DOI: 10.11817/j.ysxb.1004.0609.2020-35732
熔体结构变化对Al85Ni5Y8Co2非晶合金晶化行为的影响
王知鸷1, 2, 章星宇1,王 东1,刘 麟1,江 鹏1,吴王平1
(1. 常州大学 机械工程学院,常州 213164;
2. 常州大学 石油化工学院测试中心,常州 213164)
摘 要:
采用四电极电阻法研究Al85Ni5Y8Co2合金熔体电阻率,并结合X射线衍射仪(XRD)和差示扫描量热仪(DSC)探索熔体结构变化对Al85Ni5Y8Co2非晶晶化行为的影响。结果表明:Al85Ni5Y8Co2合金在1135~1357 K温度区间内发生熔体结构变化,而熔体结构变化导致Al85Ni5Y8Co2非晶晶化行为存在差异;在熔体温度为1673 K下制备的非晶薄带第一晶化较1323 K和1523 K的明显提前,对激活能的计算证实其初晶化更容易析出FCC-Al,且更难析出Al2Y3、Al13Co4、Al9Co2和AlNi等脆性金属间化合物;通过XRD的测试发现高的熔体温度可能会导致熔体中原子团簇的破坏,从而引起非晶晶化行为的改变。
关键词:
文章编号:1004-0609(2020)-04-0775-07 中图分类号:TG139.8 文献标志码:A
铝基非晶自1988年首次发现以来,已引起研大量究人员的注意[1-5]。它有着诸多优异的性能,如密度低[6]、成本低[7-8]、优异的耐腐蚀性、耐磨性和具有比传统高强度铝合金更高的抗拉强度等[9]。此外,通过部分晶化Al基非晶合金[2, 10-13],使其在非晶基体中析出纳米尺寸的FCC-Al,还可进一步增强其力学性能。但是,Al基非晶合金较低的非晶形成能力(Glass forming ability,GFA)和热稳定性限制了它们在工程领域中的应用。因此,提高铝基非晶合金的GFA和热稳定性一直备受关注。
以往研究者一般会通过添加稀土元素或改变熔体冷却速度等手段改善非晶的GFA和热稳定性,但是近年来,许多学者发现合适的熔体过热处理可以显著地改善合金的结构和性能。众多研究数据已经证明熔体温度会影响非晶合金的GFA和热稳定性[14]。边秀房 等[15]采用X射线衍射的方法研究了不同温度的Al85Ni10Ce5熔体,发现当熔体温度的从1173 K上升到1473 K时,原子配位数由原来的12变化到9,显然其熔体结构发生了变化。POPEL等[16]的研究发现Fe80B20合金的熔体密度随着熔体温度从1870 K升到1970 K时发生了显著变化,也证实了熔体温度对其结构的影响性。MANOV等[17]通过熔体热处理促进了非晶合金的形成并提高了非晶合金的GFA和热稳定性。MU等[18]也发现对熔体的热处理有利于消除Al85.5Ni9.5La5非晶合金的高熔点相并提高熔体稳定性。邓延波等[19]发现Al84Mg10Ce6熔体经过不同过热温度处理对其GFA有很大的影响,其中850 ℃时的GFA最强。这些研究表明,一些液态金属和合金在高于液相线温度时会出现液液结构转变[20],从而导致非晶熔体结构的变化[21-23],而熔体结构变化势必会影响非晶合金的晶化行为,进而影响合金的性能。因此,研究非晶熔体结构变化有望成为一种改善非晶性能的新手段。
