
Theoretical design of high-spin biradical molecules with heterocycles as coupling unit
WANG Li-min(王立敏)1, CHU De-qing(储德清)1 ,
ZHANG Jing-ping(张景萍)2, WANG Rong-shun(王荣顺)2
1. Faculty of Material and Chemical Engineering, Tianjin Polytechnic University, Tianjin 300160, China;
2. Faculty of Chemistry, Northeast Normal University, Changchun 130024,China
Received 10 April 2006; accepted 25 April 2006
Abstract:
Computational studies of a class of potentially stable high-spin biradicals that two-atom-three-electron spin centers SC units connected by heterocycles FC and phenyl EG were described. The geometry and character of the spin exchange interaction were obtained by means of UB3LYP/6-31G*. The results show that the molecules possessing three different arranged fashions are designed with — ?N—S as SC fragment, pyridine as FC and phenyl as EG, the spin densities on the two atoms of the SC fragment are different from the delocalization results in the specific stability of —?N—S. In these molecules, the stabilities of the triplet states decrease when the distance between the atoms of central SC (—N—) increases. Molecules with —?N—S as SC fragment, pyridine, pyrazine and triazine as FC and phenyl as EG are designed, the stability of triplet states for the molecule with pyridine as FC is the highest, and that for the molecule with pyrazine as FC is the lowest. Molecules with —?N—S, —?N—O and —?N—NH as SC fragment, pyridine as FC and phenyl as EG are designed, the stability of triplet states for the molecule with —?N—S as SC is the highest, that for the molecule with —?N—NH as SC is the lowest.
Key words:
heterocycle; ferromagnetic coupling unit; biradical molecule;
1 Introduction
The design and the synthesis of organic molecules with very high-spin ground states have been a topic of great interests [1-4]. One of the rational approaches to design high-spin molecules proposed and studied by several groups [5, 6], consists in conceptually dividing the molecules into two components: a spin-containing (SC) fragment which provides the unpaired electron and a ferromagnetic coupling (FC) unit which is connected with radical centers ferromagnetically.
The recent interest in the design of organic-based magnetic materials has spurred considerable work on the syntheses and the properties of organic open-shell molecules. The highest spin states obtained for purely organic systems were reported for systems such as polycarbenes and dendridic polyradicals. High-spin polycarbenes have not been considered although it is feasible for them to be realistic components of magnetic materials due to their high reactivity under non-cryogenic conditions, and the recent results suggested some improvements in the stability of polycarbenes via appropriate substitution [7]. Therefore, the quest for new classes of stable multiradicals with high-spin ground states has been strongly pursued.
The free radicals containing the two-atom three- electron hydrazyl [8] (—?N—S, —?N—O—, ?N—N< spin centers are exceptionally persistent and can be isolated as pure radical crystals in some cases. In the experiment, the compounds that the ferromagnetic interaction between metals and radicals were brought were found [9, 10], the metal ion was connected with radicals through heterocycle. We will make computational studies of a class of potentially stable high-spin biradicals that incorporate hydrazyl SC units connected by heterocycles FC, phenyl end ground (EG) as shown in the schemes and study the effect of heterocycles and spin centers on the spin multiplicity in high-spin molecules and select the molecules possessing stable high-spin ground state coordinated by metals.
2 Calculation models and methods
The calculation models are schematically shown in Fig.1. Novel stable high spin molecules possessing three different arranged fashions were designed using —?N—S— as SC fragment, pyridine (1), pyrazine (2), triazine (3) as a FC unit and phenyl as EG, and high spin molecules were designed with —?N—S—, —?N—O—, —?N—N< as SC, pyridine as FC and phenyl as EG. The geometry and character of the spin exchange interaction were obtained by means of UB3LYP/6-31G*. Symmetry was selected as constrains for 1am, 2an, 1b1, 1c1 with CS or C2V. All calculations were completed with PⅣ computer.
3 Results and discussion
The multiplicities of ground states and their stabilities are the most important parameters for determining whether biradical systems possess ferromagnetic coupling and their extent of stabilities, which can be obtained by calculation in terms of the energy gap between the ground state and first excited state (?ES-T=ES-ET). For biradicals, ?ES-T=2J. J>0 corresponds to ferromagnetic coupling, i.e. triplet ground state, while J<0 means antiferromagnetic coupling, i.e. singlet ground state. Additional requirements for the stabilization of a high-spin ground state in π-conjugated systems are the existence of partially occupied degenerate or near-degenerate molecular orbitals (POMOs) and strong coulomb electron-electron interaction within the subspace of near degenerate POMOs that prevent spin pairing and give rise to preference of the high-spin state. Therefore, the POMO level splitting in terms of the lowest versus the highest energy of POMOs (?EPOMO) is another important parameter. The singlet-triplet splitting energy gap ?ES-T, ?EPOMO and the spin-densities of N, S, O, NH, N-2 between FC and SC calculated by the use of DFT methods are given in Tables 1-3.
