Article ID: 1003-6326(2005)02-0257-04
Preparation of porous hydroxyapatite ceramics with starch additives
YANG Lei(杨 磊), NING Xiao-shan(宁晓山),
CHEN Ke-xin(陈克新), XIAO Qun-fang(肖群芳), ZHOU He-ping(周和平)
(National Key Laboratory of New Ceramics and Fine Processing,
Department of Materials Science and Engineering, Tsinghua University, Beijing 100084, China)
Abstract:
Porous ceramics prepared from nano-sized hydroxyapatite powders by adding water soluble starch and insoluble starch were investigated. The results show that small pores of several micrometers or less can be produced by adding water soluble starch as a pore former. Two kinds of starch have different pore forming mechanisms. The permeability of the porous ceramics can be greatly improved by adding the insoluble starch to channel the small pores rather than solely using water soluble starch. The control of permeability can be achieved by adjusting the content ratio of water soluble starch to insoluble starch. Strength tests show the ceramics have rather high strength. Therefore a kind of porous filtering material with small pores, controllable permeability and good strength can be prepared by using starch additives.
Key words:
hydroxyapatite; porous ceramics; water soluble starch; insoluble starch CLC number: TQ174;
Document code: A
1 INTRODUCTION
Hydroxyapatite(HA) can adsorb bacteria and virus effectively[1], hence it can be used as a filter to filtrate the bacteria and virus in the air or liquid. Most commonly used techniques to produce porous ceramic filter include using polymeric sponge[2-4], pore-making agent method[5, 6] and foaming processes[7, 8]. Lyckfeldt and Ferreira[9] found that the technique of starch consolidation was one of the most promising methods to produce porous ceramic filters because insoluble starch can be used as both forming consolidator/binder and pore former, and starch is nontoxic and quite cheap. This technique has been proved to be a simple one to produce porous hydroxyapatite with pore size of tens of micrometers[10]. However the pores produced by the techniques above are obviously too large to filtrate bacteria and virus.
In this work, water soluble(WS) starch is applied to produce HA porous ceramics with pore size of several micrometers or less, and this size is more appropriate for filtrating bacteria and virus. Insoluble(IS) starch is then added in order to enhance and control the permeability of the porous ceramics. The structure of the pores and pore forming mechanisms of two kinds of starch are studied. Permeability and fracture strength of ceramics are also investigated to find out the influence of the two kinds of starch on the pore structure and the properties of ceramics respectively. The influences of the combined addition of both two kinds of starch on the pore structure and the properties of the ceramics are also studied.
2 EXPERIMENTAL
HA powders were produced by a sol-gel method. To produce the powders, Ca(NO3)2 and (NH4)2HPO4 were fully reacted to form a gel, and the gel was dried at 80℃ and calcined at 550℃ for 5h. Details can be found elsewhere[11, 12].The calcined product was ground and sieved at last. The powders produced by this method have a mean granule size of about 80 μm and a mean grain size of about 90nm.
A kind of WS starch (Chengdu Hongbo Co, China, water solubility ≥96%) was dissolved in ion-free water completely, and then HA powders were mixed with the WS starch solution in a planetary mill for 4h. The slurry was dried at 60℃ for 12h to produce starch-coated HA powders. The green bodies were prepared by pressing the powders uniaxially at 4MPa for 120s. The volume fractions of starch were 30%, 60% and 80% respectively. The comparative specimens with IS starch (Sichuan LeJun Co, China, with average granule size of 43μm) were prepared in a similar way, but the volume fraction of IS starch varied from 30% to 50%. The specimens with mixed starches(MS) were prepared in the same way as the WS specimens, and the starch contents were 15%WS+15%IS(MS1), 30%WS+15%IS(MS2) and 30%WS+30%IS(MS3)(volume fraction), respectively. All green bodies were heated to 550℃ to burn out the starch at a heating rate of 0.5℃/min, and they were finally sintered at 1200℃ with a dwell time of 3h.
The total porosity of specimens was calculated by the density of the porous ceramics and that of HA powder (3.11g/cm3), while the open porosity was measured by Archimedes method. Permeability of the porous ceramics was measured by using nitrogen gas according to the Chinese government standard GB/T 3000—1999[13]. The fracture strength of the ceramics was simply obtained by measuring the critical loads when pressing the specimens uniaxially, which is shown in Fig.1, and the specimens tested are 8.60mm in diameter and 0.7mm in thickness.
