Trans. Nonferrous Met. Soc. China 26(2016) 874-881
Solvent extraction and separation of copper from base metals using bifunctional ionic liquid from sulfate medium
Niharbala Devi
Department of Chemistry, Institute of Technical Education and Research, Siksha ‘O’Anusandhan Deemed University, Khandagiri Square, OR 751030, India
Received 2 April 2015; accepted 17 November 2015
Abstract:
A novel solvent extraction process for extraction and separation of copper from other base metal ions using a bifunctional ionic liquid (IL) (trioctylmethylammonium/2,4,4-trimethylpentyl phosphinate, [A336/Cy272]) in kerosene was reported. This IL was found to extract copper more efficiently than the individual extractants Aliquat 336 or Cyanex 272. Formation of an octahedral copper-IL complex was characterized by UV-Visible spectra and metal ligand interaction was confirmed by FTIR spectra. The loading capacity of 0.1 mol/L [A336/Cy272] was found to be 1.71 g/L. Stripping studies reported that 0.298 g/L copper ions were efficiently stripped using 0.1 mol/L sulfuric acid from 0.05 mol/L loaded IL. The selectivity of copper against nickel, cadmium and iron was investigated from their equimolar binary mixtures using 0.05 mol/L [A336/Cy272] in kerosene. The highest separation factor βCu/Cd=8.41 was obtained at pH 3.56. Copper can be effectively separated from nickel over the pH range studied. The IL extracts preferentially iron over copper and the highest separation factor βFe/Cu was 3246 at pH 2.4. The extraction rate of metal ions from a synthetic solution containing copper with other metal impurities was in the order of Fe>Zn>Cu>Cd>Co>Ni.
Key words:
copper; ionic liquid; extraction; stripping; separation;
1 Introduction
Copper has made vital contribution to sustaining and improving society from the dawn of civilization to till date. It is used in domestic, industrial and also high technology applications [1]. As a result, a lot of waste is also generated which contains copper along with other metals such as zinc, cadmium, nickel, iron, manganese, and chromium. Industrial belt water was polluted due to dissolution of these metal ions. Heavy metals are hazardous and toxic. They should be removed to create safe environment. Several methods are used to remove copper ion from aqueous solutions, but our aim should recover the valuable metal which can be reused. In this aspect, hydrometallurgical route plays a vital role where copper could be recovered using commercial extractants [2-7]. Recently, ionic liquids are used as extractants for many metal ions from aqueous solutions. Ionic liquids (ILs) are liquids at room temperature having low volatility, flammability and greater stability. Mostly, they are used as solvents [8,9] and the recent trend in liquid-liquid extraction is to use the ILs as extractants [10,11] because of their unique property of extracting charged species as they consist entirely of positive and negative ions. REGEL-ROSOCKA et al [10] reported the removal of zinc and iron from chloride media using trihexyl (tetradecyl) phosphonium chloride (Cyphos IL 101) and trihexyl (tetradecyl) phosphonium bis (2,4,4-trimethylpentyl)phosphinate (Cyphos IL 104). RYBKA and REGEL-ROSOCKA [11] also reported the extraction and separation of nickel(II) and cobalt(II) using Cyphos IL 101 and Cyphos IL 104 from chloride solutions. They reported that extraction of Co(II) with Cyphos IL 101 depends on HCl concentration of the aqueous feed whilst Cyphos IL 104 extracts >95% cobalt in the absence of HCl in the aqueous feed. Cobalt can be separated from nickel using Cyphos IL 104. Some ionic liquids like Cyphos IL 104 was used as an ion carrier for Cr(VI) removal impregnated with XAD-resin [12]. Application of bifunctional ionic liquid extractants (Bif-ILEs) for extraction of metal ions was reported by some authors [13-17]. The inner synergistic extraction of [tricaprylmethylammonium][sec-octylphenoxy acetate] ([A336][CA-12]) for cobalt and nickel was studied by SUN et al [13]. They reported that the extraction of [A336][CA-12] for Co2+ or Ni2+ is much better than that of tricaprylmethylammonium sulfate ([A336]2SO4), sec-octylphenoxy acetic acid (CA-12), mixture of [A336]2SO4 and CA-12. The extraction mechanism of [A336][CA-12] for Co2+ is indicated to be an ion association mechanism. The extraction capacities of the widely used extractants can be enhanced by preparing them into Bif-ILEs, which was reported by SUN et al [15]. Using [tricapryl methyl ammonium] [di-2-ethylhexyl-phosphinate] ([A336][P204]) as an extractant for Eu(III), the extractability of [A336][P204] was compared with the mixture of A336 and P204. The distribution coefficient of [A336][P204] is much higher than that of the mixed A336 and P204. Some other Bif-ILEs are also found to have the similar inner synergistic effect. FISCHER et al [16] worked two classes of ILs based on quaternary ammonium or phosphonium cations with functionalized anions for waste water treatment. They recommended thiol- and thioether-functionalized ILs [A336][TS], [A336] [MTBA], [PR4][TS] and [PR4][MTBA] for communal waste water treatment and the use of [A336][SCN] for industrial waste water with high level Zn contamination.