本文选取非晶形成能力较好的Al85Ni5Y8Co2为研究对象,由于电阻率对结构变化较为敏感,采用恒流四电极法测试其熔体结构变化,通过DSC法探索熔体结构变化对Al85Ni5Y8Co2非晶合金晶化行为的影响。
1 实验
Al85Ni5Y8Co2母合金由高纯金属Al(99.999%,质量分数)、Ni(99.99%)、Y(99.95%)和Co(99.99%)按摩尔比配比。原料在配比前先打磨其表面,再精确称量所需的合金成分,然后放入真空熔炼炉中进行熔炼。每个合金锭需熔炼5~6次,以保证合金成分均匀。Al85Ni5Y8Co2合金的熔体电阻率采用四电极电阻法测试。由于测试的试样为Al85Ni5Y8Co2合金,为防止在高温条件下熔体和钨发生反应,将四根钨丝电极套上石墨棒。实验时,将制备好的Al85Ni5Y8Co2合金块状试样去除氧化层,然后敲碎并放入瓷舟中。管式炉的真空度为1×10-2 Pa,抽真空后通入高纯氩气,尽可能地排除氧气。
本文熔体电阻率测试和退火实验采用GSL- 1500X-50型真空管式炉,测试电阻率时由PF66M型数字多用表提供恒流源,纳伏表Keithley 2182测量待测熔体两电压极之间电压以及温度。待实验确定熔体结构转变区间之后,将母合金放入石英管内,并利用红外测温仪检测石英管中熔体温度,测温精度为±5 K。
测试Al85Ni5Y8Co2非晶薄带的热分析采用PerkinElmer-8000差示扫描量热仪,升温速率分别为20、40、80 K/min。非晶薄带以及退火后的相组成均用D/MAX2500V型X射线衍射仪分析。
2 结果与讨论
图1所示为Al85Ni5Y8Co2合金熔体的约化电阻率与温度的关系曲线,升温速率为10 K/min(由于管式炉连续升温最高温度为1673 K,故本文只加热到1673 K)。从图1中可以看出,约化电阻率随着温度的变化曲线在891 K之前近似线性上升。当Al85Ni5Y8Co2 合金达到熔点(Tm=891 K)时,Al85Ni5Y8Co2合金的约化电阻率曲线开始陡升,合金开始熔化,当温度升到1135 K时合金完全熔化。从合金完全熔化到1357 K的温度范围内,约化电阻率变化趋势平缓。在1135~1357 K温度后合金的约化电阻率开始发生突变,电阻率参数对于熔体结构的变化较为敏感,由此可以推测在该温度区间内Al85Ni5Y8Co2合金熔体结构发生了变化。大量研究[20-23]表明,合金在特定的温度范围内会发生熔体结构变化,而这一变化与合金熔体中的原子团簇有着密切的联系。
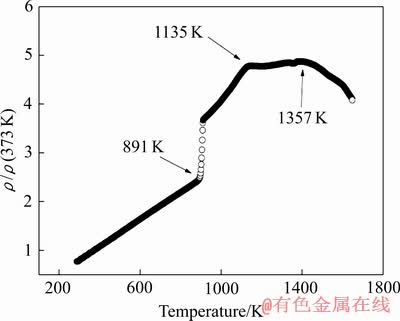
图1 Al85Ni5Y8Co2熔体约化电阻率-温度曲线
Fig. 1 Temperature dependence of reduced electrical resistivity for Al85Ni5Y8Co2 melt
根据熔体结转变化的温度区间,选择3种熔体温度制备Al85Ni5Y8Co2非晶薄带,分别为1323、1523和1673 K。图2所示为熔体温度为1323、1523和1673 K制备的Al85Ni5Y8Co2非晶薄带的XRD谱,由图2可见,每条谱线都在38°附近出现典型非晶馒头状的漫散射峰,可以判定所有试样都是非晶结构。
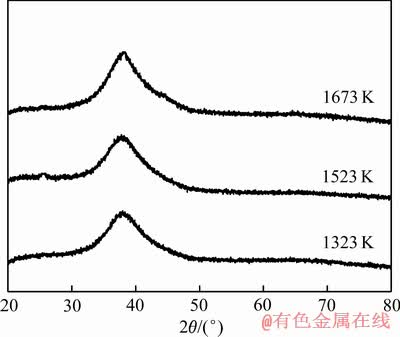
