
Elevated temperature oxidation behavior of HVOF sprayed TiB2 cermet coating
Behnam LOTFI
Department of Materials Engineering, Faculty of Engineering, Shahid Chamran University, Ahvaz, Iran
Received 19 September 2008; accepted 6 July 2009
Abstract:
Oxidation behaviors of Ni(Cr)-TiB2 coating deposited by HVOF technique were studied at 800, 900 and 1 000 ℃ in air. The microstructures of as-sprayed and oxidized coatings were characterized by X-ray diffractometry (XRD) and scanning electron microscopy (SEM). After oxidation at 800 ℃, a thin and adherent oxide layer was formed on the surface of the coating. With increasing exposure temperature, the thickness of the oxide layer increased; and at 1 000 ℃ the oxide layer separated from the coating. Investigation of the kinetics of oxidation by using the thermogravimetric analysis (TGA) technique shows a parabolic oxidation rate.
Key words:
TiB2; cermet coating; thermal spraying; oxidation;
1 Introduction
Titanium diboride (TiB2) as a refractory compound is an attractive material for a wide range of applications such as heat-resisting structural materials, wear resistant parts, and cutting tools, because of its excellent physical and chemical properties including high melting point (about 2 900 ℃), high elastic modulus (550 GPa), high hardness (33 GPa), good oxidation resistance up to 1 000 ℃ and good electrical conductivity[1-4]. This combina- tion of properties also makes TiB2 very interesting as a coating material for various applications[5].
TiB2 coatings can be deposited on various metallic substrates using several processes, such as physical and chemical vapor deposition[6], pulsed electrode surfacing, and laser surface modification[7-8]. Pure TiB2 coating is very brittle and normally has very high compressive residual stress after deposition with poor bond strength. To overcome these problems, TiB2 can be applied with a metallic binder to form a cermet coating. TiB2 cermet powders can be deposited by thermal spraying processes such as the high velocity oxy-fuel (HVOF) process, which is capable of producing high quality coating with respect to porosity, hardness, bond strength and roughness[9-10]. In HVOF technique, the thermal degradation of carbide and boride during the spraying is decreased, due to the lower flame temperature and shorter dwell time of the powder particles compared with other processes[11]. Moreover, the coatings deposited by this process contain lower porosity (<2%, volume fraction)[12]. It is, therefore, expected that HVOF coatings will exhibit a higher oxidation resistance.
The oxidation of metal-TiB2 composites has not been investigated extensively although there are a few studies on the oxidation behavior of monolithic TiB2 [5, 13].
The aim of the present study is to evaluate the oxidation behavior of a Ni(Cr)-TiB2 coating deposited by HVOF spraying.
2 Experimental
For thermal spray experiments, BS080A15 mild steel (EN3B) pieces of 100 mm×30 mm×1 mm were used as substrate. The substrates were grit blasted before spraying by alumina grit (250 mm) at blasting pressure of 0.6 MPa and blasting angle of about 90?. HVOF thermal spraying of Ni(Cr)-40%TiB2 powder (8-38 μm) was performed by using a UTP top gun. The spraying parameters are given in Table 1. Ni(Cr)-TiB2 powder was prepared by self-propagation high temperature synthesis (SHS) method. The details of the feedstock powder synthesis and spraying experiments were presented in Ref.[14].
Table 1 HVOF spraying parameters

Elevated temperature oxidation behavior of Ni(Cr)- TiB2 coating in air was evaluated by two procedures: 1) long term exposure to study the growth of oxide scale and its morphology and 2) thermogravimetric analysis (TGA) to obtain the oxidation kinetics parameters.
Long-term exposure of the Ni(Cr)-TiB2 coating was carried out to observe the integrity of the coating, coating/substrate interface, phase transformations and morphological features of the oxide scale. Coated specimens were heated in an exciton muffle furnace in air at 800, 900 and 1 000 ℃, respectively, for 50 h. The morphology, topography and cross sectioned microstructure of samples were studied by Philips XL30 field-emission scanning electron microscope(SEM). The phase compositions of the coatings were investigated by X-ray diffractometry (XRD) using a SIEMENS D500 diffractometer employing monochromatic Cu Kα radiation. XRD scans were performed with a step size of 0.01? and a dwell time per step of 9 s.
