
J. Cent. South Univ. (2019) 26: 3238-3251
DOI: https://doi.org/10.1007/s11771-019-4249-6
Effects of MgO additive on metallurgical properties of fluxed-pellet
GUO He(郭贺), SHEN Feng-man(沈峰满), JIANG Xin(姜鑫),GAO Qiang-jian(高强健), DING Guan-gen(丁关根)
School of Metallurgy, Northeastern University, Shenyang 110004, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Abstract:
As a main charging burden of blast furnace (BF) ironmaking process, pellets play an important role in ironmaking process. However, compared with sinters, there are some inevitable disadvantages for traditional acid pellets, e.g., reduction swell, low melting temperature. Therefore, the fluxed-pellets have been applied in BF, especially MgO-fluxed pellets. In the present study, the effects of category and content of MgO bearing additive on the compressive strength (CS), reduction swelling index (RSI), reduction disintegration index (RDI) and melting-dripping properties of the pellets were investigated. Minerals composition, pore distribution and microstructure of MgO-flux pellets were studied by X-ray powder diffraction (XRD), mercury intrusion method and scanning electron microscopy (SEM), respectively. The results show that the light burned magnesite (LBM) is more suitable MgO bearing additive for fluxed-pellets. With increasing LBM content from 0 to 2.0%, the CS decreases from 3066 to 2689 N, RSI decreases from 16.43% to 9.97% and RDI decreases from 19.2% to 12.99%. The most appropriate MgO bearing additive content in the fluxed- pellets is 2.0% according to principal component analysis (PCA).
Key words:
pellets; MgO bearing additive; porosity; swelling; ironmaking; principal component analysis;
Cite this article as:
GUO He, SHEN Feng-man, JIANG Xin, GAO Qiang-jian, DING Guan-gen. Effects of MgO additive on metallurgical properties of fluxed-pellet [J]. Journal of Central South University, 2019, 26(12): 3238-3251.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-019-4249-61 Introduction
Iron bearing materials should enable the reliable production of hot metal from a blast furnace (BF) or direct reduced iron (DRI) from a shaft furnace, particularly at a minimum cost and at a large scale. The requirement of iron bearing materials for modern BF has increased, especially in the case of iron bearing materials for large-scale BF. In China, the use of sinters caused serious environmental pollution. Therefore, more and more pellets were used in BF, and the quality of pellets plays a critical role in decreasing the energy consumption and increasing the production of BF [1]. But the traditional acid pellets have some inevitable poor metallurgy properties, such as the higher reduction swelling index, lower melting temperature. In order to improve the metallurgical performance of pellets at high temperature, more attention has been paid for the fluxed-pellets.
A lot of researchers have studied the properties of fluxed-pellets. DWARAPUDI et al [2-4] reported the effect of MgO and basicity on the quality and microstructure of pellets. They discovered that the pellets containing 1.5% MgO had an optimum compressive strength, and softening-melting property of the pellets improved with increased basicity. The main reason was that the FeO content in silicate melt decreased with increased basicity. FERREIRA et al [5] reported the effect of gangue and additives on the divalent iron content of magnetite pellets. The Fe2+ content increased with decreased alkalinity of the pellets.
The main reasons were that the MgO reacted with SiO2 to form silicates and reacted with free CaO to form ferrites. ILJANA et al [6] reported the effect of limestone on the reducibility of pellets, and found that the degree of reduction of pellets increased with increased limestone content. The main reason was the decomposition of limestone in calcination process of pellets, which can cause the increase of porosity in pellets. MOUSA et al [7] researched the effect of nut coke on the reduction of pellets. They found that the nut coke improved the degree of reduction of pellets by increasing the efficiency of CO and direct reduction of fayalite phase. ZHU et al [8] compared the reducibility and compressive strength of preheated pellets and fired oxide pellets. The degree of reduction of preheated pellets was higher than that of oxide pellets at the same conditions, and the porosity was the main reason for the different degree of reduction. UMADEVI et al [9] investigated the effect of basicity (CaO/SiO2) on properties and microstructure of pellets. The tumble index and cold crushing strength of pellets increased with increased basicity and the main reason was that the microstructure of pellets changed. They also pointed out the relationships between fire temperature and metallurgical properties of pellet [10]. The optimum fire temperature and carbon addition could cause better physical and metallurgical properties of pellets. FRIEL et al [11] reported the effect of basicity on minerals compositions of pellets as dolomite was added to the pellets, and indicated that oxide bonded pellets formed in the case of low basicity, oxide bonded pellets combined with slag formed in the case of intermediate basicity, and calcium ferrite bonded pellets formed in the case of high basicity. SUGIYAMA et al [12] reported the effect of magnesium on the properties of pellets at different temperatures. The Mg2+ was in unique distribution status at different temperatures. The degree of reduction decreased with increased magnesium- ferrite in the pellets. They also indicated that the softening properties of pellets was superior when w(MgO)/w(SiO2) is higher than 0.6 and w(CaO)/w(SiO2) is lower than 0.05 [13].
Based on the above literature reviews, most of the published paper focused on the CaO-fluxed pellets. But few papers focus on the MgO-fluxed pellets. Especially, the researches on the effects of MgO bearing additive category and content on the metallurgical properties of MgO-fluxed pellets were insufficient. Therefore, the present work focuses on the effect of MgO bearing additive category and content on the compressive strength (CS), reduction swelling index (RSI), reduction degradation index (RDI) and melting-dripping properties of MgO- fluxed pellets. The X-ray powder diffraction (XRD), scanning electron microscopy (SEM) and Brunauer- Emmett-Teller (BET) were carried out to investigate the type and amount of minerals of pellets made by different flux categories and different MgO contents. Also, the principal component analysis (PCA) was used to confirm the comprehensive assessment index of the metallurgical properties for MgO-fluxed pellets. This investigation will enhance our understanding of the effects of the MgO bearing additive on the metallurgical properties of fluxed-pellet.
