Trans. Nonferrous Met. Soc. China 23(2013) 3434-3439
Preparation of ammonium jarosite from clinker digestion solution of nickel oxide ore roasted using (NH4)2SO4
Xiao-yi SHEN, Hong-mei SHAO, Jia-dong WANG, Yu-chun ZHAI
School of Materials and Metallurgy, Northeastern University, Shenyang 110819, China
Received 31 December 2012; accepted 13 April 2013
Abstract:
To obtain the appropriate conditions for eliminating Fe3+ from NiSO4 solution, the digestion solution of the clinker was used as raw material, which was obtained from roasting the nickel oxide ore with (NH4)2SO4. The ammonium jarosite was successfully synthesized from the solution with analytic grade NH4HCO3. The effects of reaction temperature, reaction time, end pH value of reaction on the removal rate of iron were investigated, and the effect of the initial concentration of Fe3+ was also discussed. All of those factors had significant effects on the removal rate of Fe3+, among which the reaction temperature was the most prominent. The appropriate reaction conditions were concluded as follows: reaction temperature 95 °C, reaction time 3.5 h, end pH value of reaction 2.5 at initial concentration of Fe3+ 19.36 g/L. The physical aspect of (NH4)2Fe6(SO4)4(OH)12 was cluster figure composed of sheet or prismatic particles with smooth surface.
Key words:
nickel oxide ore; ammonium jarosite; influence factor; digestion solution;
1 Introduction
Nickel is an important nonferrous metal which is widely used in stainless steel, electroplating, etc [1-2]. Nowadays, nickel is mostly produced form sulfide ores. Because of the immoderately exploitation, the high grade ore, especially sulfide ore is exhausted gradually. The sulfide ore’s insufficient makes the utilization of nickel oxide ore urgent, especially the low grade oxide ore, which is the largest resource containing nickel [3-5]. Nickel is found in oxide/silicate minerals formed through the chemical weathering of nickeliferous peridotite rock under humid climate [6,7]. Both pyrometallurgical and hydrometallurgical routes were used in dealing with the nickel oxide ore; however, the pyrometallurgical methods were inadvisable because of high consumption of energy, as well as heavy pollution of gas and waste residue, and the pyrometallurgical route is only suitable to treat the mineral with high grade nickel [8-10]. Commonly, the hydrometallurgical routes consist of reduction roasting followed by ammonia leaching, sulfuric acid leaching and high pressure sulfuric acid leaching, among which the sulfuric acid leaching is the most development prospect process [11,12]. In acid leaching process using H2SO4, silica gel is unavoidably generated, which makes the filtration difficulty [13,14]. A new technology, which roasts laterite nickel ore using (NH4)2SO4 and by which the obtained clinker is then digested in water, is presented. After filtration, the filtrate is gained, including the valuable metal ions, such as Ni2+, Mg2+, Fe3+/Fe2+ and Al3+. The application of roasting nickel oxide ore using (NH4)2SO4 can guarantee that Fe3+ and Al3+ are extracted at a lower efficiency, but they still must be removed. The main impurities in solution are iron and aluminum, as well as trace elements of chromium, cobalt, etc.
Precipitation of iron in the chemical form of jarosite is widely adopted in hydrometallurgy, especially in zinc producing [15,16]. This process shows remarkable liquid-solid separation properties, and the minimum loss of valuable metals [17,18]. In this work, the digestion solution of the clinker obtained from roasting the nickel oxide ore using (NH4)2SO4 was used as raw material. The analytic grade NH4HCO3 was used to remove iron in solution through synthesizing ammonium jarosite. The influence factors including reaction temperature, reaction time, end pH value of reaction, as well as the initial concentration of iron ion on the removal of Fe3+ were investigated.
2 Experimental
2.1 Materials
The low-grade nickel oxide ore was dried in a drying oven, and then was crushed and grinded using a medicine grinder until the size of the ore powder was about 75 μm. The ore powder was mixed uniformly with the industrial grade (NH4)2SO4, and then was put into a roasting furnace equipped with a temperature controller with a display screen. The material was roasted under a desired temperature schedule. After that, the roasting clinker was added into water at a certain liquid to solid ratio. The digestion solution and residue were obtained after filtration. The residue was washed and dried. The as-obtained solution was used as raw material. The analytic grade ammonium bicarbonate was used as reagent to make ammonium jarosite. Distilled water was also used to wash the filter cake after each experiment.