In the present work, the extraction of copper was carried out using an home-made IL synthesized from two commercial extractants, trioctylmethylammonium chloride (Aliquat 336) and bis 2,4,4- trimethylpentyphosphinic acid (Cyanex 272). The effects of various parameters like equilibration time, aqueous phase pH, extractant and metal concentration, sulfate concentration, and organic to aqueous phase ratio were investigated along with separation feasibility of this ion in the presence of some base metals. The extraction mechanism was proposed based on the slope analysis, UV-Visible and FTIR spectra.
2 Experimental
2.1 Reagents
Aqueous phase reagents: copper(II) sulfate pentahydrate, ammonium iron(II) sulfate, nickel sulfate hexahydrate, cadmium sulfate hydrate, cobalt sulfate heptahydrate, zinc sulfate heptahydrate, sodium sulfate anhydrous, sulfuric acid, sodium carbonate, and sodium hydroxide used in the experiment are of analytical reagent grade. The stock solutions of the metals were prepared (0.1 mol/L each of copper, iron, cadmium nickel, cobalt and zinc) and the working metal solutions were prepared by diluting the stock solution with double distilled water.
Organic phase reagents: trioctylmethylammonium chloride (Aliquat 336) was purchased from sigma- Aldrich and bis 2,4,4-trimethylpentylphosphinic acid (Cyanex 272) was supplied by Cytec Inc., and they are used as received without further purification. Commercial grade kerosene and toluene were used as diluent. The ionic liquid [A336/Cy272] was prepared by adopting the published method [18]. This synthesized IL having concentration of 0.5 mol/L was considered as the stock solution and using kerosene as diluent further dilutions was made.
2.2 Liquid–liquid extraction
A glass separating funnel having capacity of 60 mL was used to perform the liquid-liquid extraction tests and the aqueous solution was equilibrated with an equal volume (except for O:A ratio variation) of [A336/Cy272] for 5 min. The pH of the aqueous solution was determined with a pH meter (Systronics) calibrated with buffer solutions of pH 4.0 and 7.0. The copper concentration before and after extraction was measured using an atomic absorption spectrometry (AAS) (Elico make) with proper dilution. The concentration of the metal ion in the organic phase was calculated by the difference of concentrations before and after extraction. The extraction rate (E) was calculated using the following relation:
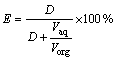 (1)
(1)
where D is the distribution ratio, i.e., the ratio of metal concentration in organic phase to that in aqueous phase after extraction. Similarly, the same procedure is followed when the separation of copper in the presence of nickel, cadmium or iron was carried out. The separation factor (β) was calculated using the following formula:
 (2)
(2)
where D1 and D2 are the distribution ratios of two metals. All the extraction and stripping experiments were carried out at (30±1) °C.
3 Results and discussion
3.1 Extraction of copper with Aliquat 336, Cyanex 272 and [A336/Cy272]
The aqueous solution containing 0.005 mol/L Cu(II) and 0.05 mol/L Na2SO4 was equilibrated with 0.1 mol/L extractant concentration of each Aliquat 336, Cyanex 272 and [A336/Cy272] in kerosene at aqueous phase pH of 5.07. The analysis of the results presented in Table 1 showed that copper was not extracted by 0.1 mol/L Aliquat 336 and Cyanex 272 whilst 100% extraction was achieved with 0.1 mol/L [A336/Cy272]. STASZAK et al [19] reported that extraction of copper was very poor with Cyanex 272 due to poor cation exchange property compared with its sodium salt while investigating the extraction of copper ion with Cyanex 272 and NaCyanex 272 in the initial pH range 2-4. Aliquat 336 can extract copper from the aqueous solution when a complex of copper like copper-EDTA is present [20]. This showed that the ionic liquid(IL) was an effective extractant under the given condition. Hence, a systematic study on the extraction of copper using the synthesized ionic liquid (IL) was investigated.
Table 1 Extraction of 0.005 mol/L Cu(II), 0.05 mol/L Na2SO4 with Aliquat 336, Cyanex 272 and ionic liquid [A336/Cy272] in kerosene

3.1.1 Effect of shaking time
The time required to attain the equilibrium extraction of Cu(II) from aqueous sulfate solution (pH=5.07) containing 0.005 mol/L Cu(II) using 0.02 mol/L [A336/Cy272] in kerosene was studied at a 1:1 phase ratio for various periods of time ranging from 0.5 to 20 min at room temperature. The data plotted in Fig. 1, showed that 5 min of contact time is sufficient to reach extraction equilibrium and extraction rate (E) of Cu(II) is 62.2%. In all other studies, the two phases were contacted for 5 min to ensure complete equilibrium.
