assessed by the linear abrasion test.
Key words:
nickel electrodeposition; cobalt oxide; composite coating; microstructure; corrosion resistance; microhardness;
1 Introduction
Electrodeposition is the most widely used, cost effective technique for the preparation of nickel matrix composite coating due to its precise controlled operation and low operating temperature at normal pressure [1]. In recent years, nickel has been extensively used to develop metal-matrix composites coating with different types of micron- and nano-sized particles because of its high hardness, corrosion resistance, anti-friction and wear resistance properties [2-5]. The nickel composites are good alternative for hard chromium coating and used in automobile industries.
Nickel coatings obtained from Watt-type bath exhibit low internal stress and good ductility, hence, Watt-type electrolytes are usually employed in nickel electrodeposition [6,7]. The insoluble solid particles are suspended in nickel plating bath, and during composite coating solid particles get embedded into nickel matrix. The incorporation of nickel into matrix plays an important role in improving the anticorrosion and mechanical properties of the coating by modifying its chemical composition and microstructure [8-10].
In order to improve the properties of the deposit, homogeneous reinforcement of large number of particles is required. The concentration of embedded materials depends on the dispersion of particles in the electrolyte and their migration to the cathode surface. Due to higher surface energy of nanoparticles, these readily tend to agglomerate in the electrolyte. The capture of particles in coating and dispersion of particles in the electrolyte are controlled by agitation as well as the use of surfactant. The addition of surfactant is found to be more effective for enhancing the dispersion of particles in electrolyte and insertion of particles into the composite coating [11].
Various types of particles, such as metal oxides (TiO2, SiO2, Al2O3, and Co3O4), metal sulphides (MoS2), metal carbides (SiC, WC), carbon nanotubes, graphene, and diamond, have been used in nickel composite electrodeposition [1-5]. The cobalt oxide (Co3O4) is an antiferromagnetic solid with chemical formula written as Co(II)Co(III)2O4. It is stable in cubic spinel structure in which Co(II) ions occupy the tetrahedral sites, and Co(III) ions occupy the octahedral sites. The Co3O4 particles are useful in many fields like photocatalysis, electrochromic devices, solid-state sensors, magnetism, solar energy absorbers and energy storage devices [12-14].
The Co3O4 particles have been used for fabricating the metal-matrix composite coating with zinc [15] and nickel [16] metals. However, studies on the use of surfactant in nickel plating bath solution to enhance the Co3O4 particle dispersion and its incorporation into nickel matrix were very limited. Additionally, no much emphasis has been given for the study of the influence of Co3O4 particles in the presence of surfactant on the morphology, orientation and corrosion resistance of developed Ni-Co3O4 composite coating.
In the present work, Co3O4 particles were synthesized and used to fabricate Ni-Co3O4 composite coating by electrodeposition using surfactant sodium lauryl sulfate (SLS). The composition, surface morphology, texture, microhardness and corrosion resistance of the Ni-Co3O4 composite coating were investigated.
2 Experimental
The Co3O4 particles were synthesized by sol-gel method as per Refs. [17-19]. 3 g of CoCl2·6H2O was dissolved in 20 mL of 1:1 ethanol-water solution through continuous mechanical stirring. Then, 50 mg of cetyltrimethyl ammonium bromide (CTAB) was added to this solution. Subsequently, 8.9 mL of propylene oxide was added dropwise with continuous stirring for 2 h. Then, it was allowed to stand for 4 h to form gel at room temperature ((27±2) °C). The gel was washed with minimum volume (3-5 mL) of ethyl alcohol three times, and later it was dried at 80 °C for 12 h in a hot air oven to remove the byproducts in the pore structure of the wet gel. Then, the gel sample was heated at 400 °C for 3 h in muffle furnace under air to obtain Co3O4 particles.
The mild steel plate (4 cm × 6 cm) and pure nickel metal (5 cm × 6 cm) were respectively used as cathode and anode in the deposition process. The mild steel cathode surface was mechanically polished using 200, 1000, 2000 grades of waterproof emery papers and degreased with 10 wt.% NaOH solution. Afterwards, it was immersed in 10 vol.% HCl to remove the dust and rust. Plates were then washed in running water and immediately transferred into the plating bath solution. Ni-Co3O4 composite coating was generated from bath solution containing 1, 2 and 3 g/L of Co3O4 particles. The particles were dispersed in the plating bath by magnetic stirring for 24 h at 200 r/min to confirm the uniform dispersion of particles in the bath. The bath constituents and operating conditions employed for the development of coating are tabulated in Table 1. Different codes used to identify the composite coatings and plating baths are given in Table 1. The pure Ni and Ni-Co3O4 coatings were fabricated by electrodeposition technique using Watts-type bath with constant stirring at 200 r/min.
Zeta potentials (surface charge) of Co3O4 particles in aqueous medium (0.2 g/L SLS) as well as in bath solutions (Table 1) were analyzed using Zeta PlusTM zeta potential analyzer at 27 °C. The size distribution of Co3O4 particles in plating bath was determined using dynamic laser light scattering technique [20]. The microscopy image of the as-synthesized Co3O4 particles was analyzed using FEI Tecnai T-20200 kV transmission electron microscope (TEM) and FEI Co. The morphologies of particles and the deposit were analyzed by using field emission scanning electron microscope (FESEM, model: LE01530-VP, Zeiss, Germany). Co3O4 particles incorporated in the coating surface were analyzed using energy-dispersive X-ray (EDX) technique. The X-ray diffraction (XRD) patterns of Co3O4 particles and composite coating were examined by using PANalytical X’pert PRO powder XRD with graphite monochromatized Cu Kα radiation (λ=0.1540 nm) source.
In order to confirm the hydrophobic nature of Ni-Co3O4 composite coating, the water contact angle (WCA) with coating surface was measured using optical contact angle meter (OCA 30 from DataPhysics Instruments GmbH, Germany). The contact angle of water on the coating surface was measured by the sessile drop method [21]. The coating surface was exposed to air and then 2 μL water droplets were placed on the coating (solid/liquid/air system) from a syringe at a slow rate. The images of water droplets on coating surface were captured and processed using the 32-bit SCA 20 software.
Table 1 Plating bath composition and operating conditions
The microhardness of the coating deposited at 4 A/dm2 for 20 min was determined by using Vickers indenter (FM-800 FUTURE-TECH). The dwell time was 10 s with test load of 50 g. Five measurements were performed at different locations on each coating surface and the average value was reported as microhardness of the coating. The mechanical durability of Ni-Co3O4 composite coatings was evaluated by linear abrasion test using 800 grit SiC sandpaper [22,23].
The corrosion resistance properties of the coatings were analyzed by electrochemical method such as Tafel polarization and electrochemical impedance spectroscopy (EIS) methods in 3.5 wt.% NaCl solution using electrochemical work station (CH Instruments, model: 6194B, CHI, USA). Pure Ni and Ni-Co3O4 composites coated mild steel surface with exposed surface area of 1 cm2 acted as working electrode. The saturated calomel electrode (SCE) and Pt wire (1 mm in diameter) served as reference and counter electrodes, respectively. Before each electrochemical measurement, a coated specimen was immersed in 3.5 wt.% NaCl solution for 30 min in order to establish steady-state open circuit potential (OCP).