图2 不同熔体温度制备的非晶薄带XRD谱
Fig. 2 XRD patterns of amorphous ribbons prepared at different temperatures
图3所示为熔体温度Al85Ni5Y8Co2非晶薄带在20 K/min升温速率下的DSC曲线。 比较3种熔体温度制备的非晶薄带DSC曲线可知,熔体温度为1673 K的非晶薄带的第一晶化峰较其他两者明显提前,这表明它的第一次晶化更早地进行了,热稳定性有所下降。此外,熔体温度为1673 K制备的Al85Ni5Y8Co2非晶薄带的第二晶化峰Tp2和第一晶化峰Tp1峰值差△Tp更大,这便意味着Al85Ni5Y8Co2非晶薄带的晶化行为的改变。
为了进一步探索熔体温度对Al85Ni5Y8Co2非晶薄带晶化行为的影响,对不同熔体温度制备的非晶薄带进行了不同速率的DSC试验。图4所示为不同熔体温度制备的Al85Ni5Y8Co2非晶薄带在20、40和80 K/min的升温速率下的DSC曲线。通过比较图4的DSC曲线,可以方便地找出Al85Ni5Y8Co2非晶的晶化特征温度值。
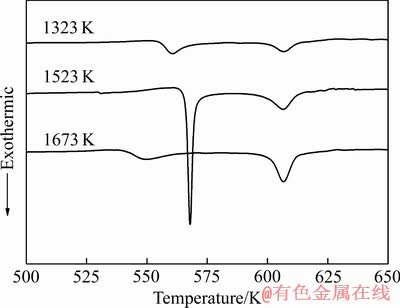
图3 不同熔体温度制备的非晶薄带在20 K/min升温速率下的DSC曲线
Fig. 3 DSC curves of amorphous ribbons prepared at different temperatures with heating rate of 20 K/min
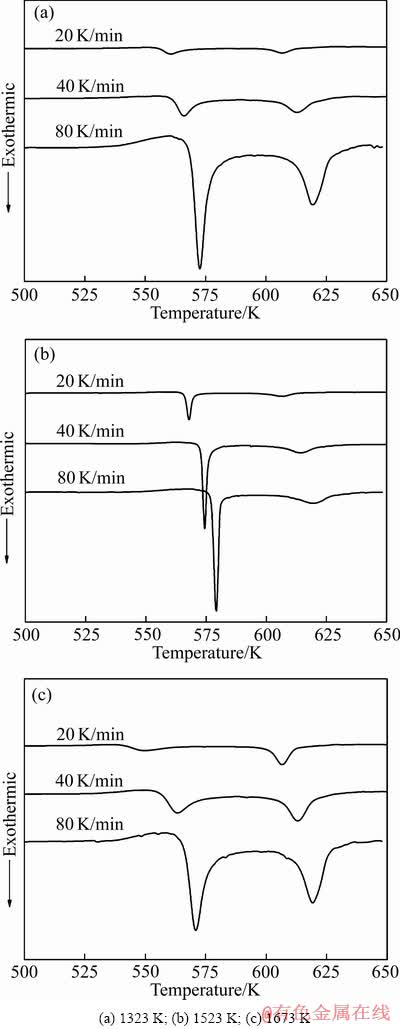
图4 Al85Ni5Y8Co2非晶薄带DSC曲线
Fig. 4 DSC curves of Al85Ni5Y8Co2 amorphous ribbons
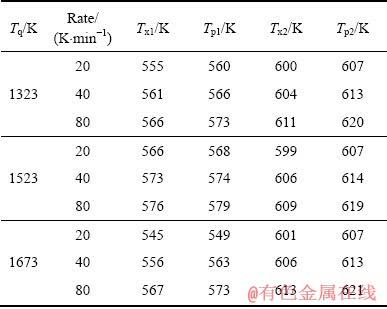
为了更直观地观察特征温度值的变化,表1列出了3种熔体温度制备的Al85Ni5Y8Co2非晶薄带的第一晶化初始温度Tx1、第一晶化峰值Tp1、第二晶化初始温度Tx2和第二晶化峰值Tp2。
表1 Al85Ni5Y8Co2非晶薄带不同升温速率下的特征温度
Table 1 Characteristic temperatures of Al85Ni5Y8Co2 amorphous ribbons with different heating rates
根据不同的特征温度值可以用Kissinger方法计算出非晶在不同晶化阶段晶化所需的激活能。Kissinger法[24-25]的数学表达式表示为:
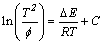 (1)
(1)
式中: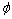 为升温速率,K/min;T为不同升温速率下的特征温度,K;△E为晶化激活能,kJ/mol;R为摩尔气体常数;C为常数。利用线性拟合得到的曲线斜率就可以算出不同熔体温度Al85Ni5Y8Co2非晶薄带相应的晶化激活能Ex1、Ep1、Ex2和Ep2。将3种熔体温度制备的Al85Ni5Y8Co2非晶薄带的激活能列于表2中。