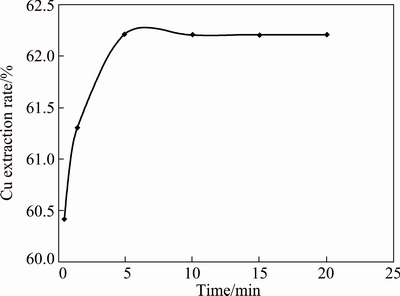
Fig. 1 Effect of contact time on extraction of 0.005 mol/L Cu(II), 0.05 mol/L Na2SO4 using 0.02 mol/L [A336/Cy272] in kerosene
3.1.2 Effect of pH
Extraction of 0.005 mol/L Cu(II), 0.05 mol/L Na2SO4 was studied using 0.02 mol/L [A336/Cy272] in kerosene at equal phase ratio in the pH range of 2.62-5.99. The variation of aqueous pH was made using dilute H2SO4/NaOH. The synthetic solution as such prepared with above concentration has pH 5.07 and the copper extraction rate was 62.2%. When the aqueous phase pH was decreased by adding dilute H2SO4, it was observed that the Cu extraction rate decreased with decrease in aqueous phase pH. For example, when the aqueous phase pH was 4.56, the extraction rate was 58.3% and it decreased to 11.3% when the pH is decreased to 2.62. One of the reasons may be that as pH is decreased, the IL has the preference to extract H+ ion from the aqueous solution instead of copper ion. On the other hand, the extraction rate was increased when the aqueous phase pH was increased by adding dilute NaOH. 81.1% extraction rate of copper was achieved when the pH was increased to 5.34 (Fig. 2) but precipitation occurred in the organic phase after equilibration when pH was increased to 5.9. CASTILLO et al [21] have investigated the extraction of Cu(II) using the same ionic liquid. They found that good extraction of copper made pH greater than 2.0 with moderate sulfate concentration using [A336/Cy272].
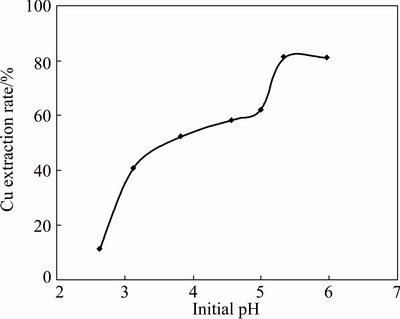
Fig. 2 Effect of aqueous phase pH on extraction of 0.005 mol/L Cu(II), 0.05 mol/L Na2SO4 using 0.02 mol/L [A336/Cy272] in kerosene
3.1.3 Effect of ionic liquid concentration
To study the effect of [A336/Cy272] concentration on the extraction of copper, the IL was varied over the range of 0.01-0.1 mol/L whilst the aqueous phase pH was 5.07 and the copper concentration was 0.005 mol/L. The plot of extraction rate versus IL concentration (Fig. 3) showed that copper extraction increased with increase in IL concentration. When the [A336/Cy272] concentration was 0.01 mol/L, the extraction rate was 35.7% and it reached 100% at 0.1 mol/L [A336/Cy272].
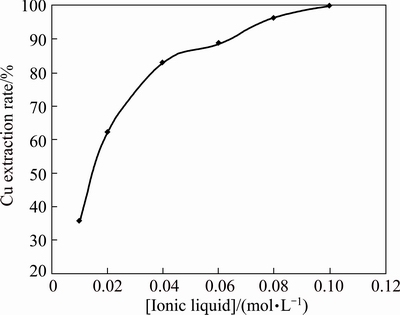
Fig. 3 Effect of ionic liquid concentration on extraction of 0.005 mol/L Cu(II), 0.05 mol/L Na2SO4 at pH 5.07
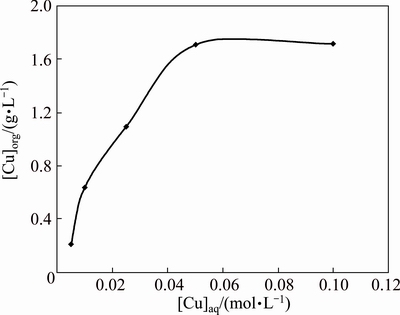
Fig. 4 Plot of [Cu]org versus [Cu]aq using 0.1 mol/L [A336/Cy272] in kerosene
3.1.4 Effect of copper concentration
The effect of copper concentration in the extraction process was studied using 0.1 mol/L [A336/Cy272] in kerosene at 0.05 mol/L Na2SO4 in the aqueous phase in the concentration range from 0.005 to 0.1 mol/L. Figure 4 represents the results obtained for extraction equilibria. The loading capacity of 0.1 mol/L [A336/Cy272] was found to be nearly 1.71 g/L under these conditions.
3.1.5 Effect of sodium sulfate concentration
The effect of sulfate concentration was studied on the extraction of copper as the IL [A336/Cy272] extracted CuSO4 from the aqueous phase. The sulfate concentration was varied from 0.05 to 0.5 mol/L and other conditions of the extractant system remained unchanged. Figure 5 indicated that the distribution coefficient (D) of copper decreased with increase in sulfate concentration. Similar type of behavior of copper with [A336/Cy272] in the presence of sulfate ion was reported by CASTILLO et al [21]. It may be due to salting out effect.
3.1.6 Effect of O:A phase ratio
The extraction rate of copper was 62.2% at 1:1 phase ratio under the experimental conditions of 0.005 mol/L Cu(II), 0.05 mol/L Na2SO4 and aqueous pH of 5.07 with 0.02 mol/L [A336/Cy272] in kerosene. To increase the extraction rate of copper, the O:A phase ratio was varied from 1:1 to 5:1 and the results are given in Fig. 6. The extraction rate was 99% at O:A ratio of 5:1.