Oxidation kinetics of those Ni(Cr)-TiB2 coatings were studied using a METTLER TOLEDO STAR thermogravimetric analyzer (TGA). TGA samples with 2 mm in diameter were prepared by a wire cut EDM machine from the coated sample. To ensure that the leftover foil was completely free of substrate, the resultant disk was ground to achieve a thin foil (<0.1 mm) of the coating. The specimen was cleaned in acetone, dried and weighed followed by exposure to air under static conditions in a furnace. TGA test was performed at a heating rate of 5 ℃/min from 25 ℃ up to 800 ℃. Samples were held at this temperature for 3 h.
3 Results and discussion
3.1 Microstructure of as-sprayed coating
The microstructures of feedstock powders were described in Ref.[14]. Fig.1 shows the cross-sectional microstructure of as-sprayed HVOF coating. Fig.1(a) shows a 200 mm-thick coating, which is well bonded to the steel substrate. The higher magnification image shows that the coating contains fine spherical TiB2 particles. The TiB2 particles are rather uniformly distributed throughout the coating. No significant pores and cracks are observed in the microstructure. A splat-like structure typically associated with thermal spraying can be seen with oxide delineating the splat boundaries.

Fig.1 Cross-sectional SEM micrographs of HVOF thermal sprayed Ni(Cr)-40% TiB2 coating: (a) Lower magnification; (b) Higher magnification
The XRD pattern of the as-sprayed coating is shown in Fig.2. The main peaks correspond to the binder (Ni solid solution) and reinforcement (TiB2). In addition to traces of TiB and Ni2B, NiTiO3 and Ti2O3 phases are observed on XRD pattern. The oxide phases are formed during spraying.

Fig.2 XRD pattern of as-sprayed Ni(Cr)-40% TiB2 coating
The amorphous halo on XRD pattern of the coating at 2θ of 40?- 50? indicates that the Ni-rich binder phase has a partially amorphous or nanocrystalline structure which is likely formed as a result of partial dissolution of B, Cr and Ti in the Ni-rich melt and subsequent rapid solidification during HVOF spraying[15].
3.2 Oxidation in air at high temperatures
Cross-sectional SEM micrograph of the coating after 50 h exposure at 800 ℃ is presented in Fig.3. A thin oxide layer with a thickness of about 5 μm is observed on the coating. Microscopic observations show that the oxide layer is continuously attached to the underlying coating, and the coating is intact with no signs of delamination or cracking.

Fig.3 Cross-sectional SEM micrograph of oxidized coating after exposure at 800 ℃ for 50 h
Fig.4 shows the cross-sectional SEM micrograph of the coating after oxidation at 900 ℃ for 50 h. A thicker oxide layer (about 20 μm) is formed in this case. The oxide layer is adherent to the coating. However, at 900 ℃ (Fig.4) the fraction of TiB2 particles in outer layers of the coating is smaller in comparison with that at 800 ℃ (Fig.3). This may be due to the higher level of oxidation in the coating. After oxidation at 1 000 ℃, the oxide layer delaminates from the coating and breaks into several pieces. Debonding of the thicker oxide layer formed at 1 000 ℃ maybe results from high stresses induced by the large Pilling-bedworth ratio of the predominant oxide phase in the oxide scale (P.BTiO2= 1.95)[16]. The results obtained in the present work demonstrate that the HVOF thermally sprayed Ni(Cr)- TiB2 coating has an improved oxidation behavior in comparison with that prepared by laser surface engineered Fe-TiB2 composite coating which spalled off from the coating after exposure at 800 ℃ for 50 h[17]. The oxidation resistance of coating prepared here is higher compared with WC-Co coatings, which oxidize in temperature range of 400-600 ℃. Oxidation rate of Ni(Cr)-TiB2 coating is similar to that reported for Ni(Cr)- Cr3C2 cermet coatings[18].