2.2 Procedure
The experiments were conducted in a 2 L Bunsen beaker, which was fixed in a thermostat water bath. The solution was agitated using a double-leaf agitating controlled by a variable speed motor. Each 1.5 L digestion solution with the pH value proximity to 2.0 was consumed and H2O2 was needed to transfer Fe2+ into Fe3+. At the desired temperature, the NH4HCO3 was slowly added into the solution agitated at a speed of 450 r/min in the whole experiment, the pH value was monitored both by an acidity meter and short range pH paper. Distilled water was added into the solution to keep the liquid quantity consistent. The solution was turned from red color into yellow color 1 h later, and the pH value was lowered during the test and was adjusted in the needed range. After the process of making ammonium jarosite, the solution was adjusted to a certain pH value. At the end of each experiment, the slurry was vacuum-filtered, and the filter residue was washed three times with distilled water and then dried. Both the residue and the solution were determined for the iron content to calculate the removal rate of iron ion.
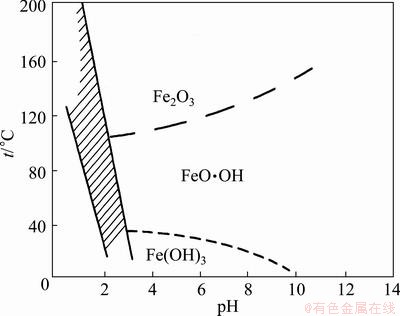
Fig. 1 Relationship between formation of jarosite and temperature and pH value
2.3 Fundamental
The formula of jarosite can be presented as A2Fe6(SO4)4(OH)12, where A represents monovalent cation, such as K+, Na+ and NH4+ [19]. The relationship between the formation of jarosite and temperature and pH value was studied by BABCAN, as shown in Fig. 1, in which the shadow area is the stable region of jarosite. We can conclude that when the pH value is lower, the high temperature is needed for the formation of jarosite. The lower the pH value is, the higher the temperature needed is. The formation of jarosite needs several factors as follows: attendance of Fe3+, existence of monovalent cation, existence of sulphate ion at pH value lower than 2. The jarosite has the advantages of stability, good sedimentation and excellent filtering performance, and its solubility in water is very low, which is very important in hydrometallurgy. The formation process is complex, but can be simply described using following chemical equations [20].
3Fe2(SO4)3+6H2O=6Fe(OH)SO4+3H2SO4 (1)
4Fe(OH)SO4+4H2O=2Fe2(OH)4SO4+2H2SO4 (2)
2Fe(OH)SO4+2Fe2(OH)4SO4+2AOH=A2Fe6(SO4)4(OH)12 (3)
3 Results and discussion
3.1 Compositions of clinker digestion solution
The compositions of clinker digestion solution are shown in Table 1. The concentration of Ni2+ is only 1.49 g/L, far below that of Fe3+/Fe2+ as well as Al3+. The Fe3+ and Al3+ as impurities must be removed to prepare the high-quality nickel products. At this point, it is similar with the zinc metallurgy, but the challenge is much great. This is because the concentration of Ni2+ is very low and the concentration of Fe3+/Fe2+ is high compared with Zn2+ and Fe3+/Fe2+ in the solution. In order to get high-quality nickel products, both the impurities of Fe3+/Fe2+ and Al3+ must be removed by preparing (NH4)2Fe6(SO4)4(OH)12 and Al(OH)3. In this work the main research purpose was the removal of iron and synthesizing ammonium jarosite. But several experiments of synthesizing (NH4)2Fe6(SO4)4(OH)12 and Al(OH)3 by hydrolysis in one step were carried out to examine the impurities removal efficiency.
Table 1 Compositions of clinker digestion solution
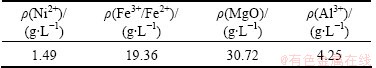
3.2 Influence of reaction temperature
Under the conditions of reaction time 4 h, end pH value of reaction 2.5, initial concentration of Fe3+ 19.36 g/L, the influence of reaction temperature on the removal rate of Fe3+ was studied in the range of 70 to 95 °C, as shown in Fig. 2. We could see from Fig. 2 that the higher the reaction temperature is, the higher the removal rate of Fe3+ is. The reaction temperature has a significant influence on the Fe3+ removal rate, which increases from 23.86% at 70 °C to 98.28% at 95 °C, and the curve is sharp. So, the reaction temperature should be higher than 90 °C in order to remove Fe3+. The reaction temperature 95 °C is selected in the following experiments.
