J. Cent. South Univ. Technol. (2007)03-0340-04
DOI: 10.1007/s11771-007-0067-3
![]()
Synthesis and characterization of triclinic structural LiVPO4F as possible 4.2 V cathode materials for lithium ion batteries
ZHONG Sheng-kui(钟胜奎)1,2, YIN Zhou-lan(尹周澜)1, WANG Zhi-xing(王志兴)3, CHEN Qi-yuan(陈启元)1
(1. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. Department of Materials and Chemistry, Guilin University of Technology, Guilin 541004, China;
3. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract:
A potential 4.2 V cathode material LiVPO4F for lithium batteries was prepared by two-step reaction method based on a carbon-thermal reduction (CTR) process. Firstly, V2O5, NH4H2PO4 and acetylene black are reacted under an Ar atmosphere to yield VPO4. The transition-metal reduction is facilitated by the CTR based on C→CO transition. These CTR conditions favor stabilization of the vanadium as V3+ as well as leaving residual carbon, which is useful in the subsequent electrode processing. Secondly, VPO4 reacts with LiF to yield LiVPO4F product. The property of the LiVPO4F was investigated by X-ray diffractometry (XRD), scanning electron microscopy (SEM) and electrochemical measurement. XRD studies show that LiVPO4F synthesized has triclinic structure(space group ![]() ), isostructural with the naturally occurring mineral tavorite, LiFePO4·OH. SEM image exhibits that the particle size is about 2 ?m together with homogenous distribution. Electrochemical test shows that the initial discharge capacity of LiVPO4F powder is 119 mA?h/g at the rate of 0.2C with an average discharge voltage of 4.2V (vs Li/Li+), and the capacity retains 89 mA?h/g after 30 cycles.
), isostructural with the naturally occurring mineral tavorite, LiFePO4·OH. SEM image exhibits that the particle size is about 2 ?m together with homogenous distribution. Electrochemical test shows that the initial discharge capacity of LiVPO4F powder is 119 mA?h/g at the rate of 0.2C with an average discharge voltage of 4.2V (vs Li/Li+), and the capacity retains 89 mA?h/g after 30 cycles.
Key words:
lithium ion batteries; cathode material; LiVPO4F; carbon-thermal reduction method;
1 Introduction
Currently, LiCoO2 is the most widely used cathode materials for lithium-ion batteries. However it is relatively expensive, especially for large-scale applications such as backup power systems and hybrid electric vehicles[1]. Many researchers are working to find a cheap, effective replacement for LiCoO2[2-7]. Since the demonstration of reversible electrochemical lithium insertion-extraction for LiFePO4 in 1997[8], lithium transition metal phosphates have attracted much great interest as promising new cathode materials for lithium-ion batteries[9-13].
Recently, BARKER et al[14] extended their investigations to include the electrochemical evaluation of a series of fluorophosphate materials represented by the general formula LiMPO4F, where M represents a 3d transition metal. Among these materials, LiVPO4F is the most promising and attractive one because of its relatively high capacity with a plateau at around 4.2 V. In this study, we synthesized LiVPO4F by two-step reaction method based on a carbothermal reduction(CTR) process. The cathode material LiVPO4F synthesized has better electrochemical performance than that reported in Ref.[15]. However, improvements must be made for the long-term cycleability in future.
2 Experimental
Appropriate amounts of V2O5, NH4H2PO4 and acetylene black were initially ground in mortar and then were thoroughly mixed by ball milling for 2 h for further use. The LiVPO4F material was prepared by a two-step reaction method based on a CTR method. In the first step of CTR, the mixed precursor was first decomposed at 300 ℃ for 4 h to disperse NH3 and H2O, reground, and then calcined at 750 ℃ for 6 h to obtain VPO4. In the second step, appropriate amounts of VPO4 and LiF were thoroughly mixed by ball milling for 2 h, and calcined at 750 ℃ for 30 min to yield LiVPO4F material. To avoid the oxidation of vanadium, each step of the process was carried out under flowing Ar atmosphere. In order to complete vanadium reduction and to leave residual carbon in the product, a 25% mass excess of carbon was used on the stoichiometric conditions based on Reaction (1). LiVPO4F preparative reaction scheme may be summarized as follows:
V2O5+2NH4H2PO4+2C→
2VPO4+2NH3+3H2O+2CO (1)
LiF+VPO4→LiVPO4F (2)
The powder X-ray diffraction (XRD, Rint-2000, Rigaku) measurement using Cu Kα radiation was employed to identify the crystalline phase and lattice parameters of the synthesized materials, recorded at room temperature. The particle size and morphology of LiVPO4F powders were observed by scanning electron microscope (JEOL, JSM-5600LV) with an accelerating voltage of 20 kV.