2 Experimental method
2.1 Materials
In this study, the raw materials were obtained from the Hansteel Company (Handan, China) with their main chemical compositions as given in Table 1. Two kinds of MgO additive categories, namely, magnesite additive and light burned magnesite (LBM), were included. The LBM was obtained by calcination of magnesite. It could be seen that the magnesite had a high content of burning loss on ignition (LOI) and a low content of MgO and the main mineral was magnesium carbonate (MgCO3) in two forms, crystalline and cryptocrystalline. LBM had a low LOI and a high content of MgO. Bentonite was a hydrous aluminosilicate that was largely composed of montmorillonite mineral and was used as a binder.
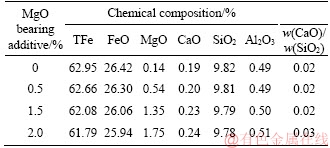
The chemical compositions of fluxed-pellets with different amounts of MgO bearing additive (LBM additive) are shown in Table 2. The MgO bearing additive contents in the fluxed-pellets were: 0, 0.5%, 1.5% and 2.0%. Under the condition of constant w(MgO)=0.8, the effects of MgO additive category (magnesite additive and LBM additive) on metallurgical properties of fluxed-pellets was studied.
Table 1 Chemical compositions of raw materials (mass fraction, %)
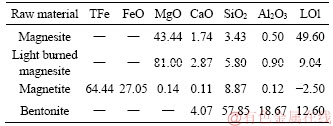
Table 2 Chemical compositions of pellets with different amount of MgO bearing additive (LBM additive)
In order to analyze the flux mineral compositions, the XRD patterns of magnesite and LBM were marked. The scanning was carried out at an angular range of 20°to 90°and scan rate of 6 (°)/min. The X-ray analysis of the MgO additive shows that the main mineral phase contained magnesium oxide (MgO), magnesium carbonate (MgCO3), magnesium silicate (MgSiO4) (Figure 1). The mineral compositions were different between magnesite and LBM. The amounts of magnesium carbonate (MgCO3), magnesium silicate (MgSiO4) in LBM are lower than those in magnesite, and the amount of magnesium oxide (MgO) in LBM is higher than that in magnesite. The particle sizes of all the raw materials, including magnetite, magnesite, LBM and bentonite, were less than 0.074 mm.
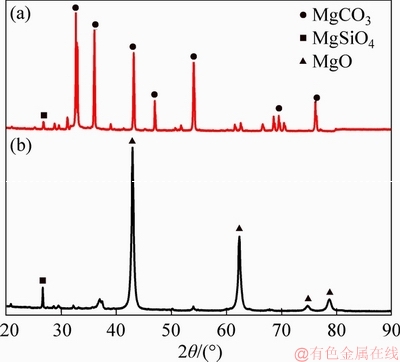
Figure 1 XRD patterns of samples magnesite (a) and LBM (b)
2.2 Procedure
The main process of preparing MgO-fluxed pellets includes balling, drying, oxidation roasting, and cooling. The binder, MgO additives and magnetite were mixed together. The bentonite was fixed at 2% of the mixture. The moisture content is 8%. The green pellets ranging from 10 to 16 mm in diameter were dried at 125 °C for 5 h in the bake out furnace. A muffle furnace was used to roast the MgO-fluxed pellets in two stages: preheating (1000 °C) and roasting. The MgO-fluxed pellets were first preheated for 20 min and then the temperature increased to the roasting temperature of 1280 °C and maintained for 1 h. Basically, we needed all of magnetite in the pellets to be oxidized and become hematite, and then the fluxed-pellets could get enough strength to meet the production requirement of BF. In the preliminary experiments, if the roasting time was less than 1 h, the oxidation would have been poorer, and there would have been more un-oxidized magnetite. If the roasting time was longer than 1 h, it would have wasted the heat energy. Therefore, based on our preliminary experiments, the roasting time was set for 1 h.
After oxidation roasting, the MgO-fluxed pellets were discharged from the muffle furnace and cooled to room temperature. The MgO-fluxed pellets were analyzed to determine their CS, RSI as per GB/T13242-91, and RDI as per GB/T13240-91. The melting-dripping experiment has been explained in previous papers [1]. We define T10 as the softening temperature and TD is defined as the dripping temperature; the softening- dripping zone is TD-T10.
The RSI is defined as follows:
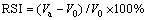 (1)
(1)
where V0 is the volume of the fluxed-pellets before reduction, mL; Va is the volume of the fluxed- pellets after reduction, mL.
3 Results
3.1 Effects of MgO bearing additive category on metallurgical properties of fluxed-pellets
The compressive strength, reduction swelling and reduction disintegration of the pellets are important metallurgical properties. Therefore, the effects of MgO bearing additive category on metallurgical properties of fluxed-pellets were investigated, as shown in Figure 2. The CS was 3015, 1805 and 2835 N for fluxed-pellets with no MgO bearing additive, magnesite bearing additive and LBM bearing additive, respectively. So, the strength of pellet with magnesite bearing additive is slightly lower than that of the LBM bearing additive, as shown in Figure 2.
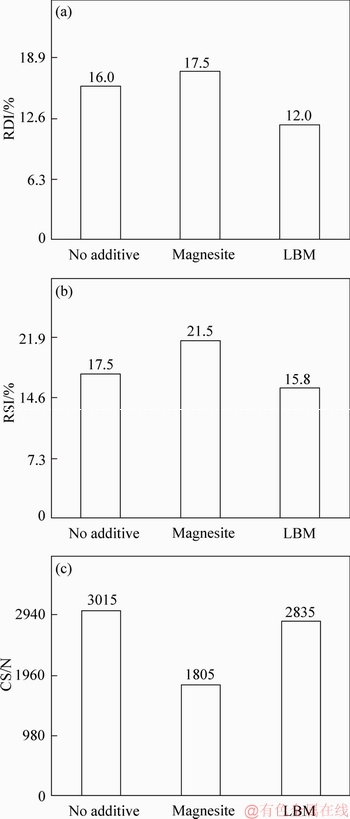
Figure 2 Effect of MgO bearing category on metallurgical performance of fluxed-pellets (w(MgO)= 0.8)
The RSIs were 17.5%, 21.5%, 15.8% for fluxed-pellets with no MgO bearing additive, with magnesite bearing additive and LBM bearing additive, respectively. The lower RSI increased the strength of reduced fluxed-pellets. From these results, it is evident that fluxed-pellets with the magnesite bearing additive displayed the highest RSI among all the fluxed-pellets tested.