为升温速率,K/min;T为不同升温速率下的特征温度,K;△E为晶化激活能,kJ/mol;R为摩尔气体常数;C为常数。利用线性拟合得到的曲线斜率就可以算出不同熔体温度Al85Ni5Y8Co2非晶薄带相应的晶化激活能Ex1、Ep1、Ex2和Ep2。将3种熔体温度制备的Al85Ni5Y8Co2非晶薄带的激活能列于表2中。
表2 不同熔体温度Al85Ni5Y8Co2非晶薄带的激活能
Table 2 Activation energies of Al85Ni5Y8Co2 amorphous ribbons prepared at different temperatures
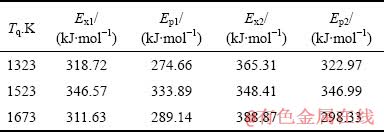
经过比较,铝基非晶薄带的激活能Ex1>Ep1,Ex2>Ep2。这表明3种非晶薄带第一阶段和第二阶段形核激活能大于长大激活能,形核较长大困难。同时,Ex2>Ex1,说明3种熔体温度制备的非晶薄带第二阶段形核激活能大于第一阶段形核激活能,第一阶段晶化较第二阶段晶化容易发生。
此外,随着熔体温度Tq的升高,第一阶段的形核激活能Ex1先从318.72 kJ/mol升高到346.57 kJ/mol,然后再下降到311.63 kJ/mol。这表明熔体温度为1673 K制备的非晶薄带的第一阶段晶化形核所需要克服的能量势垒最小,最容易发生晶化,也说明其更容易析出第一晶化相FCC-Al,与图3中的DSC曲线相吻合;而1673 K制备的非晶薄带第二阶段形核激活能Ex2相比于其他两者要高,这意味着其第二阶段晶化形核所需克服的能量势垒最大,更不容易形成脆性金属间化合物。
根据以往的研究发现,晶化行为的变化往往是由熔体中的氧含量或原子团簇的破坏引起的,但本文是在预抽真空并通以高纯氩气的条件下制备非晶薄带,并且氧化铝的生成会防止合金的进一步氧化,所以可以排除熔体中氧含量的影响。
一般来说,很难直接确定多组分合金中纳米级成分团簇的存在,然而,这些团簇可以从退火过程中非晶带样品的微观结构演变中推断出来。因此,可以通过对非晶在不同的温度和时间退火,以此确认是否是熔体温度导致了原子团簇的破坏。
根据DSC曲线选择退火温度590 K(位于第一晶化峰值和第二晶化峰值之间),将3种熔体温度制备的铝基非晶薄带以10 K/min的升温速率分别加热到590 K,然后分别保温50、60和75 min,其结果如图5所示。由图5(a)可见,在保温50 min时,3种熔体温度制备的铝基非晶薄带均只有FCC-Al析出并且明显可以看出1673 K制备的非晶晶化程度更高,这与激活能计算结果相符合。由图5(b)可见,当退火至60 min时,在1323 K和1523 K制备的非晶薄带开始析出了Al2Y3、Al13Co4、Al9Co2和AlNi等脆性金属间化合物,而在1673 K制备的非晶薄带仍然只有FCC-Al析出。由图5(c)可见,退火至75 min时,3种熔体温度制备的非晶薄带均析出了金属间化合物,产物并无差别。这也表明熔体温度为1673 K制备的非晶薄带更难析出金属间化合物,很好地验证了激活能的计算结果。