3.1.7 Extraction mechanism
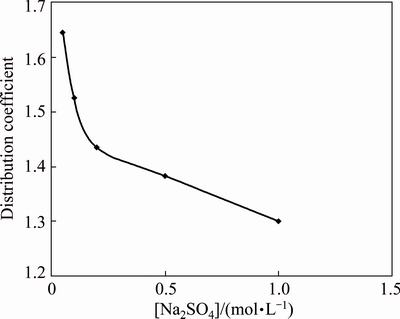
Fig. 5 Effect of sodium sulfate concentration on extraction of 0.005 mol/L Cu(II) at pH 5.07 using 0.02 mol/L [A336/Cy272] in kerosene
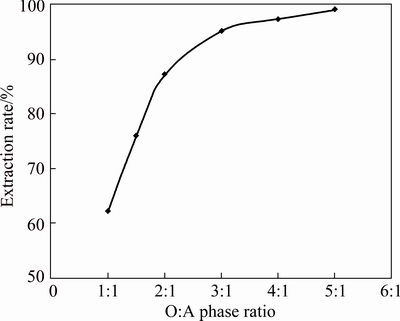
Fig. 6 Effect of organic: aqueous phase ration on extraction of 0.005 mol/L Cu(II) at pH 5.07 using 0.02 mol/L [A336/Cy272] in kerosene
Bifunctional ionic liquids can extract metal ions by ion-association mechanism [13,15] in which both cations and anions of the IL, i.e, [A336]+ and [Cy 272]- complex copper ion. The plot of lg D versus lg[A336/Cy272] gives a slope of 2.0 (Fig. 7). The extraction mechanism may be proposed as
Cu2+(aq)+2[A336/Cy272](org)+SO42-(aq) CuSO4·2[A336/Cy272](org)
CuSO4·2[A336/Cy272](org)
The copper loaded organic phase is bluish in colour. The UV-Visible spectrum of the organic phase was taken and presented in Fig. 8. The λmax value was found to be 775.5 nm. As it is known that [Cu(H2O)6]2+ is blue in colour and its λmax lies at ~800 nm [22], the complex which is formed with the IL [A336/Cy272] is also octahedral and shifting of the wavelength to 775.5 nm may be due to chelation of the IL[A336/Cy272] with copper. The FTIR spectra showed that P=O stretching vibrations observed at 1248 cm-1 [A336/Cy272] was disturbed and two new peaks appeared at 1233 cm-1 and 1217 cm-1 when copper was loaded, which indicates strong interaction of copper with P=O bond (Fig. 9).
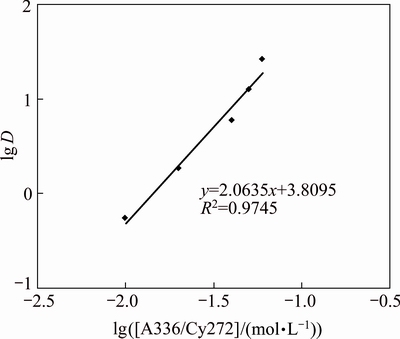
Fig. 7 Plot of lg D versus lg [A336/Cy272]
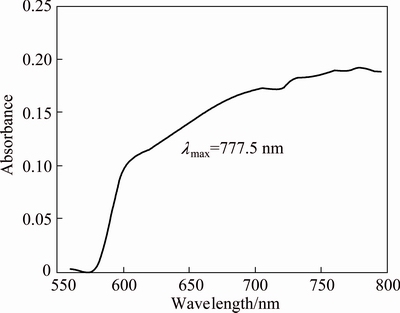
Fig. 8 UV-Visible spectra of copper loaded [A336/Cy272]

Fig. 9 FTIR spectra of [A336/Cy272] and copper loaded [A336/Cy272]
Similar results were also reported by SUN et al [15] while investigating the extraction of Eu(III) with [A336][P204]. They also reported the interaction between Eu(III) and [A336]+ from the XPS study.
Based on the slope analysis value, UV-Visible spectra and FTIR study, the structure of the copper extracted complex is proposed as Fig. 10.
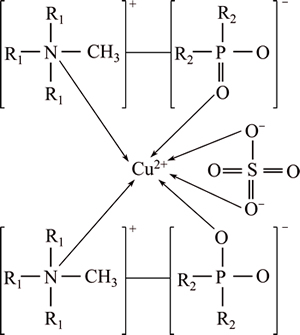
Fig. 10 Proposed structure of copper- extracted complex
3.1.8 Stripping
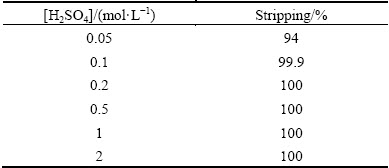
The ionic liquid [A336/Cy 272] has been proven to be a good extractant for copper whilst stripping is also an important characteristic to recover copper from the loaded organic phase. Stripping studies were carried out on the loaded organic phase of 0.05 mol/L [A336/Cy272] in kerosene using different concentrations of sulfuric acid over the range of 0.05-2.0 mol/L. The loaded organic phase was generated by equilibrating equal volume of 0.05 mol/L [A336/Cy272] with 0.005 mol/L Cu, 0.05 mol/L Na2SO4 at aqueous pH of 5.07 which was found to contain 0.298 g/L copper. As shown in Table 2, almost all copper was stripped with 0.1 mol/L H2SO4.