The RDIs were 16%, 17.5%, 12%, respectively for fluxed-pellets with no MgO bearing additive, with magnesite bearing additive and LBM bearing additive, respectively. From the results, it was noticeable that the fluxed-pellets of magnesite bearing additive exhibited the highest RDI and that of LBM bearing additive was lower.
The above results show that the LBM is more suitable MgO bearing additive for fluxed-pellets. Then, the LBM was chosen for further experiments to examine the effects of the MgO bearing additive content on the fluxed-pellets’ metallurgical properties as discussed below.
3.2 Effects of MgO bearing additive content on metallurgical properties of fluxed-pellets
The compressive strength and porosity of MgO bearing additive are critical factors in the pellets’ preparation. Therefore, the effects of MgO bearing additive content on compressive strength and porosity of fluxed-pellets are studied, as shown in Figure 3. The porosity increased, but the compressive strength decreased in the fluxed-pellets with increased MgO bearing additive content. It was cut down that the compressive strength of the fluxed-pellets gradually decreased from 3015 N to 2689 N with increased MgO bearing additive content from 0% to 2.0%.
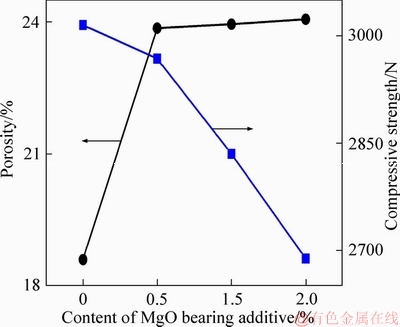
Figure 3 Effect of MgO bearing additive content on compressive strength and porosity of fluxed-pellets
The effects of MgO bearing additive content on RSI and compressive strength of reduced fluxed- pellets are shown in Figure 4. It was indicated that the RSI deceased from 17.5% to 12.99% with increased MgO bearing additive content from 0% to 2.0%. The compressive strength of reduced pellets increased with increased MgO bearing additive content. It could recognize from the results that there was a negative correlation between the compressive strength of reduced pellets and RSI.
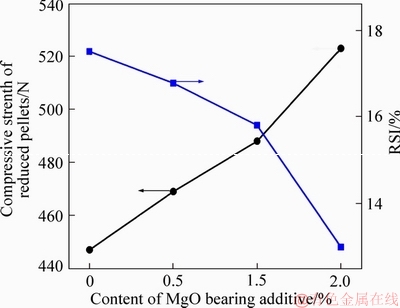
Figure 4 Effect of MgO bearing additive content on compressive strength of reduced pellets and RSI
The phenomenon of reduction disintegration of pellets is bad for BFs, and mainly occurs at low temperature in the upper part. Experimental results demonstrated that the fluxed-pellets containing high amount of the MgO bearing additive displayed the lowest RDI. However, the fluxed-pellets with low amounts of MgO bearing additive showed a higher RDI, as shown in Figure 5. With increased amount of MgO bearing additive from 0% to 2.0%, the RDI of fluxed-pellets decreased from 16% to 9.97%.
The softening-melting properties of MgO- fluxed pellets were investigated. The experimental results of softening-melting properties for the fluxed-pellets with different MgO bearing additive contents are shown in Figure 6. With MgO bearing additive content increased from 0% to 2.0%, the T10 increased from 1126 to 1136 °C, and the TD increased from 1388 to 1408 °C. The temperature range of TD-T10 was similar, from 262 to 272 °C. But the T10 and TD were obviously higher, which results in the relative position of cohesive zone decreasing in BF as shown in Figure 7. The liquidus temperature and viscosity of slag in pellets increased with increased MgO bearing additive content, and then caused the higher softening-dripping temperature of MgO-fluxed pellets in softening-dripping process. These experimental results are consistent with other literatures. For example, DWARAPUDIs et al [3] reported that MgO caused the increase of softening temperature of pellets. LIU et al [14] found more MgO-containing substances with higher melting point in un-dripped slag. Also, MgO could form the solid solution of FeO-MgO, in which the melting point of solid solution increased with increased amount of MgO [15].