3 Results and discussion
The Co3O4 particles were characterized by SEM, XRD, EDX and TEM studies before their co-deposition with nickel to electrodeposit Ni-Co3O4 composite coating.
3.1 Characterization of Co3O4 particles
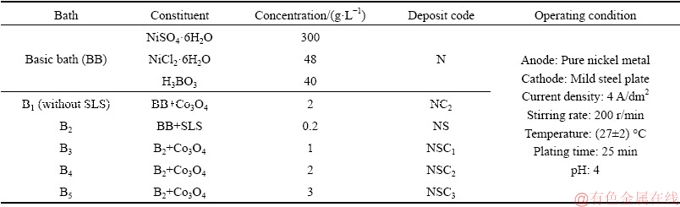
The Co3O4 particles were characterized by XRD and SEM analysis. The XRD pattern (Fig. 1(a)) shows sharp diffraction peaks at 2θ values of 19.0° (111), 31.3° (220), 36.9° (311), 38.5° (222), 44.8° (440), 55.7° (422), 59.4° (511), 65.2° (440) and 77.34° (533) which correspond to Co3O4 particles given by the standard JCPDS card No. 42-1467. The average particle size of the Co3O4 particles was determined by Scherrer equation [24] and it was found to be 57 nm. The SEM image in Fig. 1(b) confirms that the prepared Co3O4 particles have square pyramidal geometry [18,19].
The microstructures of the Co3O4 particles have been further examined by high-resolution TEM (HRTEM) and SAED images (Fig. 2). SAED image in Fig. 2(b) shows the well-defined circles inferring crystalline nature of Co3O4 particles which are also confirmed by XRD and TEM (Figs. 1 and 2). The well-ordered lattice fringes observed in Fig. 2(b) with a spacing of 0.46 nm can be efficiently assigned to the d-spacing of (111) plane of Co3O4 particles. In order to analyze the chemical composition of the particles, EDX spectrum (Fig. 2(c)) was obtained from EDX spectrometer attached to the TEM instrument. EDX spectrum confirms the presence of only cobalt and oxygen elements in the prepared particles [18]. The mass fractions of elements were found to be 69.37% Co, 27.20% O and 3.43% C. This result indicates the formation of Co3O4 particles.
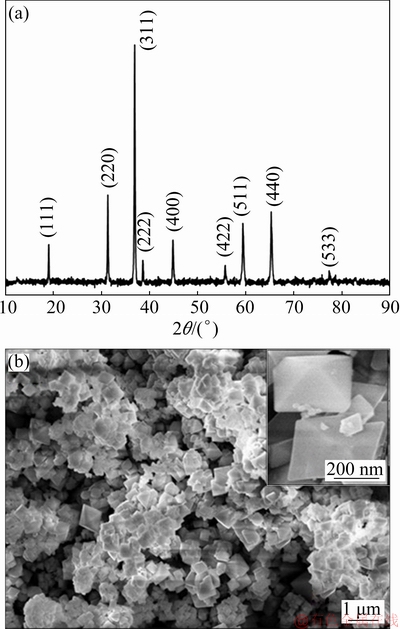
Fig. 1 XRD pattern (a) and SEM image (b) of Co3O4 particles
3.2 Electrodeposition of Ni-Co3O4 composite coating
At pH 4, zeta potential of Co3O4 particles in aqueous medium (without Ni2+ and SLS) was found to be 12 mV. However, in presence of 0.2 g/L SLS, zeta potential of the as-synthesized Co3O4 particles dispersed in aqueous medium was found to be 23.16 mV. The particles dispersed in nickel plating bath solution B3 have surface charge of 38.45 mV. These values were in agreement with the reported literature [25]. The Co3O4 particles exhibit positive surface charge in electroplating bath solution. The positive charge on the dispersed Co3O4 particles indicates that particles have a tendency to move towards the cathode and get incorporated into the growing nickel matrix.
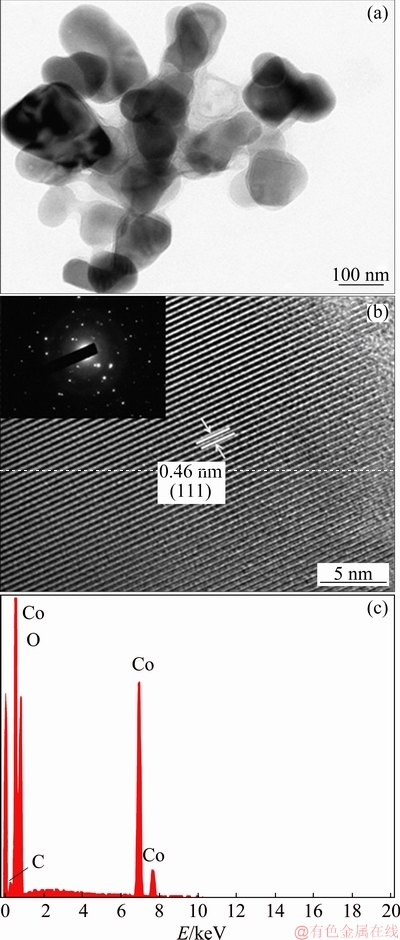
Fig. 2 HRTEM (a), SAED (b) images and EDX spectrum (c) of Co3O4 particles
The Co3O4 particles dispersed in electroplating bath solutions B1 and B4 (Table 1) were diluted to 100 times and stirred for a period of 24 h. The size distribution in the composite electrolyte has been measured immediately. Figure 3 displays the size distribution of Co3O4 particles. Magnetically homogenized composite electrolyte shows bimodal size distribution with different populations of the particles. It produces polydispersed colloidal system with polydispersity factor of 0.274 [20]. In the case of B1 bath (without SLS) the Co3O4 particles are highly populated in the size range of 1700-2200 nm with distribution up to 3100 nm, whereas in the presence SLS, the maximum distribution of Co3O4 particles was observed at 570 nm and lower distribution was noticed at 780 nm. It can be noted that, the presence of SLS keeps the Co3O4 particles in highly dispersed condition compared to its absence, hence it promotes the de- agglomeration of the particles in plating bath.