Fig.4 Cross-sectional SEM micrograph of oxidized coating after exposure at 900 ℃ for 50 h
Topographic features of the oxide scale formed on the coating after exposure at 800 ℃ and 900 ℃ are compared in Fig.5. After oxidation at 800 ℃, the coating shows a relatively smoother surface containing angular oxide crystals. The growth of oxide layer is accelerated at the higher temperature (900 ℃) as characterized by bigger oxides crystals. The oxides crystals present a prismatic feature due to faceted growth. Rod-shaped and seed-like oxides crystals were reported to form during oxidation of Al2O3-TiB2 composite and a TiB2 ceramic material, respectively[13, 5].
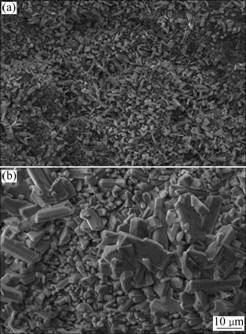
Fig.5 SEM micrographs showing morphology of oxidized surface after exposure at 800 ℃ (a) and 900 ℃ (b) for 50 h
Phase composition of the oxide scales formed on the coating was analyzed using XRD. Fig.6 presents the XRD pattern for oxide layer after oxidation at 900 ℃. The predominant phase on the surface of the coating after oxidation is rutile (TiO2) which resulted from the oxidation of TiB2 according to the following reaction:
TiB2+(5/2)O2![]() TiO2+B2O3 (1)
TiO2+B2O3 (1)
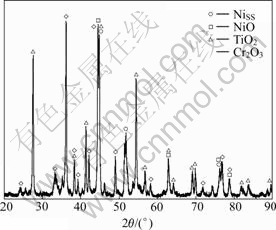
Fig.6 X-ray diffraction pattern for surface of oxidized coating after exposure at 900 ℃ for 50 h
According to Ref.[17], B2O3 amorphous phase melts at about 450 ℃ and covers the surface. However, this phase cannot be detected by X-ray analysis because of the amorphous structure[17]. Traces of NiO and Cr2O3 oxide phases are also present on XRD pattern. In contrast to Fig.2, XRD pattern of the oxide layer shows no nanocrystalline or amorphous phase in the Ni-rich matrix, suggesting that the high temperature exposure leads to the crystallization of amorphous phase. Crystallization is reported to reduce the oxidation resistance by inducing a stress field due to dimensional variation[19].
The oxidation behavior of Ni(Cr)-TiB2 cermet coating was characterized by measuring the mass change per surface area (?m/S) during exposure at 800 ℃. Fig.7 illustrates the mass gain per unit surface area during 3 h exposure to oxidation. The oxidation rate is parabolic at this temperature, which can be described by Eq.(2)[20]:
![]() (2)
(2)
where t is the oxidation time; and Kp and C represent the oxidation kinetic constants[19].
The mass gain curve (Fig.7) indicates a diffusion- controlled kinetics. The parabolic rate constant, Kp, is determined to be 6.4×10-9 g2/(cm4?min). This value is comparable to that of TiB2-base ceramics[5] and very much lower compared to that of laser surface engineered TiB2 coating oxidized at 800 ℃[17].

Fig.7 Oxidation kinetics of coating at 800 ℃ showing parabolic behavior
4 Conclusions
1) HVOF thermally sprayed Ni(Cr)-TiB2 coating exhibited a high oxidation resistance. The oxide layer is thin, and is adherent to the coating up to 900 ℃ with no signs of delamination or cracking.
2) The dominant phase in the oxide layer is rutile TiO2, which shows a prismatic crystal having larger grains at higher temperature.
3) The oxidation kinetics of the coating at 800 ?C follows a parabolic rate law.
Acknowledgements
The authors would like to acknowledge the contribution of Prof. D. G. McCARTNEY at University of Nottingham for making the coatings and helpful advice.
References
[1] RONALD G M. Material properties of titanium diboride [J]. J Res Natl Inst Stand Technol, 2000, 105: 709-720.