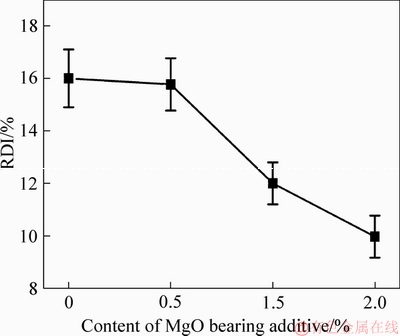
Figure 5 Effect of MgO bearing additive content on RDI of fluxed-pellets
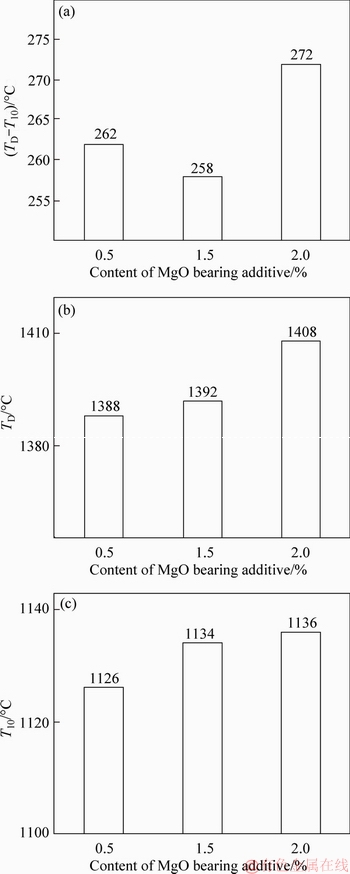
Figure 6 Effect of MgO bearing additive content on melting behavior of fluxed-pellets
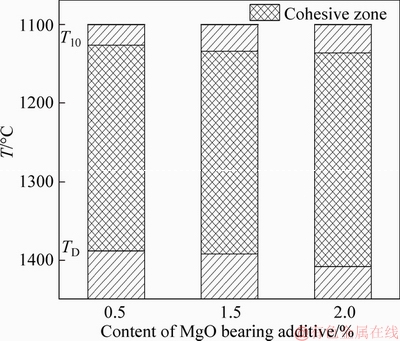
Figure 7 Effect of MgO bearing additive content on location of cohesize zone
4 Discussion
4.1 Effect of MgO bearing additive category and content on compressive strength of fluxed- pellets
The fluxed-pellets with LBM bearing additive exhibited the highest compressive strength compared with magnesite bearing additive. Magnesium carbonate (MgCO3) can decompose and generate CO2 in the fluxed-pellets containing the magnesite bearing additive, which can increase the porosity in pellets. However, the fluxed-pellets with LBM bearing additive cannot generate CO2, so their porosity is lower than the fluxed-pellets with magnesite bearing additive. This can cause different compressive strengths in the fluxed-pellets with different category MgO bearing additives. The slag or gangue also has effects on the compressive strength of fluxed-pellets with different category MgO bearing additive. The fluxed-pellets of magnesite bearing additive contain magnesium silicate (MgSiO4), and generate gangue with low strength. SIVRIKAYA and AROL [16] had proved that the compressive strength was low for containing low strength slag or gangue. The increase of porosity and the formation of low strength gangue cause the decrease of compressive strength as magnesite was added into pellets compared with LBM.
Figure 8 shows the results from the SEM analysis of typical microstructure of fluxed-pellets with different amounts of MgO bearing additive. It can be seen that the hematite (white) is the main additive (b) and 2.0% MgO bearing additive (c) (A (white): hematite; B (grey): magnetite; C (black): gangue) mineral composition for fluxed-pellets with 0% MgO bearing additive. The hematite is interconnected and its distribution is uniform; the construction has more structural capacity and there is only a small amount of magnetite (grey). The amount of hematite decreased and magnetite increased with increased MgO bearing additive content from 0% to 2.0%.
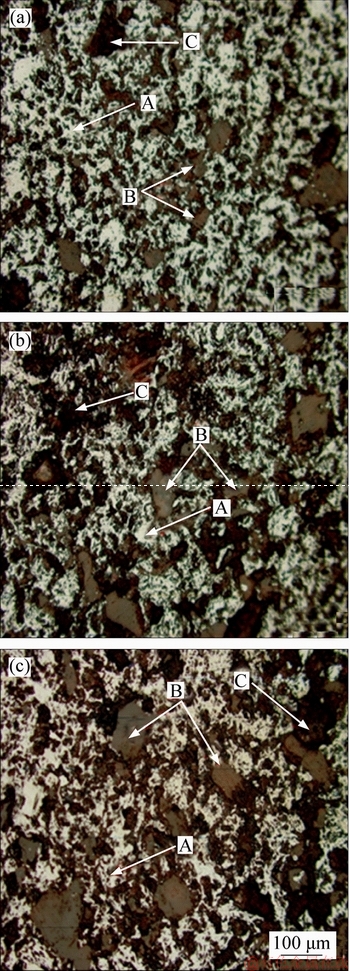
Figure 8 Microstructures of different MgO bearing additive content pellets with 0% MgO bearing additive (a), 1.5% MgO bearing
Figure 9 presents minerals compositions of the fluxed-pellets containing different amounts of MgO bearing additive. The hematite content is 85.22% in the fluxed-pellets with 0% MgO bearing additive; however, the fluxed-pellets with 1.5% and 2.0% MgO bearing additive have 75.22% and 73.34% hematite, respectively. The amount of hematite steeply decreases when there are higher amounts of MgO bearing additive. There is 5.27% magnetite in the fluxed-pellets with 0% MgO bearing additive, and there is 15.07% and 16.33% magnetite in the fluxed-pellets with 1.5% and 2.0% MgO bearing additive, respectively. The amount of magnetite slowly increases with increased amount of MgO bearing additive. The gangue mineral is almost homogenous in the fluxed-pellets even when they have different amounts of the MgO bearing additive.
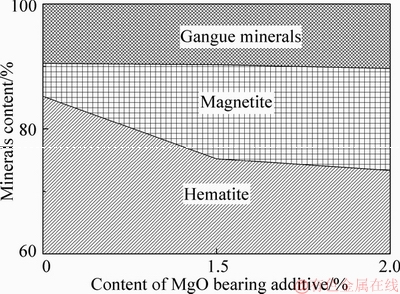
Figure 9 Effect of MgO bearing additive content on pellets’ mineral composition
Figure 10 shows the diffraction patterns of fluxed-pellets without the MgO bearing additive and with MgO bearing additive. Hematite (Fe2O3) and magnetite (Fe3O4) are the main mineral components of the fluxed-pellets without MgO bearing additive, but the MF((FexMg1-x))O·Fe 2O3), hematite (Fe2O3) and magnetite (Fe3O4) are the main components of the fluxed-pellets with the MgO bearing additive.