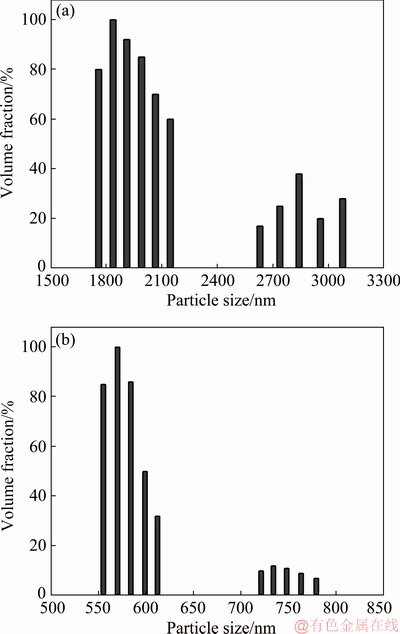
Fig. 3 Size distribution of Co3O4 particles dispersed in B1 (a) and B4 (b) electroplating baths at pH 4
PRAVEEN KUMAR et al [26] revealed that the surfactant influenced the surface properties of the particles dispersed in the plating bath. In aqueous medium at pH 4, the Co3O4 particles have been surrounded by H+ ions (Fig. 4(a)). Surfactant SLS alters the surface charge and reduces the tendency of particles agglomeration. The hydrophobic end of SLS molecule is adsorbed on Co3O4 particle, while the hydrophilic end points towards the solution. The negatively charged hydrophilic end of the SLS molecule is surrounded by H+ ion in the absence of nickel ions and it shows positive zeta potential (Fig. 4(b)). The Co3O4 particle undergoes de-agglomeration when dispersed in nickel plating bath solution containing SLS. The negatively charged hydrophilic group of SLS molecule is attracted by Ni2+ ion. Thus, more nickel ions are adsorbed on the surface of Co3O4 particles and exhibit higher positive charge (Fig. 4(c)). These results confirm that the surfactant, i.e. SLS, improves the dispersion of Co3O4 particles in bath solution and the particles have a tendency to move towards the cathode and get incorporated in the coating.
3.2.1 SEM images and XRD patterns of composite coating
The SEM images of pure Ni and Ni-Co3O4 composite coatings obtained in the absence (N and NC2) and in the presence of SLS (NS, NSC1, NSC2 and NSC3) are shown in Fig. 5. The pure Ni deposit shows large granular structure with a large gap between the grains. The surface of the Ni-Co3O4 composite coating is compact and shows smaller grains compared to pure Ni coating. In the presence of SLS, the surface of the Ni-Co3O4 composite coating is more compact and uniform with small gap between the grains. During the deposition, the Co3O4 particles are adsorbed on the growing crystals which perturb the growth of nickel matrix, resulting in a fine and compact micro- structure [24-26]. SEM images of composite coatings (NSC1, NSC2 and NSC3) obtained in the presence of SLS are shown in Figs. 5(d-f). This confirms the incorporation of Co3O4 particles in the nickel matrix and both phases are homogeneous. The higher incorporation is noticed in NSC2 coating loaded with 2 g/L Co3O4 particles (Fig. 5(e)). Figure 6 displays the SEM cross- sectional images of nickel coating obtained from NC2 and NSC2 baths. It can be seen from cross section that coatings are uniform and well adhered to the steel substrate, without pores and cracks.
The wettability of Ni and Ni-Co3O4 coating obtained in the absence and the presence of SLS was investigated. In the absence of SLS, the water contact angles (WCA) of pure Ni and Ni-Co3O4 composite coating were found to be 89° and 92° (Figs. 5(a) and (b)), respectively. Whereas in the presence of SLS pure nickel coating shows WCA of 84°, further with loading of electrolyte using 1, 2 and 3 g/L of Co3O4 particles, water contact angles on coatings increased to 105°, 110° and 116°, respectively (Figs. 5(d-f)). These studies confirm the hydrophobic nature of the developed composite coating in the presence of SLS. LIU et al [27] suggested that regular and compact surface morphology is beneficial to the increase of water contact angle. Composite coatings (NSC1, NSC2 and NSC3) derived in the presence of SLS show the compact and regular arrangement of finer granules compared to pure Ni coating. Thus, hydrophobic nature of the developed composite coating derived in the presence of SLS is closely related its surface morphology.
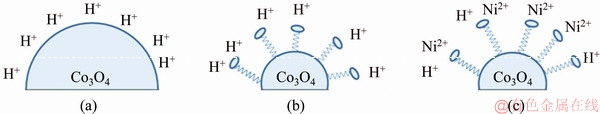
Fig. 4 Schematic representation of Co3O4 particle surface in aqueous medium in the absence (a) and presence (b) of SLS and in bath B4 (c) at pH 4
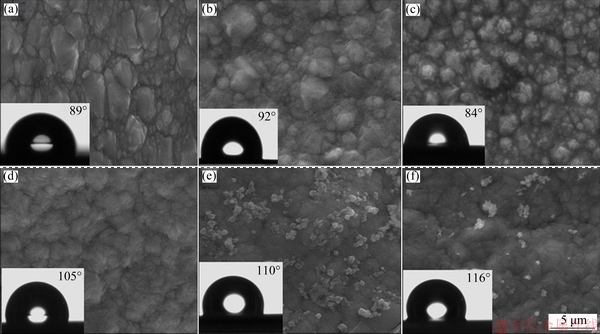
Fig. 5 SEM images and water contact angle graphs of N (a), NC2 (b), NS (c), NSC1 (d), NSC2 (e) and NSC3 (f) composite coatings
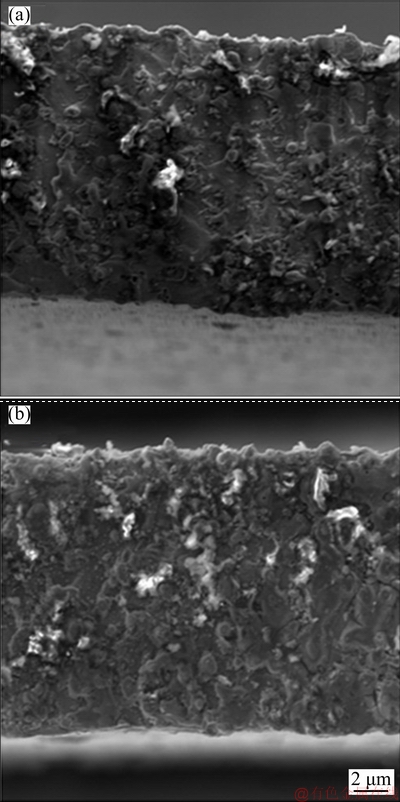
Fig. 6 SEM images of cross sections for NC2 (a) and NSC2 (b) composite coatings
The influence of SLS on the composition of Ni-Co3O4 composite coating was analyzed by EDS. The EDS analysis confirms the presence of Ni and Co elements in the composite coating. NC2 coating derived in the absence of SLS shows 2.8 wt.% Co3O4 particles incorporation, whereas NSC2 composite coating obtained in the presence of SLS contains 4.2 wt.% Co3O4 particles. Thus, particles incorporation is higher in the presence of SLS, which is attributed to higher dispersion of particles in the plating bath (Fig. 3). Figure 7 displays the variation of mass fraction of Co3O4 particles in coating as a function of concentration of Co3O4 particles in the bath. The mass fraction of particles incorporated into the composite coating increases with the increase of particle loading in the bath up to 4.2 wt.% at 2 g/L of Co3O4 particles (NSC2) and thereafter it decreases [16]. During deposition, the adsorption of particles on cathode surface from plating bath increases with the increase in particle concentration up to certain concentration limit, above which the agglomeration of particles takes place in bath solution. This property is responsible for the decrease in the particles content of the plated composite coating [28].