Fig.1 Illustration of fracture strength test
HA powders and the sintered specimens were characterized by X-ray diffractometry(XRD) using a RIGAKU diffractometer with CuKα radiation. The morphologies of starch particles were investigated by optical microscope (OLYMPUS PMG3). The microstructures of the ceramics were studied by using scanning electron microscopes (SEM, HITACHI S-450 and JSM-4060LV).
3 RESULTS AND DISCUSSION
XRD patterns of the sintered specimens prepared with different starches and HA powders shown in Fig.2 reveal that they are all composed of HA phase. The starch does not have any reactions with HA during the sintering process, and there is no thermal decomposition of HA at 1200℃[14].
Fig.3(a) shows SEM image of the fracture surfaces of the specimen prepared with 80%WS starch. There are many small pores ranged from less than a micrometre to several micrometers in the specimen prepared with WS starch, and the pores can block bacteria directly and adsorb virus. But most of the small pores are not interconnected, as shown in Fig.3(b). Contrarily, the IS speci mens have much larger and interconnected pores with the sizes from tens of micrometers to even above one hundred micrometers, which can be seen in Fig.4(a). These results reveal that the WS starch and the IS starch can produce quite different pores in the ceramics.
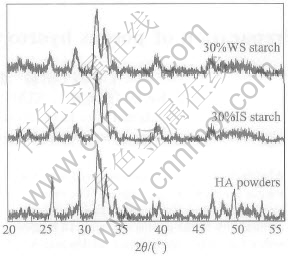
Fig.2 XRD patterns of HA powders and specimens prepared with IS and WS starch

Fig.3 SEM images of fracture surface of specimen prepared with 80%WS starch(a) and
structure of small pores(b)

Fig.4 SEM image of fracture surface of specimen with 30%IS starch(a) and
microstructure of insoluble starch particles linking into chains and agglomerating together at 60℃(b)
These different pores can be attributed to different pore forming mechanisms of two kinds of starch. The WS starch particles can dissolve completely in water when being mixed with HA powders, and then the powders can be covered by solidified starch coatings after drying. During the process of forming green body, the coatings can prevent the powders from compact stacking, which usually helps to close the pores among grains during the sintering process. Thus many pores with rather small size can be created after sintering, and this can explain why most of the small pores shown in Fig.3(b) exist on the granule boundaries and are enclosed by several granules nearby. In contrast to the dissolution of WS starch, linkages into chains and agglomerations in water at the temperature higher than room temperature are observed in the IS starch particles(see Fig.4(b)). The linked chains and agglomerations of the ball milled IS starch particles contribute to the morphology and structure of the pores created by burning out the starch particles (see Fig.4(a)).
Table 1 lists the density and porosity data of ceramics prepared with different starches. The density decreases while both the open porosity and total porosity increase as the content of the WS starch increases. But the ceramics prepared with the WS starch have comparatively low total porosity and open porosity. For example, adding 60% WS starch only obtains a total porosity of 36% and an open porosity of 20%. Permeability of the WS specimens shown in Fig.5 is much lower than that of the IS specimens, even though the fracture strength of the WS specimens is relatively higher than that of the IS specimens with the same content of starch. The low permeability can be correlated to the low open porosity of the ceramics, as the open pores determine whether the gas can penetrate.
Table 1 Density, porosity and fracture strength of ceramics prepared with different starches

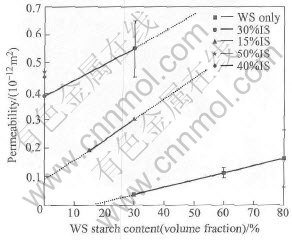
Fig.5 Permeability of specimens with different starch contents
Specimens produced with the IS starch have much higher total porosity, open porosity, and correspondingly higher permeability than the water soluble ones at the same starch content, as shown in Table 1 and Fig.5. The total and open porosity, as well as permeability of the IS specimens increase as the volume fraction of starch increases. In this case, the volume fraction of the pores is even higher than the corresponding starch content, and this may be due to the expansion of the starch particles in water[15].
The permeability of the ceramics prepared with the WS starch can be improved by adding the IS starch. As exhibited by upper lines in Fig.5, the permeability increases largely when the IS starch is added. Taking the sample with 30%WS starch as an example, it increases from 0.04×10-12m2 to 0.30 ×10-12m2 and 0.55×10-12m2 respectively when 15% and 30% IS starch are added. At the same IS content level, permeability of ceramics ascends with the increment of the WS starch content. Comparing the slopes of the curves of 15% and 30% IS starch with that without IS starch, it is obvious that the curves of IS starch have larger slopes than the one with only WS starch. If the IS and WS starch contribute to the total permeability independently, the lines should be all parallel to the one with only WS. These two steeper lines imply that more open pores must exist in the ceramics than the sum of open pores created by adding the IS and WS starch separately, in another word, lots of small closed pores formed by WS starch are interconnected or channeled by the large open pores that are formed by IS starch, as shown in Fig.6. Thus, the permeability of porous ceramics can be improved and controlled by adjusting the content ratio of WS starch to IS starch.