Table 2 Effect of sulfuric acid concentration on stripping of copper from 0.05 mol/L loaded [A336/Cy272] in kerosene ([Cu]Lo=0.298 g/L, copper concentration in loaded organic phase)
3.2 Separation of copper from other metals with [A336/Cy272]
3.2.1 In the presence of nickel
The feasibility of separation of copper in the presence of nickel was studied using the IL as some slags like copper converter slag, anode slag and alloys contain both copper and nickel. Extraction of equimolar concentrations of copper and nickel (0.005 mol/L) from 0.05 mol/L Na2SO4 was carried out using 0.05 mol/L [A336/Cy272] in kerosene at various pH values in the range of 3.01-5.36. The results plotted in Fig. 11 showed that the co-extraction of nickel was almost zero. DEVI and NAYAK [7] reported complete separation of copper from nickel from a sulfate solution containing 0.1 mol/L Cu, 0.01 mol/L Ni and 0.1 mol/L Na2SO4 using 20% LIX 984N. BELKHOUCHE et al [23] reported the extraction of copper and nickel from acetate media using an equimolar mixture of these two metals to determine the optimum conditions for their separation from each other by D2EHPA. They reported that using 4.8 mmol/L D2EHPA in n-heptane selective extraction of copper(II) over nickel(II) takes place in a single stage at pH=5.71. Comparison with the above result reveals that the IL has a greater selectivity for copper over nickel similar to commercial LIX reagents and has a better choice than D2EHPA.
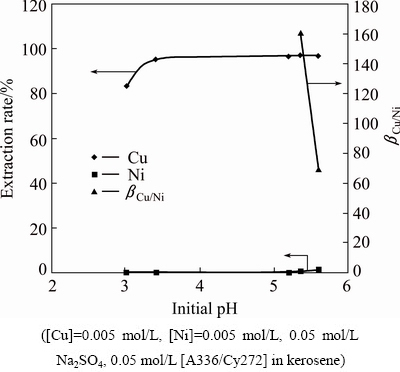
Fig. 11 Plot of extraction rate and separation factor versus initial pH
3.2.2 In the presence of cadmium
Copper and cadmium are toxic in nature and they are present in the by-products such as copper-cadmium slag, cadmium rich dust. To study the selective extraction of copper and cadmium, an aqueous solution containing 0.005 mol/L Cu(II), 0.005 mol/L Cd(II), 0.05 mol/L Na2SO4 was carried out using 0.05 mol/L [A336/Cy272] in kerosene at different pH values in the range of 3.56-5.6. The results plotted in Fig. 12 showed that extraction of both metals increased with increase in pH but copper was the better choice than cadmium for the ionic liquid. The highest separation factor of 8.41 (91.5% Cu, 10.9% Cd) was obtained at pH 3.56 and it decreased with increase in aqueous phase pH to 4.32 at pH of 5.6 (94.1% Cu, 21.8% Cd). Acidic extractants have a preference to extract cadmium over copper. BIDARI et al [24] reported that using D2EHPA, cadmium can be separated from copper from sulfate solutions as their pH0.5 values were found to be 1.6 and 3.0, respectively. They also reported that using an oxime reagent(MEX), copper can be separated over cadmium. In the present investigation, the IL behaves similarly to the oxime (MEX) reagent.
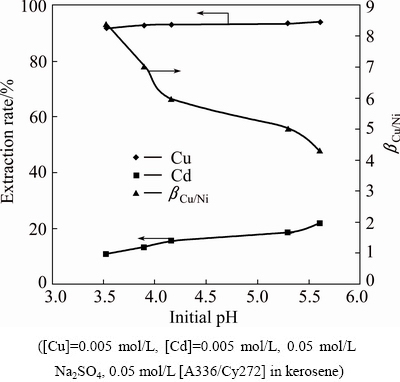
Fig. 12 Plot of extraction rate and separation factor versus initial pH
3.2.3 In the presence of iron
Copper is always associated with iron whether we consider its ore such as chalcopyrite and secondary sources such as sulfide concentrates. LIX extractants and β-diketones have the selectivity of copper over iron. ASGHARI et al [25] studied the effect of impurities on the extraction of copper using LIX 984N from sulfate solution where they found that Fe(II) had no effect on copper extraction. LOS RIOS et al [26] reported the extraction behavior of Cu, Fe, Zn and Cd from hydrochloride aqueous solutions using imidazolium and ammonium-based ionic liquids as extractants. The IL [omim+][PF6-] extracts Fe, Zn and Cd leaving copper in the aqueous phase. The extraction behavior of copper in the presence of iron from an aqueous solution containing 0.005 mol/L Cu(II) and 0.005 mol/L Fe(II), 0.05 mol/L Na2SO4 was studied at different pH values using 0.05 mol/L [A336/Cy272] in kerosene. The results plotted in Fig. 13 show that the extraction rate of Fe(II) is higher compared with Cu(II). At an aqueous phase pH of 2.4, almost all Fe(II) is extracted to the organic phase along with 46.2% co-extraction of Cu(II). The highest separation factor of 3246 was obtained under this condition similarly to the above behavior of IL.