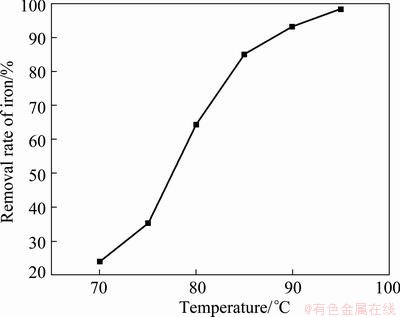
Fig. 2 Influence of reaction temperature on removal rate of iron
3.3 Influence of reaction time
The effect of reaction time on the removal rate of Fe3+ was investigated under the conditions of reaction temperature 95 °C, end pH value of reaction 2.5, initial concentration of Fe3+ 19.36 g/L. The experimental results were plotted as shown in Fig. 3. It is obvious that the influence of reaction time on the removal rate of Fe3+ is pronounced in the range of 2.5-3.5 h, but has no significant effects on the removal rate of Fe3+ when the reaction time is longer than 3.5 h. That is to say, the reaction time 3.5 h is enough for synthesizing ammonium jarosite. From the reducing energy consumption and raising productivity, 3.5 h is considered to be suitable.
3.4 Influence of end pH value of reaction
The influence of end pH value of reaction on the removal rate of Fe3+ was also studied in the experiments with initial Fe3+ concentration of 19.36 g/L at 95 °C for 3.5 h. The results are shown in Fig. 4, in which the curve displays a similar trend with the curve in Fig. 3. The removal rate of Fe3+ increases along with the end pH value of reaction in the range of 1.5-2.5. When the end pH value of reaction is above 2.5, the removal rate of Fe3+ also increases but is very slow. The suitable end pH value of reaction seems to be 2.5 in view of reducing consumption of auxiliary material, nevertheless the Al3+ in solution also must be removed to obtain pure NiSO4/MgSO4 solution, so the end pH value of reaction is adjustable for different needs.
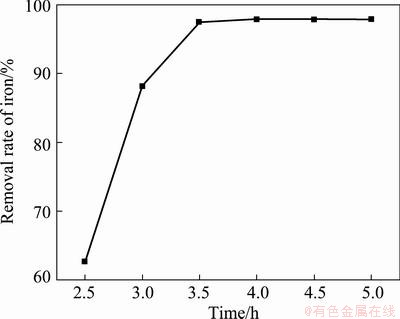
Fig. 3 Influence of reaction time on removal rate of iron
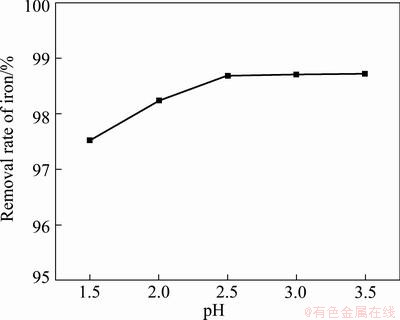
Fig. 4 Influence of pH value of reaction end on removal rate of iron
It is clear that the end pH value of reaction plays a role in removal of iron when the NH4HCO3 is enough. That is to say, the dosage of NH4HCO3 plays an important role. From the molecular formula (NH4)2Fe6(SO4)4(OH)12, the mass ratio of NH4HCO3 to Fe3+ in solution is about 0.47, but additional NH4HCO3 is needed to neutralize the H2SO4 yielded during the process of synthesizing (NH4)2Fe6(SO4)4(OH)12. The chemical equations are as follows:
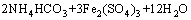 →
→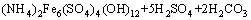 (5)
(5)
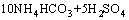 →
→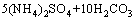 (6)
(6)
According to the equations, the calculated mass ratio of the dosage of NH4HCO3 to Fe3+ in solution is about 2.8. So, the experiments were carried out with the mass ratio of the dosage of NH4HCO3 to iron ion in solution 1.5 to 3.5. And the experimental results were in agreement with the results of end pH value of reaction.