尽管最后析出的产物没有区别,但是可以看出熔体温度却对非晶的晶化过程产生了一定影响。铝基非晶的结构特征是以溶质原子为中心的中心团簇,而 Al85Ni5Y8Co2非晶合金的晶化类型为初晶型晶化,其主要的原子团簇为Al-Ni、Al-Y和Al-Co[26]。这些原子团簇在制备非晶时的急速冷却中会被保留下来,熔体电阻率证明了高的熔体温度使非晶发生了熔体结构变化。这意味着高的熔体温度会造成Al-Ni、Al-Y等原子团簇的破坏,并且Al-Ni、Al-Y等金属间化合物的熔点与熔体结构转变区间相近也佐证了这一点。由于熔体内自由的Al原子增多,扩散能力增大,所以在初晶化过程中自由的Al原子更容易形成FCC-Al。与此同时,金属间化合物也有形核及长大的过程,当熔体中对应于这些金属间化合物的Al-Y、Al-Co等原子团簇在高温的条件下被打破时,它们晶化时的形核析出便会变得更困难。这也是1673 K制备的非晶相比于1323 K和1523 K制备的非晶更不易析出金属间化合物的原因。

图5 Al85Ni5Y8Co2非晶薄带在590 K退火不同时间的XRD谱
Fig. 5 XRD patterns of Al85Ni5Y8Co2 amorphous ribbons annealed at 590 K for different time
3 结论
1) Al85Ni5Y8Co2合金熔体约化电阻率与温度的关系曲线表明,在1135~1357 K温度区间内发生了熔体结构变化。
2) 熔体温度为1673 K制备的非晶薄带第一晶化相比于其他两者明显提前,热稳定性有所下降,但是Al2Y3、Al13Co4、Al9Co2和AlNi四种金属间化合物的析出更加困难,在这一熔体温度制备的非晶更适合制备非晶/纳米晶复合材料。
3) XRD结果证明,高的熔体温度破坏了非晶中的原子团簇,导致金属间化合物的形核受到阻碍,熔体中自由Al原子增多,为FCC-Al的析出提供了便利。
REFERENCES
[1] SHEN Y, PEREPEZKO J H. Al-based amorphous alloys: Glass-forming ability, crystallization behavior and effects of minor alloying additions[J]. Journal of Alloys and Compounds, 2017, 707: 3-11.
[2] 梁叔全, 李少强, 谭小平, 唐 艳, 张 勇. Al1.49Si1.45Zr0.26(TiCaMg)0.37O6.43非晶的非等温晶化动力 学[J]. 中国有色金属学报, 2005, 15(9): 1358-1362.
LIANG Shu-quan, LI Shao-qiang, TAN Xiao-ping, TANG Yan, ZHANG Yong. Non-isothermal crystallization kinetics on Al1.49Si1.45Zr0.26(TiCaMg)0.37O6.43amorphous bulk[J]. The Chinese Journal of Nonferrous Metals, 2005, 15(9): 1358-1362.
[3] 郭 晟, 黄伯云, 刘 咏, 刘祖铭, 刘峰晓, 黄劲松. 含非晶和纳米晶Al-Ni-Y块体合金的制备[J]. 中南大学学报(自然科学版), 2004, 35(5): 731-735.