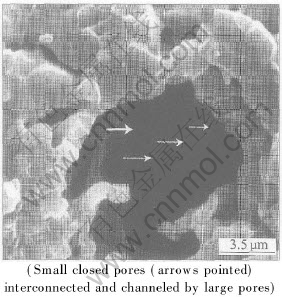
Fig.6 SEM image of pore structure in specimen with 15%WS+15%PS
The WS specimens have much higher fracture strength compared with the IS specimens at the same starch contents (Table 1), as the ceramics with small pores are much denser than the ones with large pores. Similarly the fracture strength of MS specimens is higher than that of the IS starch. The amount of two kinds of starch also has a great influence on the fracture strength of the ceramics.
4 CONCLUSIONS
Water soluble starch can be used as a pore former to produce small pores of several micrometers or less in HA ceramics. These ceramics have high fracture strength but low permeability. While insoluble starch can be used to increase the permeability of the ceramics by creating large pores distributed from tens micrometers to a hundred micrometers. These pores can channel the closed pores created by water soluble starch. The microstructure and the properties of the porous ceramics can be controlled by just changing the ratio and amount of two different starches.
REFERENCES
[1]Tomofumi M. Coating [P]. JP2002-053812A, 2002-02-19.
[2]Tian J T, Tian J M. Preparation of porous hydroxyapatite [J]. J Mater Sci, 2001, 36(10): 3061-3066.
[3]MIAO X, HU Y, LIU J, et al. Porous calcium phosphate ceramics prepared by coating polyurethane foams with calcium phosphate cements [J]. Materials Letters, 2004, 58: 397- 402.
[4]Ramay R H, Zhang M. Preparation of porous hydroxyapatite scaffolds by combination of the gel-casting and polymer sponge methods [J]. Biomaterials, 2003, 24: 3293-3302.
[5]YAO Xiu-min, TAN Shou-hong, JIANG Dong-liang. Preparation and processing of porous hydroxyapatite ceramics with controlled pore sizes [J]. Journal of Functional Materials and Devices, 2001, 7(2): 152-156.
[6]YAO Xiu-min, TAN Shou-hong, JIANG Dong-liang. Preparation of porous hydroxyapatite ceramics [J]. Journal of Inorganic Materials, 2000, 15(3): 467-472.
[7]Sepulveda P, Binner J G. Processing of cellular ceramics by foaming and in situ polymerization of organic monomers [J]. Journal of the European Ceramic Society, 1999, 19: 2059-2066.
[8]Sepulveda P, Ortega F S, Innocentini Murilo D M, et al. Properties of highly porous hydroxyapatite obtained by the gelcasting of foams [J]. J Am Ceram Soc, 2000, 83(12): 3021-3024.
[9]Lyckfeldt O, Ferreira J M F. Processing of porous ceramics by ‘Starch Consolidation’ [J]. Journal of the European Ceramic Society, 1998, 18: 131-140.
[10]Prado da Silva M H, Lemos A F, Gibson I R, et al. Porous glass reinforced hydroxyapatite materials produced with different organic additives [J]. Journal of Non-Crystalline Solids, 2002, 304: 286-292.
[11]Synthesis Processing and Properties of Calcium Phosphate Ceramics with Clinical Interests [D]. University Complutense of Madrid, 1999.
[12]Rodriguez-Lorenzo L M, Vallet-Regmi M. Controlled crystallization of calcium phosphate apatites [J]. Chem Mats, 2000, 12: 2460-2465.
[13]GB/T 3000-1999 eqv ISO 8841∶1991. Dense Shaped Refractory Products- Determination of Permeability to Gases [S]. 1999.
[14]Rodriguez-Lorenzo L M, Vallet-Regmi M, Ferreira J M F. Fabrication of hydroxyapatite bodies by uniaxial pressing from a precipitated powder [J]. Biomaterials, 2001, 22(6): 583-588.
[15]Rutenberg M W. Handbook of Water-Soluble Gums and Resins [M]. New York: McGraw-Hill, 1979. 22: 1-83.
Foundation item: Project(50342002) supported by the National Natural Science Foundation of China
Received date: 2004-12-06; Accepted date: 2005-01-18
Correspondence: YANG Lei; Tel: +86-10-62772548; E-mail: ylei00@mails.tsinghua.edu.cn