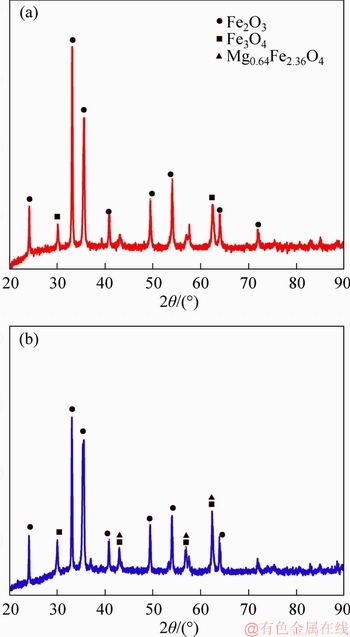
Figure 10 XRD patterns of samples without MgO bearing additive (a) and with MgO bearing additive (b)
Mg2+ reacts with Fe2+ to generate MF((FexMg1-x))O·Fe 2O3) as shown in Figure 10. The reaction can restrain the oxidation of magnetite (Fe3O4), and these fluxed-pellets contain more magnetite (Fe3O4) as shown in Figures 8 and 9. With increased MgO bearing additive, the formation of MF((FexMg1-x))O·Fe 2O3) restrains the oxidation of magnetite and densification process for pellets [17]. The porosity increases with the greater amount of MgO bearing additive. The results, as shown in Figure 11, indicate the effect of MgO bearing additive on the fluxed-pellets’ porosity. The porosity is 18.61% for the fluxed-pellets with 0% MgO bearing additive. However, the porosity is 23.86% and 24.06% for fluxed-pellets with 1.5% and 2.0% MgO bearing additive, respectively.
The main pore distribution of the fluxed- pellets without MgO bearing additive is about 5 μm and the size is small. However, the pore size increases with greater amount of MgO bearing additive. A negative correlation was found between the porosity and compressive strength. The formula is as follows [18-20]:
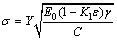 (2)
(2)
where σ is the critical rupture stress, MPa; Y is the correlation factor, dimensionless; E0 is the elastic modulus of a sample without any pores, GPa; ε is the porosity; C is the half-length of the crack, m; γ is the surface energy, J; K1 is a constant, whose value depends on shape and orientation of porosity.
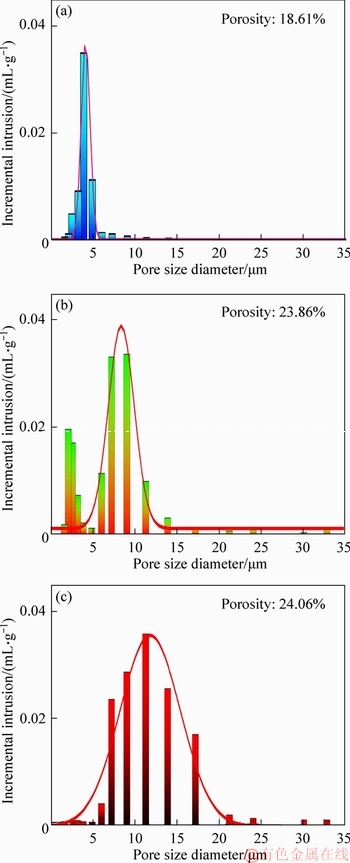
Figure 11 Pore size, distribution and porosity of pellets with 0% MgO bearing additive (a), 1.5% MgO additive bearing (b) and 2.0% MgO additive bearing (c)
One reason why compressive strength of the fluxed-pellets declined is that critical rupture stress decreases with increased porosity of the fluxed- pellets. The pore appeared at the location where was the grain juncture; the other one reason was that the stress of concentration led to crack, and crack length increased with increased MgO bearing additive in the fluxed-pellets. In summary, these results show that the compressive strength decreases with increased porosity of pellets.
The consolidation index of pellets is the parameter that represented the pellets’ degree of consolidation degree. The formula is as follows [21]:
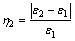 (3)
(3)
where ε1 is the porosity of the fluxed-pellets before roasting; ε2 is the porosity of the fluxed-pellets after roasting; η2 is the consolidation index of the fluxed- pellets.
The consolidation index and CS of fluxed- pellets with different amounts MgO bearing additive are shown in Figure 12. Both the consolidation index of the fluxed-pellets and their compressive strength decreased with increased amount of MgO bearing additive.
The relationship between consolidation index and CS is shown in Figure 13. The consolidation index increases with increased CS. The results show that there is basically a linear relationship between the consolidation index and the CS, and the experimental linear regression equation is given below:
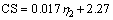 (4)
(4)
where η2 is consolidation index, %; CS is the compressive strength of the fluxed-pellets, N.
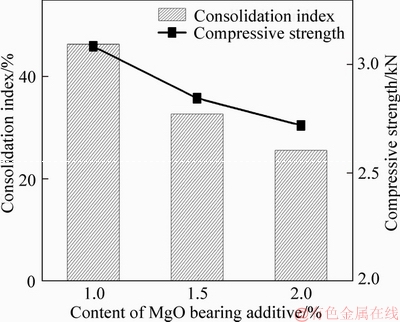
Figure 12 Effect of MgO bearing additive content on consolidation index and compressive strength of fluxed-pellets
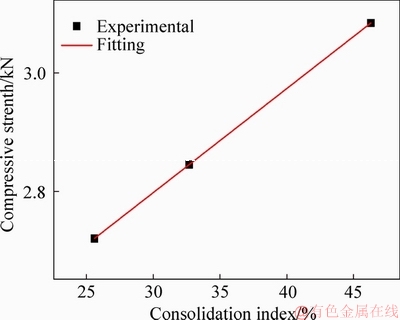
Figure 13 Relationship between consolidation index and compressive strength
4.2 Effect of MgO bearing additive on reducibility of fluxed-pellets
The reducibility properties of pellets contain reduction swelling and reduction disintegration. The main reason for reduction swelling and reduction disintegration of pellets is that the hematite is reduced into magnetite, and causes the expansion of volume [22-24].
The phase diagrams of MgO-FeO and MgO-Fe2O3 are shown in Figure 14 [25]. The FeO and MgO were replaced by each other as shown in Figure 14(a). The Fe2+ and Mg2+ can substitute each other partially, and there is a region where the phase distributes uniformly, and the roasting of pellets is in this region.
The energy spectrum analysis of the fluxed-pellets with the MgO bearing additive is shown in Figure 15. Mg2+ and Fe3+ have generated the solid solution as shown in Figure 14(b).During roasting and reduction process, the mechanisms occurring that are suggested and summarized in Figure 16. These results further support the idea that the RSI and RDI decreased with increased MgO bearing additive content of fluxed-pellets.