GUO Sheng, HUANG Bai-yun, LIU Yong, LIU Zu-ming, LIU Feng-xiao,HUANG Jin-song. Fabrication of bulk Al-Ni-Y Alloy with amorphous and nanocrystalline structure[J]. Journal of Central South University(Science and Technology), 2004, 35(5): 731-735.
[4] SWEITZER J E, SHIFLET G J, SCULLY J R. Localized corrosion of Al90Fe5Gd5 and Al87Ni8.7Y4.3 alloys in the amorphous, nanocrystalline and crystalline states: Resistance to micrometer-scale pit formation[J]. Electrochimica Acta, 2003, 48(9): 1223-1234.
[5] JAKAB M A, SCULLY J R. On-demand release of corrosion-inhibiting ions from amorphous Al-Co-Ce alloys[J]. Nature materials, 2005, 4(9): 667-670.
[6] KUBALL A, STOLPE M, BUSCH R. Crystallization behavior of the Al86Ni8Y6 metallic glass forming alloy upon rapid cooling[J]. Journal of Alloys and Compounds, 2018, 737: 398-404.
[7] 徐 燕, 张玉凤, 高 湉, 张 研, 张惠然, 刘永生. Al基非晶合金表征参数的支持向量回归分析[J]. 中国有色金属学报, 2016, 26(4): 836-843.
XU Yan, ZHANG Yu-feng, GAO Tian, ZHANG Yan, ZHANG Hui-ran, LIU Yong-sheng. Parameters analysis of Al-based amorphous alloys using support vector regression[J]. The Chinese Journal of Nonferrous Metals, 2016, 26(4): 836-843.
[8] ZHANG S D, WANG Z M, CHANG X C, HOU W L, WANG J Q. Identifying the role of nanoscale heterogeneities in pitting behavior of Al-based metallic glass[J]. Corrosion Science, 2011, 53(9): 3007-3015.
[9] 王 玉, 张文礼, 孙冬柏, 李辉勤. 非晶纳米晶复合材料的性能与制备[J]. 材料科学与工程学报, 2006, 24(2): 292-296.
WANG Yu, ZHANG Wen-li, SUN Dong-bai, LI Hui-qin. Properties and fabrication of amorphous-nanocrystalline composites[J]. Journal of Materials Science and Engineering, 2006, 24(2): 292-296.
[10] TAN Zhen, XUE Yun-fei, CHENG Xing-wang, ZHANG Long, CHEN Wei-wei, WANG Lu, ZHANG Hai-feng, FU Hua-meng. Effect of element fitting on composition optimization of Al-Cu-Ti amorphous alloy by mechanical alloying[J]. Transactions of Nonferrous Metals Society of China, 2015, 25(10): 3348-3353.
[11] JIANG JIAN-ZHONG, HOFMANN D, JARVIS D J, FECHT H J. Low-density high-strength bulk metallic glasses and their composites: A review[J]. Advanced Engineering Materials, 2015, 17(6): 761-780.
[12] PRASHANTH K G, SHAHABI H S, ATTAR H, SRIVASTAVA V C, ELLENDT N, UHLENWINKEL V, ECKERT J, SCUDINO S. Production of high strength Al85Nd8Ni5Co2 alloy by selective laser melting[J]. Additive Manufacturing, 2015, 6: 1-5.
[13] CUEVAS F G, LOZANO-PEREZ S, ARANDA R M, TERNERO F. Crystallisation of amorphous Al-Y-Ni-(Cu) alloys[J]. Journal of Non-Crystalline Solids, 2019, 512: 15-24.
[14] JIA P, ZHANG J, GENG H, YANG Z, TENG X, ZHAO D, WANG Y, ZUO M, SUN N. Effect of melt superheating treatment on solidification structures of Al75Bi9Sn16 immiscible alloy[J]. Journal of Molecular Liquids, 2017, 232: 457-461.
[15] BIAN X, SUN M. Thermal-rate treatment and critical thickness for formation of amorphous Al85Ni10Ce5 alloy[J]. Materials Letters, 2003, 57(16/17): 2460-2465.