[2] SCHNEIDER S J. Engineering materials handbook: Ceramic and glasses [M]. Ohio: Materials Park, ASM International, 1995: 787.
[3] MARTIN C, CALES B, VIVER P, MATHIEU P. Electrical discharge machinable ceramic composites [J]. Mater Sci Eng A, 1989, 109: 351-356.
[4] MATKOVICH V I. Boron and refractory borides [M]. New York: Springer-Verlag, 1977.
[5] LEE B D, LEE Y C, KIM D J. The oxidation of TiB2 ceramics containing Cr and Fe [J]. Oxid Met, 2001, 56: 177-189.
[6] CHOY K L, DERBY B. Evaluation of the efficiency of TiB2 and TiC as protective coatings for SiC monofilament in titanium-based composites [J]. J Mater Sci, 1994, 29: 3774-3780.
[7] AGARWAL A, DAHORTRE N B. Pulse electrode deposition of superhard boride coatings on ferrous alloy [J]. Surf Coating Technol, 1998, 106: 242-250.
[8] AGARWAL A, DAHORTRE N B. Synthesis of boride coating on steel using high energy density processes: Comparative study of evolution of microstructure [J]. Mater Charact, 1999, 42: 31-44.
[9] JONES M, HORLOCK A J, SHIPWAY P H, MCCARTNEY D G, WOOD J V. A comparison of the abrasive wear behaviour of HVOF sprayed titanium carbide- and titanium boride-based cermet coatings [J]. Wear, 2001, 250/251: 1009-1016.
[10] STURGEON A J. High velocity oxyfuel spraying promises better coatings [J]. Met Mater, 1992, 8: 547-548.
[11] WANG B Q, LUER K. The erosion-oxidation behaviour of HVOF Cr3C2-NiCr cermet coating [J]. Wear, 1994, 174: 177-185.
[12] HARVEY D. HVOF takes off [J]. TWI Bulletin, 1991, 5: 107-109.
[13] TAMPIERI A, BELLOSI A. Oxidation of monolithic TiB2 and of Al2O3-TiB2 composite [J]. J Mater Sci, 1993, 28: 649-653.
[14] LOTFI B, SHIPWAY P H, McCARTNEY D G, EDRIS H. Abrasive wear behaviour of Ni(Cr)-TiB2 coatings deposited by HVOF spraying of SHS-derived cermet powders [J]. Wear, 2003, 254: 340-349.
[15] DENT A H, HORLOCK A J, McCARTNEY D G, HARRIS S J. Microstructure formation in high velocity oxy-fuel thermally sprayed Ni-Cr-Mo-B alloys [J]. Mater Sci Eng A, 2000, 283: 242-250.
[16] XU C, GAO W. Pilling-Bedworth ratio for oxidation of alloy [J]. Mat Res Innovat, 2000, 3: 231-235.
[17] AGARWAL A, KATIPELLI L R, DAHORTE B. Elevated temperature oxidation of laser surface engineered composite boride coating on steel [J]. Met Mat Trans A, 2000, 31: 461-473.
[18] BERGER L M, HERMEL W, VUORISTO P, MANTYLA T, LENGAUER W, ETTMAYER P. Structure, properties and potentials of WC-Co, Cr3C2-NiCr and TiC-Ni-based hardmetal-like coatings [C]// 9th National Thermal Spray Conf. Cincinnati, 1996: 89-96.
[19] LOURO C, CAVALEIRO A. Oxidation behaviour of W-N-M(M=Ni, Ti) sputtered films [J]. Surf Coating Technol, 1995, 74/75: 998-1004.
[20] BIRKS N, MEIER G H, PETTIT F S. Introduction to the high temperature oxidation of metals [M]. Cambridge: Cambridge University Press, 2006: 75.
Corresponding author: Behnam LOTFI; Tel: +98-913-1024677; E-mail: behnaml@scu.ac.ir
DOI: 10.1016/S1003-6326(09)60129-1
(Edited by YANG Hua)