3.2.4 In the presence of nickel, cadmium, iron, cobalt and zinc
Many leach liquors like polymetallic nodules and sulfide concentrates contain copper with other metals such as nickel, iron, cobalt and zinc, [27,28] which were extracted and separated using acidic and chelating extractants but the behavior of such metal ions using bifunctional ionic liquids was not reported. So, the extraction of copper in the presence of other metal impurities was investigated as a function of pH using the ionic liquid [A336/Cy272] diluted in kerosene. Experiments were carried out from a synthetic leach liquor containing 0.005 mol/L each of Cu(II), Fe(II), Cd(II) and 0.001 mol/L each of Co(II) and Zn(II) along with 0.05 mol/L Na2SO4 using 0.1 mol/L [A336/Cy272] in kerosene in the aqueous pH range of 2.3-4.03. The extraction rate of the metal ions versus initial pH was plotted in Fig. 14 which revealed that iron and zinc extractions were 100% and were extracted in preference to copper in the entire pH range studied. Though the extraction of cadmium and cobalt ions were increased with increase in pH from 5.3%-50.4% for cadmium and 9.7%-14.5% for cobalt, respectively, but they were less preferred over copper (70.6%-94.2%) by the ionic liquid. Extraction of nickel was negligible over the pH range and the order of extraction of the metal ions was Fe>Zn>Cu>Cd>Co>Ni.
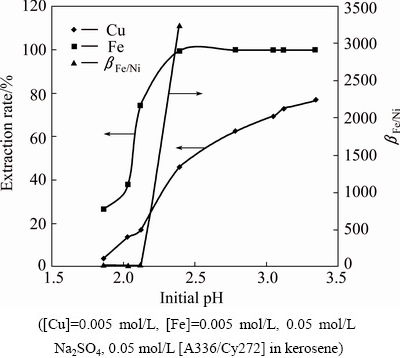
Fig. 13 Plot of extraction rate and separation factor versus initial pH
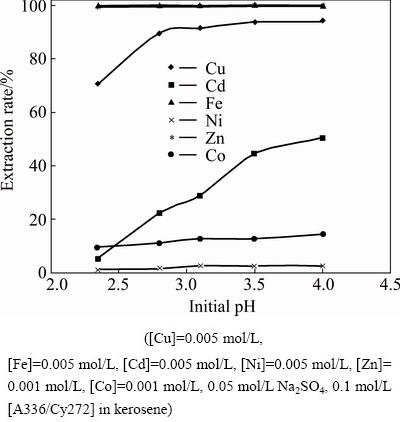
Fig. 14 Plot of extraction versus initial pH
4 Conclusions
It was found that the ionic liquid [A336/Cy272] is able to extract copper from sulfate media quite efficiently than the commercial extractants, Aliquat 336 and Cyanex 272 from which it is synthesized. 100% extraction of copper was achieved with 0.1 mol/L IL and the loading capacity of 0.1 mol/L IL was found to be 1.71 g/L. Increase in sodium sulfate concentration decreased the copper extraction due to salting out effect. The extraction of copper was increased with increase in O:A ratio and 99.9% copper was extracted at O:A ratio of 5:1. The proposed extraction mechanism was found to be ion-associative and the nature of the extracted complex is octahedral. Back extraction of copper could be achieved with and above 0.1 mol/L H2SO4. Copper can be effectively separated from nickel and cadmium from their respective binary mixtures using 0.05 mol/L [A336/Cy272] in kerosene. The highest value 8.41 of βCu/Cd was found at aqueous phase pH of 3.56 corresponding 91.5% Cu and 10.9% Cd extraction. The IL has the reverse choice of extraction when binary mixtures of copper and iron(II) were investigated. It prefers to extract Fe(II) over Cu(II) and the highest separation factor of 3246 was obtained corresponding to pH of 2.4. The order of extraction of metal ions from an aqueous sulfate solution containing copper, cadmium, iron, nickel, cobalt and zinc follows the pattern of Fe>Zn>Cu>Cd>Co>Ni.
The overall conclusion is that the IL [A336/Cy272] is proven to be an efficient extractant for copper from sulfate media and can be used as an extractant for copper/cadmium, copper/nickel copper/cobalt separation.
Acknowledgements
The author is thankful to Cytec, Canada for providing the Cyanex 272. This research work was carried out with the encouragement and support of the authorities of Siksha ‘O’Anusandhan University.
References
[1] DOEBRICH J. Copper—A metal for ages [R]. U.S.: U.S. Geological Survey, 2009.
[2] OKEWOLE A I, WALMSLEY R S, VALTANCOLI B, BIANCHI A, TSHENTU Z R. Separation of copper(II) from base metals in an acidic synthetic sulfate leach solution using a novel 1-octylimidazole-2-aldoxime extractant [J]. Solvent Extraction and Ion Exchange, 2013, 31: 61-78.