3.5 Influence of initial concentration of Fe3+
The appropriate conditions for synthesizing ammonium jarosite using NH4HCO3 at the initial Fe3+ concentration of 19.36 g/L are reaction temperature 95 °C, reaction time 3.5h and end pH value of reaction 2.5. Since the components in laterite nickel ore and theirs contents usually are variable, it is necessary to examine the influence of the concentration of iron ion in solution. Based on the experimental data, the initial concentration of Fe3+ was also investigated, and the result is shown in Fig. 5. It is obvious that the removal rate of Fe3+ increases along with the initial concentration of Fe3+ increasing. The removal rate of Fe3+ increases from 83.72% at initial Fe3+ concentration 4.97 g/L to 98.36% at initial Fe3+ concentration 23.97 g/L. The variation of removal rate of Fe3+ is slight when the initial Fe3+ concentration is in the range of 23.97-45.22 g/L. Although the initial concentration of Fe3+ varies from 4.97 g/L to 45.22 g/L, the residual Fe3+ in solution is about 0.3 g/L after each test, which can be removed during the removal of Al3+. If both the Fe3+ and Al3+ are removed in one step consisting of synthesizing (NH4)2Fe6(SO4)4(OH)12 and making Al(OH)3 by hydrolysis, the concentration of Fe3+ could be lowered to 0.01 g/L or even less.
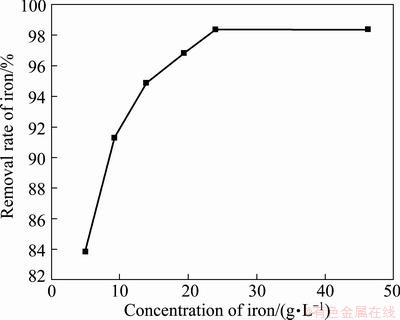
Fig. 5 Influence of initial concentration of Fe3+ on removal rate of iron
3.6 Characterization of as-synthesized ammonium jarosite
The XRD pattern and SEM image of ammonium jarosite are displayed in Fig. 6, which was prepared at the conditions of reaction temperature 95 °C, reaction time 3.5 h, end pH value of reaction 2.5 at initial concentration of Fe3+ 19.36 g/L. The diffraction data are in good agreement with the standard diffraction peaks and intensity of the (NH4)2Fe6(SO4)4(OH)12. No other phases are detected and the diffraction peaks are sharp. The morphology of ammonium jarosite could be seen clearly in SEM image. The physical aspect of the sample presents cluster figure comprising of many small particles or crystals, which displays a sheet or prismatic morphology with smooth surface. The average particle size of (NH4)2Fe6(SO4)4(OH)12 is about 15 μm.
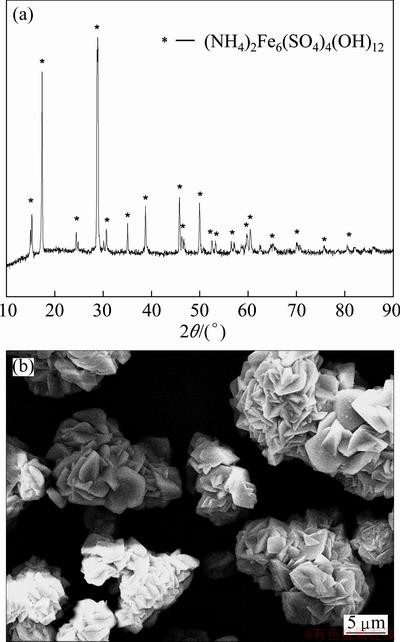
Fig. 6 XRD pattern (a) and SEM image (b) of as-synthesized ammonium jarosite
The main chemical components of (NH4)2Fe6(SO4)4(OH)12 that was washed three times with distilled water and dried for 12 h in an oven are shown in Table 2. The contents of Fe2O3 and SO3 are 48.86% and 31.95%, respectively. According to the formula of (NH4)2Fe6(SO4)4(OH)12, the contents of Fe2O3 and SO3 are 50% and 33.33%, respectively, which are slightly higher than the determined data. There are two reasons for that. On one hand, a small amount of metal ions such as Al3+ in solution are precipitated during the process of synthesizing (NH4)2Fe6(SO4)4(OH)12 because of high local pH value when NH4HCO3 is added into the solution, which were mingled in (NH4)2Fe6(SO4)4(OH)12. On the other hand, when the local pH value of the solution during the experiments is high, Fe(OH)3 is almost certainly generated, which could be transferred into ammonium jarosite but needed a very long time. The presences of Fe(OH)3 and other metal ion’s precipitations play a role in chemical components of (NH4)2Fe6(SO4)4(OH)12 so that the contents of Fe2O3 and SO3 are slightly lower than the theoretical data.