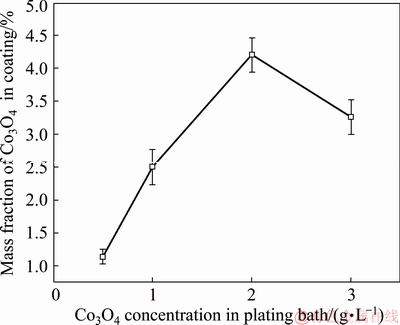
Fig. 7 Variation of mass fraction of Co3O4 particles in coating as function of concentration of Co3O4 particles in bath
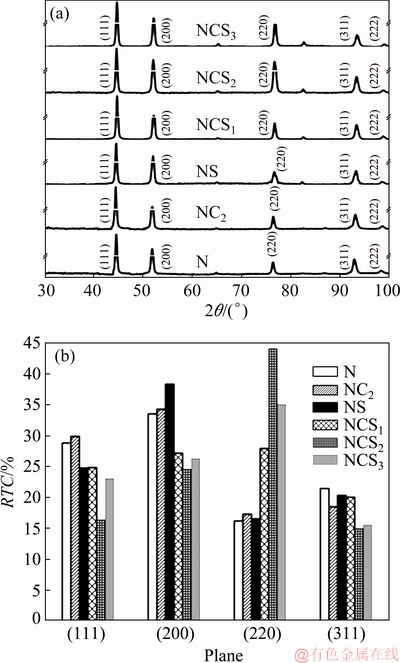
Fig. 8 XRD patterns (a) and RTC values as function of crystallographic planes (b) of Ni and Ni-Co3O4 composite coatings derived in the absence and presence of SLS
In order to investigate further, XRD analysis was carried out for pure Ni and Ni-Co3O4 composite coatings in the absence and presence of SLS (Fig. 8(a)). The diffraction peaks correspond to the FCC structure of nickel. All coatings show characteristic nickel peaks at (111), (200), (220), (311) and (222) crystallographic planes with different intensities (JCPDS card No. 70–0989). The XRD peak corresponding to Co3O4 was not observed due to lower amount of the insertion of dispersed Co3O4 particles. The crystallite sizes of coatings were calculated by using Scherrer equation (FWHM of prominent (111), (200) and (220) reflection peaks) [29]. The average crystallite sizes of N, NC2, NS and NSC2 coatings were found to be 43.85, 41.17, 42.7 and 39.2 nm, respectively. The crystallite sizes confirm that the Ni-Co3O4 composite coatings obtained in the presence of SLS show smaller grain size than other coating.
The preferred orientation of the deposits was determined by calculating the texture coefficient, TC(hkl), and the relative texture coefficient, RTC(hkl) as follows [30,31]. The ratio of peak intensity (hkl) to the sum of the intensities of all XRD peaks of nickel electrodeposit, R(hkl), is calculated by using Eq. (1):
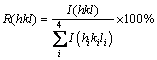 (1)
(1)
where I(hkl) is the peak intensity of nickel electrodeposit and 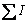 is the sum of the intensities of four main nickel reflection peaks (111), (200), (220) and (311).
is the sum of the intensities of four main nickel reflection peaks (111), (200), (220) and (311).
The texture coefficient TC(hkl) of each reflection peak is defined by
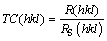 (2)
(2)
where RS(hkl) is defined in the same way as given by Eq. (1), but related to standard nickel sample (JCPDS card No. 70-0989) [29]. More precise quantitative information about the absolute reflection intensity of XRD peaks can be derived from texture coefficient. The R, RS and TC(hkl) values calculated for (111), (200), (220) and (311) planes are given in Table 2.
Finally, the relative texture coefficient, RTC(hkl), is given by Eq. (3):
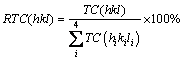 (3)
(3)
The RTC(hkl) coefficient describes the reflection intensity (hkl) relative to the standard nickel sample (included in the TC values).
The determined RTC(hkl) values are shown as bar diagram in Fig. 8(b). Since four reflection peaks of (111), (200), (220) and (311) were used in the analysis, the RTC(hkl) value greater than 25% confirms preferred orientation of the deposit. A majority of the crystals were oriented parallel to (111) and (200) planes in the case of pure Ni and Ni-Co3O4 coatings derived in the absence of SLS. Thus, (111) and (200) are the preferred orientations of N and NC2 coatings, respectively. RTC values of (111) and (200) planes are slightly higher in composite NC2 coating compared to those in the N coating. In the presence of SLS, pure nickel coating (NS) shows 38.33% crystals along (200) plane, thus (200) is the preferred orientation of the deposit [32]. Whereas, in composite coating the TC value of (220) plane increases with the increase of Co3O4 particles concentration in the plating bath. The maximum TC value of 44.08% was observed in NSC2 coating loaded with 2 g/L particles and RTC value decreases with further increase in particle concentration to 3 g/L. The maximum RTC value for (220) plane confirms that (220) is the preferred orientation of the Ni-Co3O4 composite coatings derived in the presence of SLS [33]. Thus, the presence of SLS alters the preferred orientation of the composite coating from (200) to (220). The SLS increases the dispersion of Co3O4 particles in the plating electrolyte and hence improves the adsorption of particles on the coating surface, thereby exhibiting a shielding effect by surrounding the growth center to modify the direction of the crystal growth and increasing the nickel deposition over-potential which results in the change in preferred orientation of the deposit. Thus, the presence of SLS promotes the growth of crystals along (220) plane in Ni-Co3O4 composite coating. (220) plane is a lower surface energy plane formed at higher deposition over-potential [34,35].
3.2.2 Abrasion resistance
The micro- and nano-structures are important for hydrophobic nature of coating, but such surfaces are more vulnerable to mechanical abrasion. Hence, TIAN et al [22] suggested a method to evaluate the mechanical permanence of hydrophobic coatings by adopting linear abrasion test. A simple, available, wear-test method was employed to assess the mechanical durability of the hydrophobic Ni-Co3O4 composite coatings derived in the absence (NC2) and presence (NSC2) of SLS [23]. The experimental setup used to conduct linear abrasion test is shown in Fig. 9. The coatings were abraded using pin on disc tribometer (Ducom, Bengaluru) in linear mode. The pin (8 mm) surface covered with 800 grit silicon carbide sandpaper was used as an abradant. The abradant moved on the stationary coated sample and no lubricant was used. The tests were carried out at (27±2) °C and 1 Hz without applying any load. After abrasion for a distance of 1.2 m, the water contact angles with coatings were maintained at around 86° (NC2) and 107° (NSC2). The SEM images of hydrophobic Ni-Co3O4 composite coating obtained in the absence and presence of SLS after abrasion for 2.4 m are displayed in Fig. 10. The prominent micro- and nano-structures are worn and scratches are observed on the coating surface. The water contact angle drops to 79° in the case of NC2 coating and 100° in the case of NSC2 coating after abrasion distance of 2.4 m. Further, when abrasion distance is 3.6 m the water contact angle decreases to 73° for NC2 and 95° for NSC2 coatings. The hydrophobic nature of the coating reduces with abrasion. The Ni-Co3O4 coating derived in the presence of SLS maintains its hydrophobic nature up to abrasion distance of 3.6 m whereas NC2 coating obtained in the absence of SLS loses its hydrophobic nature at a abrasion distance of 1.2 m.