[3] REDDY B R, PARK K H, MOHAPATRA D. Process development for the separation and recovery of copper from sulfate leach liquors of synthetic Cu-Ni-Co-Fe matte using LIX 84 and LIX 973N [J]. Hydrometallurgy, 2007, 87: 51-57.
[4] KINOSHITA T, AKITA S, KOBAYASHI N, KAWAIZUMI F, TAKAHASHI K. Metal recovery from non-mounted printed wiring boards via hydrometallurgical processing [J]. Hydrometallurgy, 2003, 69(1-3): 73-79.
[5] LE H L, JEONG J, LEE J C, PANDEY B D, YOO J M, HUYUNH T H. Hydrometallurgical process for recovery from waste printed circuit boards(PCBs) [J]. Mineral Processing ExtractiveMetallurgy Review, 2011, 32: 90-104.
[6] MISHRA S, DEVI N B. Extraction of copper(II) from hydrochloric acid solution by Cyanex 921 [J]. Hydrometallurgy, 2011, 107: 29-33.
[7] DEVI N B, NAYAK B. Liquid-liquid extraction and separation of copper(II) and nickel(II) using LIX 984N [J]. The Journal of South African Institute of Mining and Metallurgy, 2014, 114: 937-943.
984N [J]. The Journal of South African Institute of Mining and Metallurgy, 2014, 114: 937-943.
[8] ROUT A, KARMAKAR K A, VENKATEASAN K A, SRINIVASAN T G, VASUDEV RAO P R. Room temperature ionic liquid diluents for the mutual separation of europium(III) from americium(III) [J]. Separation and Purification Technology, 2011, 81: 109-115.
[9] ZHAO Hua, XIA Shu-qian, MA Pei-sheng. Use of ionic liquids as ‘green’ solvents for extractions [J]. Journal Chemical Technology and Biotechnology, 2005 80: 1089-1096.
[10] REGEL-ROSOCKA M, NOWAK L, WISNIEWSKI M. Removal of zinc(II) and iron ions from chloride solutions with phosphonium ionic liquids [J]. Separation and Purification Technology, 2012, 97: 158-163.
[11] RYBKA P, REGEL-ROSOCKA M. Nickel(II) and cobalt(II) extraction from chloride solutions with quaternary phosphonium salts [J]. Separation Science and Technology, 2012, 47: 1296-1302.
[12] YANG Xiu-yun, ZHANG Jian-ping, GUO Lin, ZHAO He, ZHANG Yang, CHEN Ji. Solvent impregnated resin prepared using ionic liquid Cyphos IL 104 for Cr(VI) removal [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(12): 3126-3130.
[13] SUN Xiao-qi, JI Yang, ZHANG Li-na, CHEN Ji, LI De-qian. Separation of cobalt and nickel using inner synergistic extraction from bifunctional ionic liquid extractant (Bif-ILE) [J]. Journal of Hazardous Materials, 2010, 182: 447-452.
[14] LIU Ying-hul, ZHU Li-li, SUN Xiao-qi, CHEN Ji. Towards greener separations of rare earths: Bifunctional ionic liquid extractants in biodiesel [J]. AIChE Jounal, 2010, 56(9): 2338-2346.
[15] SUN Xiao-qi, JI Yang, HU Feng-chun, HE Bo, CHEN Ji, LI De-qian. The inner synergistic effect of bifunctional ionic liquid extractant for solvent extraction [J]. Talanta, 2010, 81: 1877-1883.
[16] FISCHER L, FALTA T, KOELLENSPERGER G, STOJANOVIC A, KOGELNIG D, GALANSKI M, KRACHLER R, KEPPLER, B K, HANN S. Ionic liquids for extraction of metals and metal containing compounds from communal and industrial waste water [J]. Water Research, 2011, 45: 4601-4614.
[17] YANG Hua-ling, CHEN Ji, ZHANG Dong-li, WANG Wei, CUI Hong-min, LIU Yu. Kinetics of cerium(IV) and fluoride extraction from sulfuric solutions using bifunctional ionic liquid extractant (Bif-ILE) [A336][P204] [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(6): 1937-1945.
[18] FORTUNY A, COLL, M T, SASTRE A M. Use of methyltrioctyl/ decylammonium bis 2,4,4-(trimethylpentyl) phosphinate ionic liquid (ALiCY IL) on the boron extraction in chloride media [J]. Separation and Purification Technology, 2012, 97: 137-141.
[19] STASZAK K, REGEL-ROSOCKA M, WIESZCZYCKA K, BURMISTRZAK P. Copper(II) sulfate solutions treatment by solvent extraction with Na-Cyanex 272 [J]. Separation and Purification Technology, 2012, 85: 183-192.
[20] JUANG R S, CHEN Y J, HUANG I P. Amine-based extraction and recovery of Cu(II) from aqueous solutions in the presence of EDTA, equilibrium studies [J]. Separation Science and Technology, 1999, 34(15): 3099-3112.