For the sake of simplicity, only the spin densities for the SCs and ρ(N-2) are listed, it can be seen that these are the major sites of positive spin density. These variations in spin density can be correlated with the variation of ?ES-T, and the combination of the two results reveals useful trends about the degree of preference of the high spin state as a function of structural variation. From Tables 1-3, it can be seen that all the molecules in which the singlet-triplet splitting energy gap ?ES-T>0 corresponds to ferromagnetic coupling possess high-spin ground states. Since ?EPOMO is very small in all the molecules, there are near-degenerate molecular orbitals and strong coulomb electron-electron interaction in them, their high-spin ground states are very stable.
From Table 1, it can be seen that for the biradical systems composed of phenyl (EG) and —?N—S (SC) and heterocycles (FC) the spin densities on the two atoms of the SC have the same sign, but with considerably larger spin densities on the hypovalent nitrogen atoms than on the —S— atom. The normally valent atoms therefore tend to insulate the spin-bearing hypovalent sites from delocalization. The choice of connectivity of the FC to the hypovalent or the normally valent atom of the SC strongly affects the spin density ρ(N-2) of the FC unit, which is a useful measure of the degree of spin delocalization from the SC to the FC. In which the hypovalent spin density sites are directly attached to the FC, the high-spin state is considerably
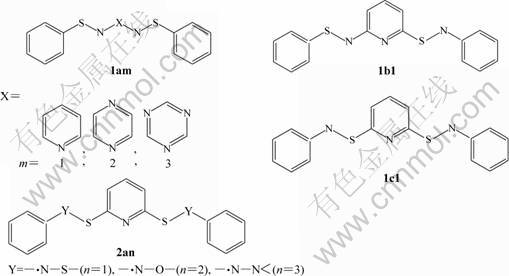
Fig.1 Calculation models
Table 1 E(T), E(S), ?ES-T, ?EPOMO and spin densities of N, S, N-2 for 1a1, 1b1 and 1c1
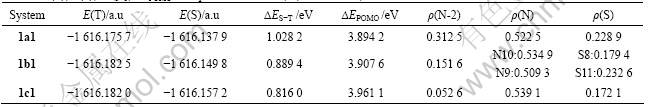
Table 2 E(T), E(S), ?ES-T, ?EPOMO and spin densities of N, S, N-2 for 1am
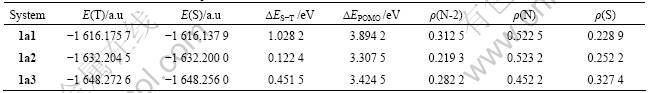
Table 3 E(T), E(S), ?ES-T, ?EPOMO and spin densities of N, S, O, NH, N-2 for 2an

more favorable than the cases in which neither of these hypovalent sites is connected to the FC. So the spin densities on the two atoms of the SC are different from delocalization results in the specific stability of — ?N—S.
The diradicals can be ranked in the order of decreasing ?ES-T as 1a1>1b1>1c1. The decreasing high spin preference not only connects with an effect of distance as the hypovalent spin-bearing sites on the SC fragments become further apart, but also is due to the spin-insulating effects of the normally valent atoms in the SC fragments. Because spin density does not delocalize well through the sites in the SC fragments, less spin density is delocalized on to the FC units in 1b1, and even less in 1c1, resulting in the decrease in ?ES-T and the spin densities of N-2, So the stability of triplet states decreases with the increasing distance between central SC fragments (—N—).
Table 2 shows that the order of the stability of triplet states for 1am (the singlet- triplet splitting energy gap) is 1a1>1a3>1a2, since ?EPOMO for 1am is very small, there are near-degenerate molecular orbitals in all the molecules. Because the order of the spin density of N-2 for 1am is 1a1>1a3>1a2, that is consistent with the results discussed before. The orders of the stability of triplet states for 1am, 1a1, 1a2, 1a3, 2an show the stability of triplet states for —?N—S as SC fragment, phenyl as EG and pyridine as FC is the highest, and that for the molecule with pyrazine as FC is the lowest.