[16] POPEL P S, CALVO-DAHLBORG M, DAHLBORG U. Metastable microheterogeneity of melts in eutectic and monotectic systems and its influence on the properties of the solidified alloy[J]. Journal of Non-Crystalline Solids, 2007, 353(32/40): 3243-3253.
[17] MANOV V, POPEL P, BROOK-LEVINSON E, MOLOKANOV V, CALVO-DAHLBORG, DAHLBORG U, SIDOROV V, SON L, TARAKANOV Y U. Influence of the treatment of melt on the properties of amorphous materials: ribbons, bulks and glass coated microwires[J]. Materials Science and Engineering A, 2001, 304/306: 54-60.
[18] MU J, FU H, ZHU Z, WANG A, LI H, HU Z, ZHANG, H. The effect of melt treatment on glass forming ability and thermal stability of Al-Based amorphous alloy[J]. Advanced Engineering Materials, 2010, 12(11): 1127-1130.
[19] 邓延波, 王学亮, 耿浩然, 王致明, 张 蕾. 熔体过热处理对铝基合金非晶形成能力的影响[J]. 特种铸造及有色合金, 2009, 29(8): 756-759.
DENG Yan-bo,WANG Xue-liang,GENG Hao-ran,WANG Zhi-ming,ZHANG Lei. Effects of melt over-heating treatment on glass forming ability of Al-based alloys[J]. Special Casting & Nonferrous Alloys, 2009, 29(8): 756-759.
[20] ZU F Q, ZHU Z G, GUO L J, QIN X B, YANG H, SHAN W J. Observation of an anomalous discontinuous liquid- structure change with temperature[J]. Physical Review Letters, 2002, 89(12): 125505.
[21] MUDRY S, SHTABLAVYI I. The influence of doping with tin on the structure of Cu0.70Si0.30 eutectic melt[J]. Journal of Non-Crystalline Solids, 2006, 352(40/41): 4287-4291.
[22] DUTKIEWICZ M, DUTKIEWICZ E. Study of microheterogeneous structure of non-aqueous mixed solvents by non-linear dielectric and viscometric methods[J]. Electrochimica acta, 2006, 51(11): 2346-2350.
[23] JIA E G, WU A Q, GUO L J, LIU C S, SHAN W J, ZHU Z G. Experimental evidence of the transformation from microheterogeneous to microhomogeneous states in Ga-Sn melts[J]. Physics Letters A, 2007, 364(6): 505-509.
[24] 邵 阳, 陈 刚, 赵玉涛. CuYSi-Mg60Cu30Y10块体非晶合金复合材料的晶化行为[J]. 中国有色金属学报, 2013, 23(1): 212-218.
SHAO Yang, CHEN Gang, ZHAO Yu-tao. Crystallization behavior of CuYSi-Mg60Cu30Y10 bulk amorphous alloy matrix composite[J]. The Chinese Journal of Nonferrous Metals, 2013, 23(1): 212-218.
[25] MINOUEI H, AKBARI G H, ENAYATI M H, HONG S I. Non-isothermal nano-crystallization kinetics in amorphous Ni55Nb35Si10alloy[J]. Transactions of Nonferrous Metals Society of China, 2019, 29(2): 358-364.
[26] SHENG H W, CHENG Y Q, LEE P L, SHASTRI S D, MA E. Atomic packing in multicomponent aluminum-based metallic glasses[J]. Acta Materialia, 2008, 56(20): 6264-6272.