Table 2 Values of R(hkl) and TC(hkl) calculated for Ni and Ni-Co3O4 composite coatings obtained in the absence and presence of SLS
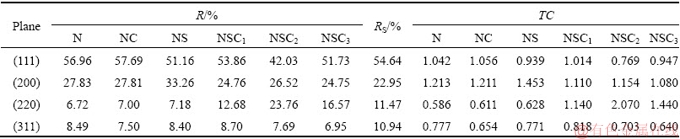
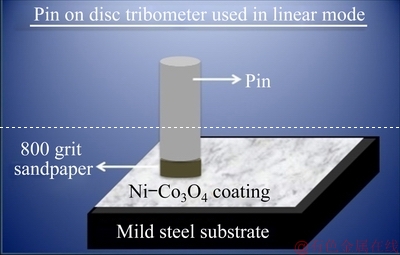
Fig. 9 Schematic representation of experimental setup for linear abrasion test
3.2.3 Microhardness
Ni and Ni-Co3O4 coatings derived in the absence of SLS at test load of 0.5 N show microhardness of HV 315 and HV 480, respectively. The higher hardness of Ni-Co3O4 composite coating compared to pure nickel coating is attributed to incorporation of particles into nickel matrix. The microhardness values of the pure Ni and Ni-Co3O4 composite coatings derived in the presence of SLS and different concentrations of Co3O4 (0-3 g/L) are given in Fig. 11. The Vicker microhardness values of nickel coating with 1, 2 and 3 g/L Co3O4 particles are found to be HV 570, HV 623 and HV 485, respectively. The hardness is higher for all Ni-Co3O4 composite coatings compared to Ni coating. In the presence of SLS, higher incorporation of Co3O4 particles in nickel matrix results in higher microhardness of the Ni-Co3O4 composite coating (Fig. 5). The less particle incorporation in bath loaded with 3 g/L Co3O4 particles results in the decrease in microhardness of NSC3 coating compared to NSC2 deposit.
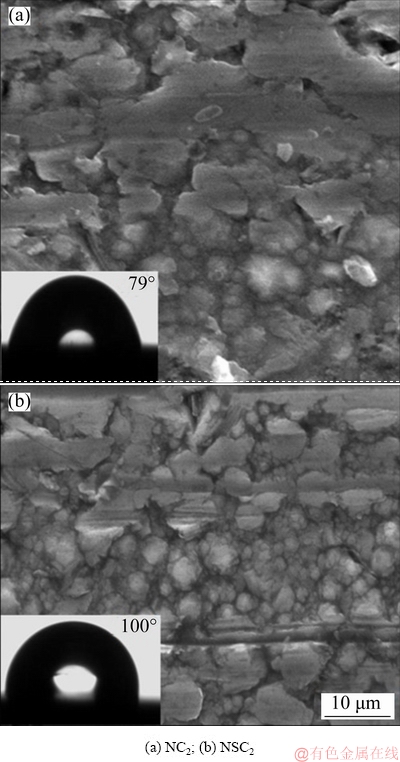
Fig. 10 SEM images of hydrophobic Ni-Co3O4 composite coating after abrasion distance of 2.4 m
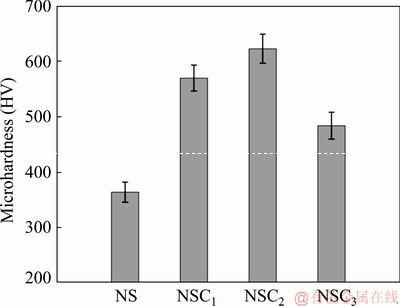
Fig. 11 Microhardness of different coatings
The increase of hardness is due to the dispersion-strengthening and grain filling effects caused by higher incorporation of Co3O4 particles in nickel matrix in the presence of SLS. Nickel carries the load and particles obstruct the dislocation movement. Strengthening is attained as particles control the metal matrix deformation by nanomechanical restraint (Orowan mechanism) [36]. The higher dispersion of Co3O4 particles in the presence of SLS increases the adsorption on cathode surface which enhances the nucleation rate and hinders grain growth, thus resulting in the decrease of grain size of the deposit. The grain refinement also impedes the dislocation motion, which results in the increase of microhardness of the composite coating (Hall-Petch relation) [37].
3.2.4 Electrochemical corrosion
In order to evaluate the corrosion performance of developed Ni-Co3O4 composite coating, electrochemical corrosion studies were carried out in 3.5 wt.% NaCl solution.
3.2.4.1 Tafel plots
The influence of Co3O4 particles on the corrosion behavior of nickel deposit obtained in the absence and presence of SLS was analyzed by Tafel polarization studies. The polarization measurements were carried out in the potential range from -0.2 to 0.2 V using 3.5 wt.% NaCl solution. The Tafel plots of pure Ni and Ni-Co3O4 composite coatings are given in Fig. 12. The corrosion parameters such as corrosion potential (φcorr), corrosion current density (Jcorr), corrosion rate (CR) and anodic/cathodic Tafel slopes (βa and βc) derived from each Tafel plot are tabulated in Table 3.
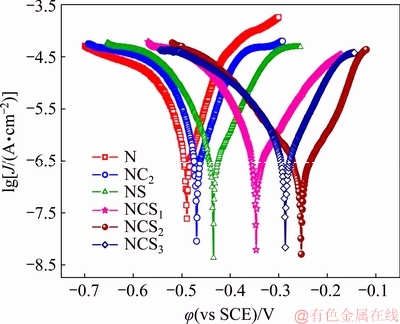
Fig. 12 Tafel plots for coatings in 3.5 wt.% NaCl solution
Table 3 shows that Ni-Co3O4 composite coatings derived in the presence of SLS exhibit higher corrosion potential and lower corrosion current density than other coating. This indicates the nobler character of Ni-Co3O4 composite coating derived in the presence of SLS.
SEM images of Ni and Ni-Co3O4 coatings derived in the presence of SLS obtained after Tafel study are shown in Fig. 13. The pure Ni coating derived in the presence of SLS (NS) possesses defects, large gap and micro-cracks between the grains that assist the diffusion of corrosion medium into coating, thereby accelerating corrosion at active sites [38]. Hence, localized corrosion with higher deterioration of coating is noticed on the SEM image of the pure nickel (NS) coating obtained after Tafel studies (Fig. 13(a)). During the electrodepositon of Ni-Co3O4 composite coating obtained in the presence of SLS, the incorporation of Co3O4 particles is larger, which fills the defects, gap between the grains and micro-cracks. This leads to the decrease of number of active sites for Ni dissolution, thus preventing the internal diffusion of corrosion medium into coating and reducing corrosion current density. Hence, the developed Ni-Co3O4 composite coating derived in the presence of SLS is highly stable, intact and exhibits good protection against corrosion as observed in SEM image (Fig. 13(b)) [36].