Table 2 Main components of ammonium jarosite
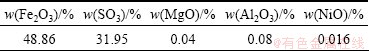
From Table 2, we also found the existence of NiO. That is to say the valuable metal Ni is lost more or less in the process of synthesizing ammonium jarosite. From the results of quantitative analysis of the jarosite residue, the calculated loss rate of Ni is less than 0.6%, which is acceptable.
4 Conclusions
1) In the synthesizing process of (NH4)2Fe6(SO4)4(OH)12 using digestion solution of roasting nickel oxide ore clinker and NH4HCO3, the factors consisting of reaction temperature, reaction time, end pH value of reaction have significant effects on the removal rate of Fe3+, among which the reaction temperature is the most prominent. The initial concentration of Fe3+ in solution also has effect on the removal rate. At the end of each experiment, the residual Fe3+ in solution is about 0.3 g/L. The loss rate of Ni is less than 0.6%.
2) The appropriate reaction conditions are concluded as follows: reaction temperature 95 °C, reaction time 3.5 h, end pH value of reaction 2.5 at the initial concentration of Fe3+ 19.36 g/L.
3) The (NH4)2Fe6(SO4)4(OH)12 shows cluster figure comsisting of sheet or prismatic particles with smooth surface.
References
[1] GUO Xue-yi, SHI Wen-tang, LI Dong, TIAN Qing-hua. Leaching behavior of metals from limonitic laterite ore by high pressure acid leaching [J]. Transactions of Nonferrous Metals Society China, 2011, 21: 191-195.
[2] ZHAI Yu-chun, MU Wen-ning, LIU Yan, XU Qian. A green process for recovering nickel from nickeliferous laterite ores [J]. Transactions of Nonferrous Metals Society China, 2010, 20: 65-77.
[3] KAYA S, TOPKAYA Y A. High pressure acid leaching of a refractory lateritic nickel ore [J]. Miner Eng, 2011, 24(11): 1188-1197.
[4] MU Wen-ning, ZHAI Yu-chun. Desiliconization kinetics of nickeliferous laterite ores in molten sodium hydroxide system [J]. Transactions of Nonferrous Metals Society China, 2010, 20: 330-335.
[5] MU Wen-ning, ZHAI Yu-chun, LIU Yan. Leaching of magnesium from desiliconization slag of nickel laterite ores by carbonation process [J]. Transactions of Nonferrous Metals Society China, 2010, 20(S): s87-s91.
[6] SOLER J M, CAMA J,  A, ESTANGA J. Composition and dissolution kinetics of garnierite from the Loma de Hierro Ni-laterite deposit, Venezuela [J]. Chem Geol, 2008, 249(1-2): 191-202.
A, ESTANGA J. Composition and dissolution kinetics of garnierite from the Loma de Hierro Ni-laterite deposit, Venezuela [J]. Chem Geol, 2008, 249(1-2): 191-202.
[7] BRAND N W, BUTT C R M, ELIAS M. Nickel laterites: Classification and features [J]. AGSO Journal of Australian Geology and Geophysics, 1998, 17(4): 81-88.
[8] GUO X Y, LI D, PARK K H, TIAN Q H, WU Z. Leaching behavior of metals from a limonitic nickel laterite using a sulfation-roasting- leaching process [J]. Hydrometallurgy, 2009, 99(3-4): 144-150.
[9] LI B, WANG H, WEI Y G. The reduction of nickel from low-grade nickel laterite ore using a solid-state deoxidisation method [J]. Miner Eng, 2011, 24(3-4): 1556-1562.
[10] GIRGIN I, OBUT A, UCYILDIZ A. Dissolution behaviour of a Turkish lateritic nickel ore [J]. Miner Eng, 2011, 24(7): 603-609.
[11] LUO W, FENG Q M, OU L M, ZHANG G F, LU Y P. Fast dissolution of nickel from a lizardite-rich saprolitic laterite by sulphuric acid at atmospheric pressure [J]. Hydrometallurgy, 2009, 96(1-2): 171-175.