[21] CASTILLO J, COLL M T, FORTUNY A, DONOSO P N, SEPULVEDA R, SASTRE A M. Cu(II) extraction using quaternary ammonium and quaternary phosphonium based ionic liquid [J]. Hydrometallurgy, 2014, 141: 89-96.
[22] COTTON F A, WILKINSON G. Advanced inorganic chemistry [M]. 5th ed. New York: John Wiley & Sons, 1988.
[23] BELKHOUCHE N E, DIDI M A, VILLEMIN D. Separation of nickel and copper by solvent extraction using Di-2- ethylhexylphosphoric acid-based synergistic mixture [J]. Solvent Extraction and Ion Exchange, 2005, 23: 677-693.
[24] BIDARI E, IRANNEJAD M, GHARABAGHI M. Solvent extraction recovery and separation of cadmium and copper from sulfate solution [J]. Journal of Environmental Chemical Engineering, 2014, 1: 1269-1274.
[25] ASGHARI H, SAFARZADEH M S, ASGHARI G, MORADKHAM D. The effect of impurities on the extraction of copper from sulfate medium using LIX 984N in kerosene [J]. Russian Journal of Non-Ferrous Metals, 2009, 50(2): 89-96.
984N in kerosene [J]. Russian Journal of Non-Ferrous Metals, 2009, 50(2): 89-96.
[26] de LOS RIOS A P, HERNANDEZ-FERNANDEZ F J, ALGUACIL F J, LOZANO L J, GINESTA A, GARCIA-DIAZ I, SANCHEZ- SEGADO S, LOPEZ F A, GODINEZ C. On the use of imidazolium and ammonium-based ionic liquids as green solvents for the selective recovery of Zn(II), Cd(II), Cu(II) and Fe(III) from hydrochloride aqueous solutions [J]. Separation and Purification Technology, 2012, 97: 150-157.
[27] SHEN Yong-feng, XUE Wen-ying, LI Wei, LI Shan-de, LIU Xiang-hua. Recovery of Mn2+, Co2+ and Ni2+ from manganese nodules by redox leaching and solvent extraction [J]. Transactions of Nonferrous Metals Society of China, 2007, 17(5): 1105-1111.
[28] CONIC V T, RAJCIC VUJASINOVIC M M, TRUJIC V K, CVETKOVSKI V B. Copper, zinc, and iron bioleaching from polymetallic sulphide concentrate [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(11): 3688-3695.
在硫酸盐介质中采用双官能团离子液体从母材中提取和分离铜
Niharbala Devi
Department of Chemistry, Institute of Technical Education and Research, Siksha ‘O’Anusandhan Deemed University, Khandagiri Square, OR 751030, India
摘 要:研究了一种在煤油中采用双官能团[A336/Cy272]离子液体(IL),从其他母材离子溶剂萃取与分离铜的新工艺。采用这种双官能团IL比单独采用Aliquat 336或Cyanex 272萃取剂能更有效地萃取铜。采用紫外-可见光谱表征八面体铜-IL复合物的形成,同时采用FTIR光谱验证金属配体的相互作用。0.1 mol/L [A336/Cy272]的负载容量为1.71 g/L。剥离研究表明,采用0.1 mol/L 硫酸从0.05 mol/L IL中有效剥离0.298 g/L铜离子。在煤油中采用0.05 mol/L [A336/Cy272]等摩尔二元混合物研究铜对镍、镉、铁的选择性。在pH 3.56时得到最高分离因子8.41。在pH研究范围内铜能有效地从镍中分离,铁比铜优先被IL萃取,在pH 2.4时,得到最高分离因子3246。含有其他金属杂质的铜合成溶液中金属离子萃取率顺序为Fe>Zn>Cu>Cd>Co>Ni。
关键词:铜;离子液体;萃取;剥离;分离
(Edited by Xiang-qun LI)
Corresponding author: Niharbala Devi; Tel: +91-674-2351777; E-mail: drnbdevi@gmail.com
DOI: 10.1016/S1003-6326(16)64179-1
Abstract: A novel solvent extraction process for extraction and separation of copper from other base metal ions using a bifunctional ionic liquid (IL) (trioctylmethylammonium/2,4,4-trimethylpentyl phosphinate, [A336/Cy272]) in kerosene was reported. This IL was found to extract copper more efficiently than the individual extractants Aliquat 336 or Cyanex 272. Formation of an octahedral copper-IL complex was characterized by UV-Visible spectra and metal ligand interaction was confirmed by FTIR spectra. The loading capacity of 0.1 mol/L [A336/Cy272] was found to be 1.71 g/L. Stripping studies reported that 0.298 g/L copper ions were efficiently stripped using 0.1 mol/L sulfuric acid from 0.05 mol/L loaded IL. The selectivity of copper against nickel, cadmium and iron was investigated from their equimolar binary mixtures using 0.05 mol/L [A336/Cy272] in kerosene. The highest separation factor βCu/Cd=8.41 was obtained at pH 3.56. Copper can be effectively separated from nickel over the pH range studied. The IL extracts preferentially iron over copper and the highest separation factor βFe/Cu was 3246 at pH 2.4. The extraction rate of metal ions from a synthetic solution containing copper with other metal impurities was in the order of Fe>Zn>Cu>Cd>Co>Ni.