Table 3 Corrosion parameters from Tafel plots
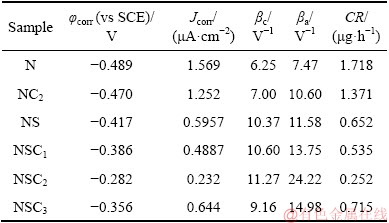
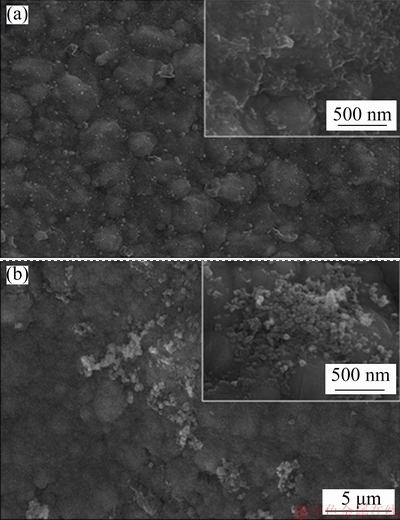
Fig. 13 SEM images of pure Ni (a) and Ni-Co3O4 (b) composite coatings derived in the presence of SLS after Tafel test in 3.5 wt.% NaCl solution
The corrosion resistance of Ni-Co3O4 composite coating derived in the presence of SLS increases with Co3O4 particles loading in bath. Higher corrosion resistance is noticed for NSC2 composite coating derived from 2 g/L Co3O4 particles loaded to the bath. When 3 g/L particles are loaded to bath the NSC3 coating obtained shows less corrosion protection (lower φcorr and higher Jcorr) than NSC1 and NSC2 coatings. At higher load, the agglomeration and nonuniform distribution of Co3O4 particles create chemical heterogeneity in the nickel matrix, resulting in the acceleration of corrosion in NSC3 coating.
3.2.4.2 Electrochemical impedance spectra
The corrosion behavior of composite coating was investigated by non-destructive electrochemical impedance spectroscopy (EIS). Impedance measurements were carried out at OCP in the frequency range from 10 mHz to 100 kHz with a 5 mV sine wave as an excitation signal. The experimentally determined impedance data were analyzed using ZSimp-Win 3.21 software by fitting with a suitable equivalent circuit. The data were represented as Nyquist (-Z'' versus Z') and Bode (|Z|/phase angle versus frequency) plots as shown in Fig. 14. The proposed electrically equivalent circuit (EEC) for impedance analysis is given in Fig. 14(d). It contains a charge transfer resistance (Rct) which is parallel to CPE (constant phase element), Qdl, both of which are in series with solution resistance (Rs). The proposed equivalent circuit is a simple charge transfer controlled model for corrosion system (single semicircle). The impedance parameters derived from fitting the data using ZSimpWin 3.21 software are tabulated in Table 4.
The CPE is used in the electrically equivalent circuit instead of capacitors due to roughness and nonhomogeneity of the coating surface. The impedance of CPE is given by Z(jω)=Q-1(jω)-n, where Q is a CPE constant independent of frequency, ω is angular frequency, j=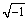 , and n is an exponential index. n=1 gives a real capacitive sense to electrical element and n<1 shows non-uniform distribution of current due to surface defects and surface roughness [39-41].
, and n is an exponential index. n=1 gives a real capacitive sense to electrical element and n<1 shows non-uniform distribution of current due to surface defects and surface roughness [39-41].
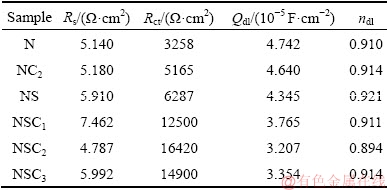
Table 4 confirms that Ni-Co3O4 composite coatings derived in the presence of SLS exhibit higher charge transfer resistance and lower double-layer capacitance between deposit-solution (corrosion medium) interfaces than other coatings. The Rct value of coating increases with bath loading and it is higher for NSC2 coating derived at 2 g/L Co3O4 particle loading, further Rct value decreases with the increase in particle concentration to 3 g/L Co3O4 (NSC3). At higher bath loading, NSC3 composite coating shows lower corrosion resistance with smaller Rct value than NSC1 and NSC2 coatings. This is due to particles agglomeration in plating bath which leads to non-uniform distribution of Co3O4 particles in coating which is in accordance with the results reported by other authors [42,43].
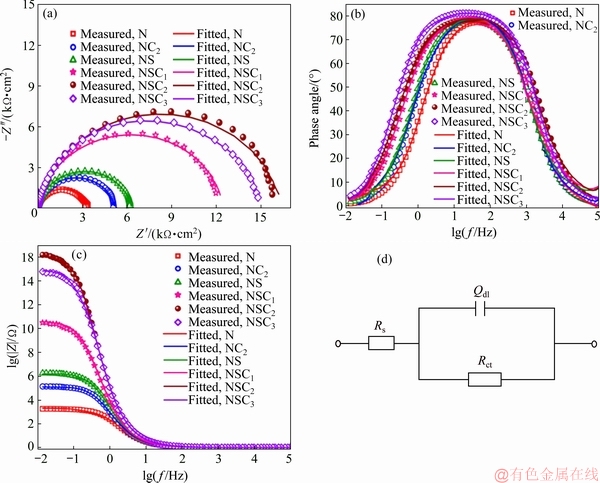
Fig. 14 Nyquist (a), Bode phase angle (b) and impedance modulus (c) plots obtained for coatings in 3.5 wt.% NaCl solution and corresponding equivalent circuit used to simulate recorded EIS data (d)
Table 4 Electrochemical impedance analysis data
The phase angle versus lg f plot is shown in Fig. 14(b). All the coatings show a single phase angle maximum of 80°, which indicates that the process involves single time constant. The behavior of coating is capacitive, when resistance and/or capacitance is high, then current density generally passes through the capacitor and the phase angle would be close to 90°. Figure 14(c) shows that |Z| value is higher for NSC2 composite coating derived from bath loaded with 2 g/L Co3O4 in the presence of SLS and the observed trend is similar to the results obtained from Tafel and Nyquist plots analysis.
In the presence of surfactant SLS, the Co3O4 particles incorporation was higher and more particles were incorporated in the nickel matrix during deposition. The higher incorporation was observed in NSC2 composite coating. The incorporation of more number of inert particles in the coating impeded the metal dissolution by hindering the movement of electrons from anode to cathode in corrosion cell. Furthermore, these particles covered the pores formed in the coating, and controlled corrosion. Thus, NSC2 composite coating exhibited higher corrosion resistance.
The morphological change makes the surface hydrophobic, which hinders the contact of aqueous corrosion medium (3.5 wt.% NaCl) with nickel coating, which has an additional effect of enhancing the corrosion resistance of composite coatings [44,45].
4 Conclusions
(1) The Ni-Co3O4 composite coating was successfully fabricated on mild steel surface in the presence of SLS from a Watts type bath.
(2) The surface studies in the presence of SLS confirm the formation of compact and finer nickel granules with (220) preferred orientation and the surface is hydrophobic in nature.
(3) The surfactant SLS enhances the co-deposition of the Co3O4 particles in the coating and higher incorporation is observed when 2 g/L Co3O4 particles are loaded into the plating bath.
(4) Composite thin film obtained in the presence of SLS and 2 g/L Co3O4 particles show good corrosion resistance and high microhardness.