After the reduction, the appearances of fluxed- pellets containing different amounts of MgO bearing additive are shown in Figure 17. The pellets without the MgO bearing additive show a lot of cracks, and disintegration has occurred in some of the pellets. But the appearance of the pellets with the MgO bearing additive basically remains unchanged and the cracks do not occur. The appearance of these pellets demonstrates that the MgO bearing additive can improve the RSI and RDI.
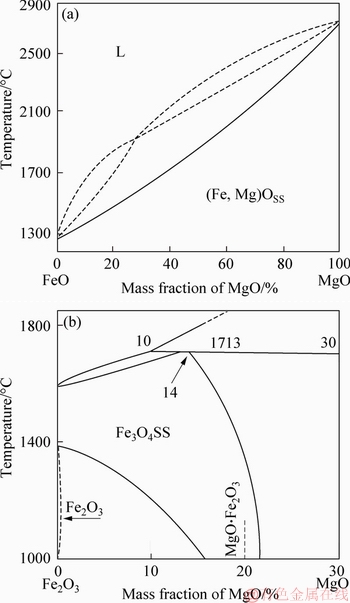
Figure 14 Phase diagram (a) FeO-MgO (b) Fe2O3-MgO
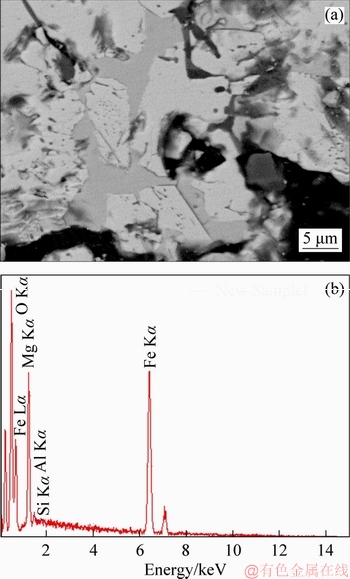
Figure 15 Energy spectrum of MgO bearing additive pellets
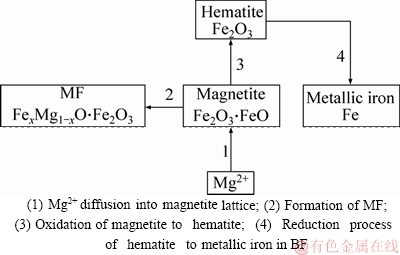
Figure 16 Schematic of influence of MgO on pellets’ mineral compositions:
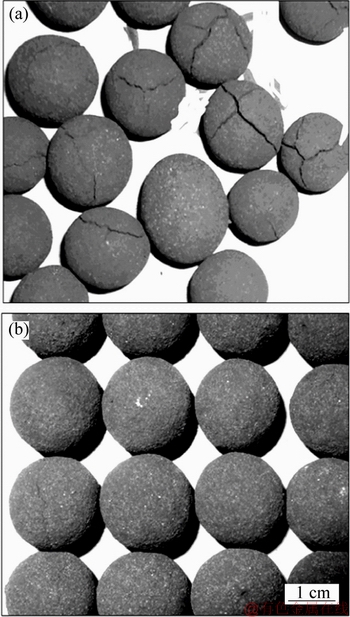
Figure 17 Materialization after reduction without MgO bearing additive pellets (a) and with MgO bearing additive pellets (b)
The relationship between RSI and CS of reduced pellets with MgO bearing additive is shown in Figure 18. As shown in Figure 18, the RSI decreases with increased CS of reduced pellets. The results show that there is a good linearity relationship between RSI and CS of reduced pellets. The degree of fitting reaches 0.94395 for the relationship and the result is reliable. The experimental linear regression equation is:
RSI=-0.06020·CS+44.77995 (5)
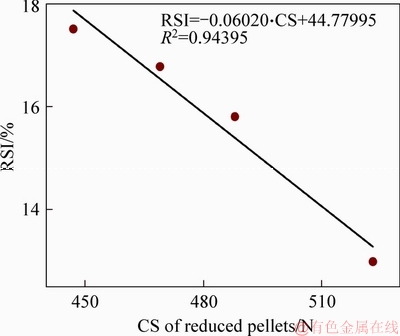
Figure 18 Relationship between RSI and CS of reduced MgO-fluxed pellets
To further explain the mechanism of the MgO bearing additive on RSI, the reduced pellets with different amounts of MgO bearing additive were broken and examined using SEM to check the fluxed-pellets’ morphology and the results are shown in Figure 19. There is a significant difference between the three groups of fluxed-pellets containing different amounts of MgO bearing additives. Some pores and little metallic iron fibers formed for reduced pellet with no MgO bearing additive (Figure 19(a)), causing the highest RSI. However, the pores become smaller and clustered together and the metallic iron fibers become shorter in the reduced pellets with increased amounts of MgO bearing additive (Figures 19(b) and (c)). This phenomenon caused the decrease of RSI and the increase of CS of reduced pellets with increased MgO bearing additive. This conclusion is consistent with the results of other researchers [26, 27]. This discrepancy should be attributed to the ionic radius of Mg2+. The ionic radius of Mg2+ (0.65  ) is smaller than that of Fe2+ (0.76
) is smaller than that of Fe2+ (0.76  ), and Mg2+ fills the ample vacancies in the wustite lattice [28, 29]. The lattice is stabilized and alleviates the crystal defect, and the diffusion coefficient of the Fe2+ decreases in the wustite. This is the main reason why the pores have become smaller and metallic iron fibers restrain with increased MgO bearing additive content in the fluxed-pellets.
), and Mg2+ fills the ample vacancies in the wustite lattice [28, 29]. The lattice is stabilized and alleviates the crystal defect, and the diffusion coefficient of the Fe2+ decreases in the wustite. This is the main reason why the pores have become smaller and metallic iron fibers restrain with increased MgO bearing additive content in the fluxed-pellets.