The electrochemical characterizations were performed using CR2025 coin-type cell. For positive electrode fabrication, the prepared powders were mixed with 10%(mass fraction) carbon black and 10%(mass fraction) polyvinylidene fluoride in N-methyl pyrrolidinone until slurry was obtained. And then, the blended slurries were pasted onto an aluminum current collector, and the electrode was dried at 120 ℃ for 10 h in vacuum. The coin cell consisted of the positive electrode and lithium foil negative electrode separated by a porous polypropylene film, and 1 mol/L LiPF6 in EC+EMC+DMC (1:1:1 in volume) as the electrolyte. The assembly of the cells was carried out in a dry Ar-filled glove box. The cells were charged and discharged over a voltage range of 3.0-4.4 V versus Li/Li+ electrode at room temperature. Cyclic voltammograms were tested at a scanning rate of 0.1 mV/s in the voltage range of 3.0-4.9 V.
3 Results and discussion
Fig.1 shows the XRD pattern of VPO4 powders. All fundamental peaks can be indexed to the orthorhombic structure with space group Cmcm (JCPDS card No.34-1336). The crystallographic parameters of VPO4 powders: a=0.523 8 nm, b=0.779 0 nm , c=0.628 8 nm, and cell volume of 0.256 6 nm3, are close to literature data of VPO4 that made by conventional solid-state synthesis methods[16].

Fig.1 XRD pattern of VPO4 powder
Fig.2 shows the XRD pattern collected for the prepared LiVPO4F powders. It’s evident that all fundamental peaks can be indexed to the triclinic structure with space group![]() (JCPDS card No.42-1412). Excess carbon (1.83%, determined by carbon-sulphur analyzer) left in LiVPO4F powders is not detected in the XRD pattern, indicating that excess carbon exists in amorphous form. As expected, no diffraction peaks are identifiable as LiF, which confirms complete LiF incorporation. The incorporation reaction of the orthorhombic VPO4 with LiF results in a loss of crystal symmetry so that the LiVPO4F product phase adopts a triclinic structure. The following crystallographic parameters of the prepared LiVPO4F powders, a=0.514 9 nm, b=0.529 2 nm, c=0.744 5 nm, α=67.397?, β=67.518?, γ=82.008?, and cell volume of 0.173 0 nm3, are in good accordance with literature sample[17]. The framework structure of LiVPO4F comprises a three-dimensional framework built up from [PO4] tetrahedron and [VO4F2] octahedron with the oxygen atoms shared between PO4 and VO4F2. Within this framework structure, there exist two crystallographic positions, within which the lithium ions are statistically distributed.
(JCPDS card No.42-1412). Excess carbon (1.83%, determined by carbon-sulphur analyzer) left in LiVPO4F powders is not detected in the XRD pattern, indicating that excess carbon exists in amorphous form. As expected, no diffraction peaks are identifiable as LiF, which confirms complete LiF incorporation. The incorporation reaction of the orthorhombic VPO4 with LiF results in a loss of crystal symmetry so that the LiVPO4F product phase adopts a triclinic structure. The following crystallographic parameters of the prepared LiVPO4F powders, a=0.514 9 nm, b=0.529 2 nm, c=0.744 5 nm, α=67.397?, β=67.518?, γ=82.008?, and cell volume of 0.173 0 nm3, are in good accordance with literature sample[17]. The framework structure of LiVPO4F comprises a three-dimensional framework built up from [PO4] tetrahedron and [VO4F2] octahedron with the oxygen atoms shared between PO4 and VO4F2. Within this framework structure, there exist two crystallographic positions, within which the lithium ions are statistically distributed.
The SEM image of VPO4 powders is shown in Fig.3. The irregular and slight agglomeration morphology of VPO4 is clearly seen in the image.