Table 3 shows that the order of the stability of triplet states for 2an (the singlet-triplet splitting energy gap) is 2a1>2a2>2a3, ?EPOMO of 2a1 is the smallest, namely, the degenerate degree of two frontier orbitals for 2a1 is the largest, so the stability of triplet states for 2a1 is the largest. Because the order of the spin density of N-2 for 2an is 2a1>2a2>2a3, that is consistent with the results discussed before. The orders of the stability of triplet states for 2an show the stability of triplet states for pyridine as FC, phenyl as EG and — ?N—S as SC is the highest, that for the molecule with — ?N—NH as SC is the lowest.
4 ConclusionsAll the molecules correspond to ferromagnetic coupling and possess high-spin ground states. For the biradical systems with —?N—S as SC fragment, pyridine as FC and phenyl as EG, the spin densities on the two atoms of the SC are different from delocalization results in the specific stability of —?N—S, the stability of triplet states decreases with the increasing distance between central SC fragments (—N—). The orders of the stability of triplet states for 1am show the stability of triplet states for —?N—S as SC fragment, phenyl as EG and pyridine as FC is the highest, and that for the molecule with pyrazine as FC is the lowest. The orders of the stability of triplet states for 2an show the stability of triplet states for pyridine as FC, phenyl as EG and —?N—S as SC is the highest, that for the molecule with —?N—NH as SC is the lowest.
References[1] ITOH K, KINOSHITA M, Molecular Magnetism New Magnetic Materials [M]. Tokyo: Gordon and Breach, 2000.
[2] MILLER J S, DRILLON M. Magnetism-Molecules to Materials [M]. Weinheim: Willey-VCH, 2001.
[3] ZHENG S J, LAN J, KHAN S I, LAN J, KHAN S I, RUBIN Y. Synthesis, characterization, and coordination chemistry of the 2-Azaphenalenyl radical [J]. J Am Chem Soc, 2003, 125(19), 5786-5791.
[4] MAEKAWA K, SHIOMI D, ISE T. Exchange interaction in covalently bonded biradical-monoradical composite molecules [J]. J Phys Chem, 2005, B109(8): 3303-3309.
[5] OYCHINNIKOV A A, OYCHINNIKOV A A. Multiplicity of the ground state of large alternant organic molecules with conjugated bonds[J]. Theor Chim Acta, 1978, 47: 297-301.
[6] YAMAGUCHI K, TOYODA Y, FUENO T. A generalized MO (GMO) approach to unstable molecules with quasi-degenerate electronic states: ab initio GMO calculations of intramolecular effective exchange integrals and designing of organic magnetic polymers [J]. Synth Met, 1987, 19: 81.
[7] TOMIOKA H, HATTORI M, HIRAI K, SATO K, SHIOMI D, TAKUI T, ITOH K. Persistent high-spin polycarbene. Generation of polybrominated 1,3,5-Tris-[2-[4- (phenylcarbeno)-phenyl]ethynyl] benzene (S=3) and spin identification by two-dimensional electron spin transient nutation spectroscopy [J]. J Am Chem Soc, 1998, 120: 1106-1112.
[8] HAIRE D L, JAMEN E G. Enhanced diagnostic EPR and ENDOR spectroscopy of radical spin adducts of deuterated a-Phenyl N-tert-Butyl Nitrone [J]. Magnetic Resonance in Chemistry, 1994, 32: 151-157.
[9] ISHIMARU Y, KITANO M, KUMADA H, KOGA N, IWAMURA H. Regiospecificity in the exchange coupling of the spins of copper(II) ion coordinated with the ring nitrogen atoms and N-tert-butylaminoxyl radical attached as a substituent on the pyridine and N-phenylimidazole rings [J]. Inorg Chem, 1998, 37: 2273-2280.
[10] BARCLAY T M, HICKS R G, LEMAIRE M T. Structure and magnetic properties of a nickel (II): complex of a tridentate verdazyl radical: strong ferromagnetic metal-radical exchange coupling [J]. Chem Comm, 2000,21:2141-2142.
Foundation item: Projects(29804002, 20274006) supported by the National Natural Science Foundation of China; Projects(029307, 029302) supported by Tianjin Polytechnic University, China
Corresponding author: WANG Li-min; Tel: +86-22-81669245; E-mail: wanglm326@nenu.edu.cn