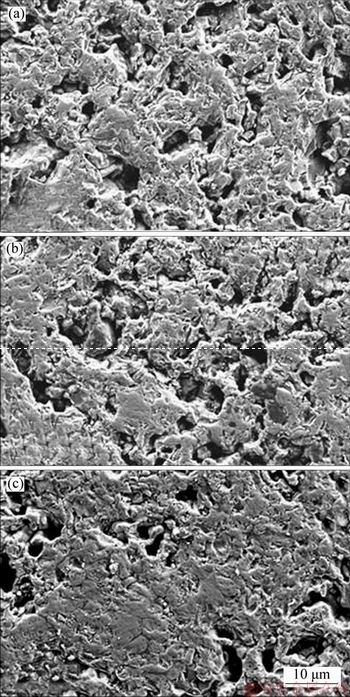
Figure 19 SEM morphology of reduced MgO-fluxed pellets with 0% MgO bearing additive (a), 1.5% MgO bearing additive (b), and 2.0% MgO bearing additive (c)
4.3 Comprehensive assessment index of metallurgical properties
The metallurgical properties of MgO-fluxed pellets could be improved by adding an appropriate amount of MgO. A principal component analysis (PCA) was used to analyze the fluxed-pellets. The method of PCA was used to corroborate the composite indicator of fluxed-pellets. The MgO content of the fluxed-pellets was calculated using the PCA. The calculation process is detailed below.
The parameters of the fluxed-pellets are as follows: primitive matrix=X; canonical matrix=X′; covariance matrix=C; eigenvector of canonical matrix=λ;
The original data that constitute the primitive matrix are the RDI, RSI, CS, T10 and TD. The primitive matrix is shown in Eq. (6).
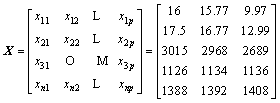 (6)
(6)
The primitive matrix is normalized and the canonical matrix is shown in Eq. (7).
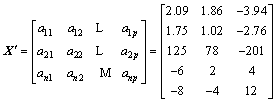 ,
,
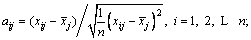
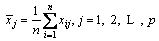 (7)
(7)
The covariance matrix is defined as generalization from randomly variables of scalar to high dimension. The equation is shown in Eq. (8) and calculation result of covariance matrix is shown in Eq. (9)
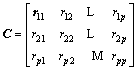
 (8)
(8)
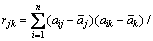
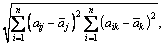
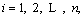
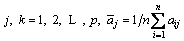 (9)
(9)
The eigenvalue of the covariance matrix is λ1, λ2, λ3, λ4, λ5 as shown in Eq. (10), and the corresponding eignvector for different eigenvalue is shown in Eq. (11)
λ1=20800, λ2=80, λ3=λ4=λ5=0 (10)
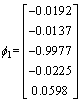 ,
, 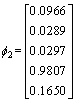 ,
, 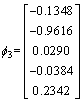 ,
,
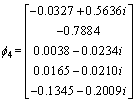 ,
, 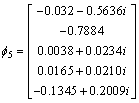 (11)
(11)
The corresponding eignvector of the maximum eignvalue is selected as the matrix of dimensionality reduction as shown in Eq. (12).
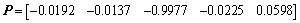 (12)
(12)
The comprehensive assessment of the metallurgical properties for different MgO bearing additive content pellets is shown in Eq. (13).
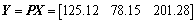 (13)
(13)
The comprehensive assessment index is shown in Figure 20. The results indicate that the comprehensive assessment index is 201.28 for the 2.0% MgO bearing additive pellets.
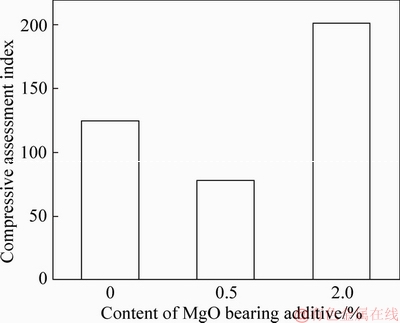
Figure 20 Compression assessment index of different MgO bearing additive pellets
5 Conclusions
The effects of MgO bearing additive category and content on the metallurgical performance of pellets were studied. The main findings can be summarized as follows:
1) For w(MgO)=0.8 in fluxed-pellets, in the case of magnesite additive, the compressive strength (CS) is 1805 N, the reduction swelling index (RSI) is 21.5% and reduction disintegration index (RDI) is 17.5%. In the case of light burned magnesite (LBM), the CS is 2835 N, RSI is 15.8% and RDI is 12.0%. Therefore, LBM is more suitable MgO-bearing additive for fluxed-pellets.
2) With the increase of MgO bearing additive from 0% to 2.0%, the CS decreases from 3066 N to 2689 N, RSI decreases from 16.43% to 9.97%, and RDI decreases from 19.2% to 12.99%. Therefore, the MgO bearing additive is benefit for RSI and RDI- of fluxed-pellets, but it has negative effect on CS of fluxed-pellets.
3) The SEM analysis shows that MgO additive can restrain the growth of metallic iron fibers, and more pores appear in the fluxed-pellets. Consequently, the reduction swelling index (RSI) of MgO-fluxed pellets decreases compared with traditional pellets.
4) With the increase of MgO bearing additive in fluxed-pellets, the softening temperature of fluxed-pellets increases from 1126 °C to 1136 °C and the dripping temperature of fluxed-pellets increases from 1388 °C to 1408 °C. However, the cohesive zone firstly decreases from 262 °C to 256 °C, and then increases to 272 °C.
5) The most appropriate MgO bearing additive content in the fluxed-pellets is 2.0% according to principal component analysis (PCA).
Acknowledgement
The authors wish to acknowledge the contributions of associates and colleagues in Northeastern University of China.
References
[1] SHEN Feng-man, JIANG Xin, WU Gang-shen, WEI Guo, LI Xiao-gang, SHEN Yang-song. Proper MgO addition in blast furnace operation [J]. ISIJ International, 2006, 46(1): 65-69. DOI:10.2355/isijinternational.46.65.