[12] MCDONALD R G, WHITTINGTON B I. Atmospheric acid leaching of nickel laterites review part I. Sulphuric acid technologies [J]. Hydrometallurgy, 2008, 91(1-4): 35-55.
[13] SENANAYAKE G, CHILDS J, AKERSTROM B D, PUGAEV D. Reductive acid leaching of laterite and metal oxides—A review with new data for Fe(Ni,Co)OOH and a limonitic ore [J]. Hydrometallurgy, 2011, 110(1-4): 13-32.
[14] DAS G K, de LANGE J A B. Reductive atmospheric acid leaching of West Australian smectitic nickel laterite in the presence of sulphur dioxide and copper (II) [J]. Hydrometallurgy, 2011, 105(3-4): 264-269.
[15] DUTRIZAC J E. The effect of seeding on the rate of precipitation of ammonium jarosite and sodium jarosite [J]. Hydrometallurgy, 1996, 42(3): 293-312.
[16] DAS G K, ANAND S, ACHARYA S, DAS R P. Preparation and decomposition of ammoniojarosite at elevated temperatures in H2O-(NH4)2SO4-H2SO4 media [J]. Hydrometallurgy, 1995, 38(3): 263-276.
[17] RISTIC’ M, MUSIC’ S, OREHOVEC Z. Thermal decomposition of synthetic ammonium jarosite [J]. J Mol Struct, 2005, 744-747(3): 295-300.
[18] CHEN Jia-yong, YU Shu-qin, WU Zhi-chun. Separation and utilization of iron in hydrometallurgy [M]. Beijing: Metallurgical Industry Press, 1991. (in Chinese)
[19] MA Rong-jun. Principle of hydrometallurgy [M]. Beijing: Metallurgical Industry Press, 2007. (in Chinese)
[20] CHEN Jia-yong. Handbook of hydrometallurgy [M]. Beijing: Metallurgical Industry Press, 2005. (in Chinese).
硫酸铵焙烧红土镍矿的溶出液制备黄铵铁矾
申晓毅, 邵鸿媚, 王佳东, 翟玉春
东北大学 材料与冶金学院,沈阳 110819
摘 要:为得到硫酸镍溶液除铁的合适工艺条件,以硫酸铵焙烧红土镍矿的熟料溶出液为原料,采用NH4HCO3合成黄铵铁矾。考查了反应温度、反应时间、反应终点pH以及Fe3+初始浓度对除铁率的影响。以上因素均对Fe3+的去除率有显著影响,其中反应温度的影响最为显著。合适的反应条件为:Fe3+初始浓度19.36 g/L、反应温度95 °C、反应时间3.5 h、反应终点pH2.5。在此条件下所得到的黄铵铁矾为包含片状或棱形颗粒的花簇结构。
关键词:红土镍矿;黄铵铁矾;影响因素;溶出液
(Edited by Hua YANG)
Foundation item: Project (51204054) supported by the National Natural Science Foundation of China; Project (N110402012) supported by Fundamental Research Funds for the Central Universities, China; Project (2007CB613603) supported by the National Basic Research Program of China
Corresponding author: Yu-chun ZHAI; Tel: +86-24-83673860; E-mail: shenxy@smm.neu.edu.cn
DOI: 10.1016/S1003-6326(13)62885-X
Abstract: To obtain the appropriate conditions for eliminating Fe3+ from NiSO4 solution, the digestion solution of the clinker was used as raw material, which was obtained from roasting the nickel oxide ore with (NH4)2SO4. The ammonium jarosite was successfully synthesized from the solution with analytic grade NH4HCO3. The effects of reaction temperature, reaction time, end pH value of reaction on the removal rate of iron were investigated, and the effect of the initial concentration of Fe3+ was also discussed. All of those factors had significant effects on the removal rate of Fe3+, among which the reaction temperature was the most prominent. The appropriate reaction conditions were concluded as follows: reaction temperature 95 °C, reaction time 3.5 h, end pH value of reaction 2.5 at initial concentration of Fe3+ 19.36 g/L. The physical aspect of (NH4)2Fe6(SO4)4(OH)12 was cluster figure composed of sheet or prismatic particles with smooth surface.