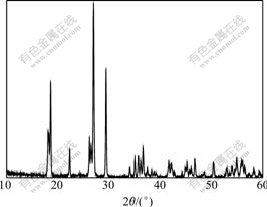
Fig.2 XRD pattern of LiVPO4F
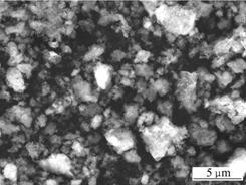
Fig.3 SEM image of VPO4 powder
Fig.4 shows the SEM image of LiVPO4F powder. The sphere-like particles are observed, and the particle size is about 2 ?m together with homogenous distribution. The notable morphology difference between VPO4 and LiVPO4F is that the surfaces of LiVPO4F are smoother and compacter than those of VPO4. The composite product morphology can also be seen from the SEM micrograph, and the floc-like particles enclosing LiVPO4F maybe excess carbon left.
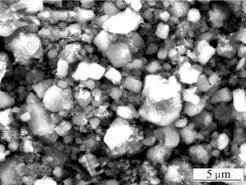
Fig.4 SEM image of LiVPO4F powder
The first charge-discharge curves of LiVPO4F cycled between 3.0 V and 4.4 V at the rate of 0.2C are shown in Fig.5. The initial charge and discharge capacities are about 140 mA?h/g and 119 mA?h/g, respectively. It is clear that the columbic efficiency of the initial charge-discharge cycle is only about 85%. The charge-discharge curves of LiVPO4F powders exhibit the presence of a small inflection in the charge curve, reflecting two energetically inequivalent reactions. This phenomenon presumably corresponds to accessing of the lithium ions located in the two known crystallographic sites within the LiVPO4F structure.
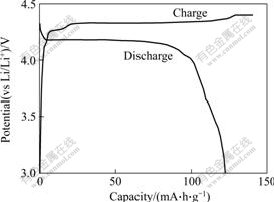
Fig.5 First charge-discharge curves of LiVPO4F at rate of 0.2C
The electrochemical cycling performance of LiVPO4F compound is evaluated in Li/LiVPO4F cell configuration in the voltage range of 3.0-4.4 V at room temperature. Fig.6 shows the cyclic charge/discharge profiles for LiVPO4F cathode materials at 0.2C rate. As seen in Fig.6, the initial discharge capacity of LiVPO4F is about 119 mA?h/g, and the discharge capacity drops to about 89 mA?h/g after 30 cycles. The capacity loss is about 25.2% after 30 cycles. From above results, although LiVPO4F synthesized has better cycling performance than the result reported by Ref.[15], the capacity loss should be reduced.
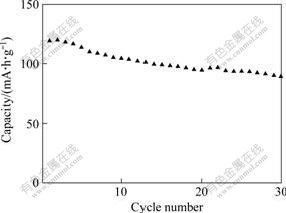
Fig.6 Electrochemical cycling performance of LiVPO4F
The cyclic voltammetry (CV) for the LiVPO4F electrode in the first cycle at a scanning rate of 0.1 mV/s is shown in Fig.7. The cyclic voltammetry profile indicates the oxidation/reduction potential, at which lithium ion is extracted from or inserted into the lattice. As shown in Fig.7, the electrode of LiVPO4F exhibits oxidation and reduction peak at 4.38 and 4.18 V, respectively. The appearance of a small oxidation peak located at 4.27 V presumably corresponds to accessing of Li ions located in the two known crystallographic sites within the LiVPO4F structure, which is in good agreement with the first charge-discharge results.
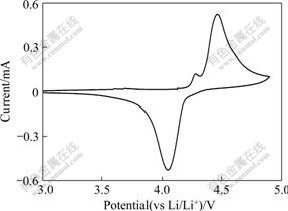
Fig.7 CV profile of LiVPO4F electrode in first cycle
LiVPO4F powder has been prepared by a two-step reaction method based on a carbon-thermal reduction(CTR) process under Ar atmosphere. X-ray diffraction (XRD) patterns show that LiVPO4F has triclinic structure. Scanning electron microscopic (SEM) images show that the particle size is about 2 ?m. A initial charge and discharge capacity is about 140 mA·h/g and 119 mA·h/g, respectively. In summary, the demonstrated performance of the synthesized LiVPO4F material offers some properties favorable for commercial application.