(5) The co-deposition of particles in nickel matrix and hydrophobic nature of composite coating in the presence of SLS improve the corrosion resistance property of Ni-Co3O4 (2 g/L) composite coating.
(6) The microhardness of the nickel matrix is enhanced by the higher incorporation of Co3O4 particles in Ni-Co3O4 (2 g/L) composite coating.
(7) The linear abrasion test confirms that the hydrophobic property of Ni-Co3O4 composite coating deposited in presence of SLS is well retained at abrasion distance of 3.6 m.
Acknowledgments
The authors are grateful to UGC, New Delhi, India, for the award of Post-Doctoral Fellowship to K. O. Nayana (Award No: F.15-1/2015-16/PDFWM-2015-17- KAR-31527(SA-II)). The authors thank the Department of Chemistry, Bangalore University, India, for providing the research facilities.
References
[1] JEYARAJ S, ARULSHRI K P, SIVASANKARAN S. Investigations on effect of process parameters of electrodeposited Ni-Al2O3 composite coating using orthogonal array approach and mathematical modeling [J]. Archives of Civil and Mechanical Engineering, 2016, 16: 168-177.
[2] GYFTOU P, PAVLATOU E A, SPYRELLIS N. Effect of pulse electrodeposition parameters on the properties of Ni/nano-SiC composites [J]. Applied Surface Science, 2008, 254: 5910-5916.
[3] SHI L, SUN C F, ZHOU F, LIU W M. Electrodeposited nickel– cobalt composite coating containing nano-sized Si3N4 [J]. Materials Science and Engineering A, 2005, 397: 190-194.
[4] LI J, SUN Y, SUN X, QIAO J. Mechanical and corrosion-resistance performance of electrodeposited titania–nickel nanocomposite coatings [J]. Surface and Coatings Technology, 2005, 192: 331-335.
[5] JEON Y S, BYUN J Y, OH T S. Electrodeposition and mechanical properties of Ni–carbon nanotube nanocomposite coatings [J]. Journal of Physics and Chemistry of Solids, 2008, 69: 1391-1394.
[6] CISZEWSKI A, POSLUSZNY S, MILCZAREK G, BARANIAK M. Effects of saccharin and quaternary ammonium chlorides on the electrodeposition of nickel from a Watts-type electrolyte [J]. Surface and Coatings Technology, 2004, 183: 127-133.
[7] REINTJES A S, FLEISCHMANN M. Kinetics of electrodeposition of nickel from Watts baths [J]. Electrochimica Acta, 1984, 29: 557-566.
[8] GARCIA I, CONDE A, LANGELAAN G, FRANSAER J, CELIS J P. Improved corrosion resistance through microstructural modifications induced by codepositing SiC-particles with electrolytic nickel [J]. Corrosion Science, 2003, 45: 1173-1189.
[9] HU F, CHAN K C, SONG S Z, YANG X J. Enhancement of corrosion resistance of electrocodeposited Ni-SiC composites by magnetic field [J]. Journal of Solid State Electrochemistry, 2007, 11: 745-750.
[10] FENG Q, LI T, TENG H, ZHANG X, ZHANG Y, LIU C, JIN J. Investigation on the corrosion and oxidation resistance of Ni-Al2O3 nano-composite coatings prepared by sediment co-deposition [J]. Surface and Coatings Technology, 2008, 202: 4137-4144.
[11] WALSH F C, PONCE LEON C. A review of the electrodeposition of metal matrix composite coatings by inclusion of particles in a metal layer: An established and diversifying technology [J]. Transactions of the Institute of Metal Finishing, 2014, 92: 83-98.
[12] LOU X, HAN J, CHU W, WANG X Q. Synthesis and photocatalytic property of Co3O4 nanorods [J]. Materials Science and Engineering B, 2007, 137: 268-271.
[13] MAKHLOUF S A, BAKR Z H, ALY K I, MOUSTAFA M S. Structural, electrical and optical properties of Co3O4 nanoparticles [J]. Superlattices and Microstructures, 2013, 64: 107-117.
[14] MAKHLOUF S A. Fabrication and investigation of the magnetic properties of Co and Co3O4 nanoparticles [J]. Journal of Magnetism and Magnetic Materials, 2002, 246: 184-190.
[15] CHANDRAPPA K G, VENKATESHA T V. Generation of Co3O4 microparticles by solution combustion method and its Zn-Co3O4 composite thin films for corrosion protection [J]. Journal of Alloys and Compounds, 2012, 542: 68-77.
[16] AKHTAR K, KHALID H, UL I, ZUBAIR H N, KHAN Z U, HUSSAIN A. Tribological properties of electrodeposited Ni-Co3O4 nanocomposite coating on steel substrate [J]. Journal of Tribology, 2017, 139: 061302.
[17] CONWAY B E. Electrochemical supercapacitors: Scientific fundamentals and technological applications [M]. New York: Kluwer Academic/Plenum Publishers, 1999.
[18] RANGANATHA S, KUMAR S, PENKI T R, KISHORE B, MUNICHANDRAIAH N. Co2(OH)3Cl xerogels with 3D inter- connected mesoporous structures as a novel high-performance supercapacitor material [J]. Journal of Solid State Electrochemistry, 2017, 21: 133-143.
[19] ZHANG Z, YIN L. Mn-doped Co2(OH)3Cl xerogels with 3D interconnected mesoporous structures as lithium ion battery anodes with improved electrochemical performance [J]. Journal of Materials Chemistry A, 2015, 3: 17659-17668.
[20] VATHSALA K, VENKATESHA T V. Zn–ZrO2 nanocomposite coatings: Elecrodeposition and evaluation of corrosion resistance [J]. Applied Surface Science, 2011, 257: 8929-8936.
[21] SHUBHA H N, VENKATESHA T V, VATHSALA K, PAVITRA M K, PUNITH KUMAR M K. Preparation of self assembled sodium oleate monolayer on mild steel and its corrosion inhibition behavior in saline water [J]. ACS Applied Materials and Interfaces, 2013, 5: 10738-10744.
[22] TIAN X, VERHO T, RAS R H A. Moving superhydrophobic surfaces toward real-world applications [J]. Science, 2016, 352: 142-143.
[23] XUE Y, WANG S, BI P, ZHAO G, JIN Y. Super-hydrophobic Co-Ni coating with high abrasion resistance prepared by electrodeposition [J]. Coatings, 2019, 9: 232(1-14).
[24] LEITNER A, SAKEYE M, ZIMMERLIA C E, SMATT J H. Insights into chemoselectivity principles in metal oxide affinity chromatography using tailored nanocast metal oxide microspheres and mass spectrometry-based phosphoproteomics [J]. Analyst, 2017, 142: 1993-2003.
[25] LARIBA GHAL S M, AMADEH A, HEYDARZADEH SOHI M, HADAVI S M M. The effect of SDS surfactant on tensile properties of electrodeposited Ni-Co/SiC nanocomposites [J]. Materials Science and Engineering A, 2013, 559: 583-590.
[26] PRAVEEN KUMAR C M, VENKATESHA T V, SHABADI R, Preparation and corrosion behavior of Ni and Ni–graphene composite coatings [J]. Materials Research Bulletin, 2013, 48: 1477-1483.