Effect of melt structure transition on crystallization behavior of Al85Ni5Y8Co2 amorphous alloys
WANG Zhi-zhi1, 2, ZHANG Xing-yu1, WANG Dong1, LIU Lin1, JIANG Peng1, WU Wang-ping1
(1. College of Mechanical Engineering, Changzhou University, Changzhou 213164, China;
2. Testing Center of Petrochemical College, Changzhou University, Changzhou 213164, China)
Abstract: The electrical resistivity of Al85Ni5Y8Co2 melt was studied by four-electrode electrical resistivity method. The effect of melt structure transition on the crystallization behavior of Al85Ni5Y8Co2 amorphous alloys was investigated by both X-ray diffractometer(XRD) and differential scanning calorimetry(DSC) measurements. The result shows that the melt structure of Al85Ni5Y8Co2 alloy changes within temperature range from 1135 K to 1357 K. The crystallization behavior of Al85Ni5Y8Co2 amorphous alloys is different due to the change of melt structure: the first crystallization of amorphous ribbon prepared at 1673 K is obviously earlier than that prepared at 1323 K and 1523 K. The calculation of activation energy confirms that the primary crystallization of Al85Ni5Y8Co2 alloy is easier to precipitate FCC-Al and harder to precipitate brittle intermetallic compounds, such as Al2Y3, Al13Co4 and AlNi. The XRD measurements show that high melt temperature may lead to the destruction of atomic clusters in the melt, which may lead to the change of crystallization behavior.
Key words: melt structure; crystallization behavior; activation energy; atomic cluster
Foundation item: Project(51705038) supported by the National Natural Science Foundation of China; Project (BK20160279) supported by the National Natural Science Foundation of Jiangsu Province, China; Project(CM20173001) supported by the Funding of Changzhou High Technology Research Key Laboratory of Mould Advanced Manufacturing, China
Received date: 2019-04-02; Accepted date: 2019-09-05
Corresponding author: WANG Zhi-zhi; Tel: +86-18796955138; E-mail: zzwang@cczu.edu.cn
(编辑 王 超)
基金项目:国家自然科学基金资助项目(51705038);江苏省国家自然科学基金资助项目(BK20160279);常州模具先进制造高技术研究重点实验室资助项目(CM20173001)
收稿日期:2019-04-02;修订日期:2019-09-05
通信作者:王知鸷,讲师,博士;电话:18796955138;E-mail:zzwang@cczu.edu.cn
摘 要:采用四电极电阻法研究Al85Ni5Y8Co2合金熔体电阻率,并结合X射线衍射仪(XRD)和差示扫描量热仪(DSC)探索熔体结构变化对Al85Ni5Y8Co2非晶晶化行为的影响。结果表明:Al85Ni5Y8Co2合金在1135~1357 K温度区间内发生熔体结构变化,而熔体结构变化导致Al85Ni5Y8Co2非晶晶化行为存在差异;在熔体温度为1673 K下制备的非晶薄带第一晶化较1323 K和1523 K的明显提前,对激活能的计算证实其初晶化更容易析出FCC-Al,且更难析出Al2Y3、Al13Co4、Al9Co2和AlNi等脆性金属间化合物;通过XRD的测试发现高的熔体温度可能会导致熔体中原子团簇的破坏,从而引起非晶晶化行为的改变。
[3] 郭 晟, 黄伯云, 刘 咏, 刘祖铭, 刘峰晓, 黄劲松. 含非晶和纳米晶Al-Ni-Y块体合金的制备[J]. 中南大学学报(自然科学版), 2004, 35(5): 731-735.
[7] 徐 燕, 张玉凤, 高 湉, 张 研, 张惠然, 刘永生. Al基非晶合金表征参数的支持向量回归分析[J]. 中国有色金属学报, 2016, 26(4): 836-843.
[9] 王 玉, 张文礼, 孙冬柏, 李辉勤. 非晶纳米晶复合材料的性能与制备[J]. 材料科学与工程学报, 2006, 24(2): 292-296.
[19] 邓延波, 王学亮, 耿浩然, 王致明, 张 蕾. 熔体过热处理对铝基合金非晶形成能力的影响[J]. 特种铸造及有色合金, 2009, 29(8): 756-759.
[24] 邵 阳, 陈 刚, 赵玉涛. CuYSi-Mg60Cu30Y10块体非晶合金复合材料的晶化行为[J]. 中国有色金属学报, 2013, 23(1): 212-218.