References
[1] CHEN Z H, DAHN J R. Reducing carbon in LiFePO4/C composite electrodes to maximize specific energy, volumetric energy, and tap density[J]. J Electrochem Soc, 2002, 149(9): A1184-A1189.
[2] TAKAHASHI K, SAITOH M, ASAKURA N, et al. Electrochemical properties of lithium manganese oxides with different surface areas for lithium ion batteries[J]. J Power Sources, 2004, 136: 115-121.
[3] ARACHI Y, KOBAYASHI H, EMURA S, et al. Li de-intercalation mechanism in LiNi0.5Mn0.5O2 cathode material for Li-ion batteries[J]. Solid State Ionics, 2005, 176(9/10): 895-903.
[4] LIU Q Y, LIU H W, ZHOU X W, et al. A soft chemistry synthesis and electrochemical properties of LiV3O8 as cathode material for lithium secondary batteries[J]. Solid State Ionics, 2005, 176(17/18): 1549-1554.
[5] PARK S H, SUN Y K. Synthesis and electrochemical properties of 5 V spinel LiNi0.5Mn0.5O4 cathode material prepared by ultrasonic spray pyrolysis method[J]. Electrochemica Acta, 2004, 50(2/3): 427-430.
[6] PARK S H, OH S W, MYUNG S T, et al. Effects of synthesis condition on LiNi1/2Mn3/2O4 cathode material prepared by ultrasonic spray pyrolysis method[J]. Solid State Ionics, 2005, 176(5/6): 481-486.
[7] DENG Ling-feng, LI Xin-hai, XIAO Li-xin, et al. Synthesis and electrochemical properties of polyradical cathode material for lithium second batteries[J]. J Cent South Univ Technol, 2003, 10(3): 190-193.
[8] PANHI A K, NANJUNDASWAMY K S, GOODENPUGH J B. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries[J]. J Electrochem Soc, 1997, 144(4): 1188-1194.
[9] HU Y Q, DOEFF M M. KOSTECKI R, et al. Electrochemical performance of sol-gel synthesized LiFePO4 in lithium batteries[J]. J Electrochem Soc, 2004, 151(8): A1279- A1285.
[10] PATOUX S, WURM C, MORCRETTE M, et al. A comparative structural and electrochemical study of monoclinic Li3Fe2(PO4)3 and Li3V2(PO4)3[J]. J Power Sources, 2003, 119-121: 278-284.
[11] RICHARDSON T J. Phosphate-stabilized lithium intercalation compounds[J]. J Power Sources, 2003, 119-121: 262-265.
[12] ZHOU F, KANG K, MAXISCH T, et al. The electronic structure and band gap of LiFePO4 and LiMnPO4[J]. Solid State Communications, 2004, 132(3/4):181-186.
[13] ZANE D, CAREWSKA M, SCACIA S, et al. Factor affecting rate performance of undoped LiFePO4[J]. Electrochimica Acta, 2004, 49(25): 4259-4271.
[14] BARKER J, SAIDI M Y, SWOYER J.Lithium Metal Fluorophosphates Materials and Preparation Thereof[P]. US 6387568, 2002.
[15] BARKER J, SAIDI M Y, SWOYER J, et al. Electrochemical insertion properties of the novel lithium vanadium fluorophosphates, LiVPO4F[J]. J Electrochem Soc, 2003,150(10): A1394-A1398.
[16] BARAN E J. Materials belonging to the CrVO4 type: preparation, crystal chemistry and physicochemical properties[J]. J Mater Sci, 1998, 33: 2479-2497.
[17] BARKER J, GOVER R K B, BURNS P, et al. Structural and electrochemical properties of lithium vanadium fluorophosphates, LiVPO4F [J]. J Electrochem Soc, 2005, 152(9): A1776-A1779.
(Edited by YANG Bing)
Foundation item: Project(50302016) supported by the National Natural Science Foundation of China
Received date: 2006-07-24; Accepted date: 2006-09-27
Corresponding author: ZHONG Sheng-kui, PhD; Tel: +86-731-8836633; E-mail: zskui74@163.com
- Synthesis and characterization of triclinic structural LiVPO4F as possible 4.2 V cathode materials for lithium ion batteries