[27] LIU C J, FENG X Y, LI N, LUO C W, CHAO Z S. Super- hydrophobic Co3O4-loaded nickel foam with corrosion-resistant property prepared by combination of hydrothermal synthesis and PFAS modification [J]. Surface and Coatings Technology, 2017, 309: 1111-1118.
[28] GUGLIELMI N. Kinetics of the deposition of inert particles from electrolytic baths [J]. Journal of the Electrochemical Society, 1972, 119: 1009-1012.
[29] PATTERSON A L. The Scherrer formula for X-ray particle size determination [J]. Physical Review, 1939, 56: 978-982.
[30] BERUBE L P, LESPERANCE G. A quantitative method of determining the degree of texture of zinc electrodeposits [J]. Journal of the Electrochemical Society, 1989, 136: 2314-2315.
[31] AVRAMOVIC L, IVANOVIC E R, MAKSIMOVIC V M, PAVLOVIC M M, VUKOVIC M, STEVANOVIC J S, NIKOLIC N D. Correlation between crystal structure and morphology of potentiostatically electrodeposited silver dendritic nanostructures [J]. Transactions of Nonferrous Metals Society of China, 2018, 28: 1903-1912.
[32] PAVLATOU E A, STROUMBOULI M, GYFTOU P, SPYRELLIS N. Hardening effect induced by incorporation of SiC particles in nickel electrodeposits [J]. Journal of Applied Electrochemistry, 2006, 36: 385-394.
[33] UL-HAMIDA A, QUDDUS A, SARICIMEN H, DAFALLA H. Corrosion behavior of coarse- and fine-grain Ni coatings incorporating NaH2PO4·H2O inhibitor treated substrates [J]. Materials Research, 2015, 18: 20-26.
[34] ZHOU Y B, QIAN B Y, ZHANG H J. Al particles size effect on the microstructure of the co-deposited Ni-Al composite coatings [J]. Thin Solid Films, 2009, 517: 3287-3291.
[35] TIAN Z J, WANG D S, WANG G F, SHEN L D, LIU Z D, HUANG Y H. Microstructure and properties of nanocrystalline nickel coatings prepared by pulse jet electrodeposition [J]. Transactions of Nonferrous Metals Society of China, 2010, 20: 1037-1042.
[36] ABDEL A A. Hard and corrosion resistant nanocomposite coating for Al alloy [J]. Materials Science and Engineering A, 2008, 474: 181-187.
[37] BAKHIR B, AKBARI A. Effect of particle size and co-deposition technique on hardness and corrosion properties of Ni-Co/SiC composite coatings [J]. Surface and Coatings Technology, 2012, 206: 4964-4975.
[38] ZHOU X, SHEN Y. Beneficial effects of CeO2 addition on microstructure and corrosion behavior of electrodeposited Ni nanocrystalline coatings [J]. Surface and Coatings Technology, 2013, 235: 433-446.
[39] LIU W, ZHANG H, QU Z, ZHANG Y, LI J. Corrosion behavior of the steel used as a huge storage tank in seawater [J]. Journal of Solid State Electrochemistry, 2010, 14: 965-973.
[40] MACAK J, SAJDL P, KUCERA P, NOVOTNY R, VOSTA J. In situ electrochemical impedance and noise measurements of corroding stainless steel in high temperature water [J]. Electrochimica Acta, 2006, 51: 3566-3577.
[41] RANGANATHA S, VENKATESHA T V, VATHSALA K. Development of electroless Ni-Zn-P/nano-TiO2 composite coatings and their properties [J]. Applied Surface Science, 2010, 256: 7377-7383.
[42] VLASA A, VARAVARA S, POP A, BULEA C, MURESAN L M. Electrodeposited Zn-TiO2 nanocomposite coatings and their corrosion behavior [J]. Journal of Applied Electrochemistry, 2010, 40: 1519-1527.
[43] PUNITH KUMAR M K, VENKATESHA T V, PAVITHRA M K, NITHYANANDA SHETTY A. The fabrication, characterization and electrochemical corrosion behavior of Zn-TiO2 composite coatings [J]. Physica Scripta, 2011, 84: 1-10.
[44] WANGV Y, GU Z, XIN Y, YUAN N, DING J. Facile formation of super-hydrophobic nickel coating on magnesium alloy with improved corrosion resistance [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2018, 538: 500-505.
[45] RADWAN A B, ABDULLAH A, M, ALNUAIMI N A. Recent advances in corrosion resistant superhydrophobic coatings [J]. Corrosion Reviews, 2017, 36: 127-153.
十二烷基硫酸钠对Ni-Co3O4复合电沉积镀层显微组织、耐蚀性能和显微硬度的影响
K. O. NAYANA1, S. RANGANATHA2, H. N. SHUBHA3, M. PANDURANGAPPA1
1. Department of Chemistry, Bangalore University, Bengaluru 560001, India;
2. Department of Chemistry, School of Engineering, Presidency University, Bengaluru 560064, India;
3. Department of Mechanical Engineering, Indian Institute of Science, Bengaluru 560012, India
摘 要:在添加十二烷基硫酸钠(SLS)的瓦特型镀液中,于低碳钢表面电沉积Ni-Co3O4复合镀层。当镀液中存在SLS时,分散的Co3O4颗粒向阴极迁移的趋势更大,并与镀层结合。SLS改变Ni-Co3O4复合镀层的化学成分、表面形貌和显微组织。与纯镍镀层相比,所开发的复合镀层具有更高的耐蚀性能和显微硬度。在SLS存在条件下,在镀液中装载不同浓度的Co3O4颗粒使镀层表面具有疏水性,这对于提高Ni-Co3O4复合镀层的耐蚀性能非常有效。在镀液高Co3O4颗粒载荷量(>3 g/L)的情况下,Co3O4颗粒发生团聚,在镀层表面形成缺陷和位错,导致镀层耐腐蚀性能下降。通过线性磨损试验对疏水镀层的力学性能进行评价。
关键词:镍电沉积;氧化钴;复合镀层;显微组织;耐蚀性能;显微硬度
(Edited by Wei-ping CHEN)
Corresponding author: M. PANDURANGAPPA; E-mail: mprangachem@gmail.com
DOI: 10.1016/S1003-6326(19)65143-5
Abstract: Ni-Co3O4 composite coatings were electrodeposited on mild steel surface from a Watts-type bath in the presence of sodium lauryl sulfate (SLS). The dispersed Co3O4 particles in the presence of SLS have a greater tendency to move towards cathode and get incorporated in the coating. SLS modifies chemical composition, surface morphology and microstructure of the Ni-Co3O4 composite coating. The developed composite coating exhibits higher corrosion resistance and microhardness than the pure nickel coating. The loadings of bath solution with different concentrations of Co3O4 particles in the presence of SLS provide hydrophobic nature to the coating surface, which is much effective in enhancing the corrosion resistance of Ni-Co3O4 composite coating. The agglomeration of Co3O4 particles (>3 g/L) under high bath load condition develops defects and dislocation on the coating surface, which results in lower corrosion resistance of the deposit. The mechanical properties of the hydrophobic coatings were assessed by the linear abrasion test.