[2] DWARAPUDI S, GHOSHT K, SHANKAR A, TATHAVADKAR V, BHATTACHARJEE D, VENUGOPAL R. Effect of pyroxenite flux on the quality and microstructure of hematite pellets [J]. International Journal of Mineral Processing, 2010, 96(1-4): 45-53. DOI: 10.1016/j.minpro. 2010.06.002.
[3] DWARAPUDI S, GHOSH T K, SHANKAR A, TATHAVADKAR V, BHATTACHARJEE D, VENUGOPAL R. Effect of pellet basicity and MgO content on the quality and microstructure of hematite pellets [J]. International Journal of Mineral Processing, 2011, 99(1): 43-53. DOI: 10.1016/j.minpro.2011.03.004.
[4] DWARAPUDI S, GHOSH T K, TATHAVADKAR V, DENYS M B, BHATTACHARJEE D, VENUGOPAL R. Effect of MgO in the form of magnesite on the quality and microstructure of hematite pellets [J]. International Journal of Mineral Processing, 2012, 112-113(4): 55-62. DOI: 10.1016/j.minpro.2012.06.006.
[5] FERREIRA S, CORES A, ROBLA J I, VERDEJA L F, RUIZ-BUSTINZA I, GARCIA-CARCEDO F, MOCHON J. The influence of gangue and additives on the divalent iron content of magnetite pellets [J]. Steel Research International, 2014, 85(2): 261-272. DOI: 10.1002/srin.201300370.
[6] ILJANA M, KEMPPAINEN A, PAANANEN T, MATTILA O, PISILA E, KONDRAKOV M, FABRITIUS T. Effect of adding limestone on the metallurgical properties of iron ore pellets [J]. International Journal of Mineral Processing, 2015, 141: 34-43. DOI: 10.1016/j.minpro.2015.06.004.
[7] MOUSA E, SENK D, BABICH A. Reduction of pellets-nut coke mixture under simulating blast furnace conditions [J]. Steel Research International, 2010, 81(9): 706-715. DOI: 10.1002/srin.201000047.
[8] ZHU De-qing, MENDES V, CHUN Tie-jun, PAN Jian, LI Qi-hou, LI Jian, QIU Guan-zhou. Direct reduction behaviors of composite binder magnetite pellets in coal-based grate-rotary kiln process [J]. ISIJ International, 2011, 51(2): 214-219. DOI: 10.2355/isijinternational.51.214.
[9] UMADEVI T, KUMAR P, LOBO N F, PRABHU M, MAHAPATRAP C, RANJAN M. Influence of pellet basicity (CaO/SiO2) on iron ore pellet properties and microstructure [J]. ISIJ International, 2011, 51(1): 14-20. DOI: 10.2355/isijinterna-tional.51.14.
[10] UMADEVI T, LOBO N F, DESAI S, MAHAPATRA P C, SAH R, PRABHU M. Optimization of firing temperature for hematite pellets [J]. ISIJ International, 2013, 53(9): 1673-1682. DOI: 10.2355/isijinternational.53.1673.
[11] FRIEL J J, ERICKSON E S. Chemistry, microstructure, and reduction characteristics of dolomite-fluxed magnetite pellets [J]. Matallurgical Transactions B, 1980, 11B(2): 233-243. DOI: 10.1007/BF02668407.
[12] SUGIYAMA T, SHIROUCHI S, TSUCHIYA O, ONODA M, FUJITA I. Effect of Magnesite on the properties of pellets at room and low (900 °C) temperatures [J]. Transactions ISIJ, 1983, 23(2): 146-152. DOI: https://doi.org/10.2355/isijinter national1966.23.146.
[13] SUGIYAMA T, SHIROUCHI S, TSUCHIYA O, ONODA M, FUJITA I. High temperature reduction and softening properties of pellets with magnesite [J]. ISIJ International, 2006, 23(2): 153-160. DOI: https://doi.org/10.2355/ isijinternational1966.23.153.
[14] LIU Zheng-gen, CHU Man-sheng, WANG Hong-tao, ZHAO Wei, XUE Xiang-xin. Effect of MgO content in sinter on the softening–melting behavior of mixed burden made from chromium-bearing vanadium–titanium magnetite [J]. International Journal of Minerals Metallurgy and Materials, 2016, 23(1): 25-32. DOI: 10.1007/s12613-016-1207-2.
[15] LI Ting-le, SUN Chang-yu, LIU Xue-yan, SONG S, WANG Qi. The effects of MgO and Al2O3 behaviors on softening– melting properties of high basicitysinter [J]. Ironmaking & Steelmaking, 2018, 45(8): 755-763. DOI: 10.1080/03019233. 2017.1337263.
[16] SIVRIKAYA O, AROL A I. An investigation of the relationship between compressive strength and dust generation potential of magnetite pellets [J]. International Journal of Mineral Processing, 2013, 123(9): 158-164. DOI: 10.1016/j.minpro.2013.06.006.
[17] GAO Qiang-jian, WEI Guo, SHEN Feng-man. Effect of MgO on compressive strength of pellet [J]. Journal of Northeastern Universuty, 2013, 34(1): 103-106. (in Chinese)
[18] SURESH S. Fatigue of materials [M]. New York: Cambridge University Press, 1998.
[19] GIBSON L J, ASHBY M F. Cellular solids structure and properties [M]. Cambridge: Cambridge University Press, 1997.
[20] GAO Qiang-jian, JIANG Xin, WEI Guo, SHEN Feng-man. Characterization of consolidation degree of iron ore pellet by mercury injection method [J]. Journal of Northeastern University, 2013, 34(6): 832-835. (in Chinese)
[21] DONSKOI E, MCELWAIN D L C. Mathematical modeling of non-isothermal reduction in highly swelling iron ore–coal char composite pellet [J]. Ironmaking & Steelmaking, 2013, 28(5): 384-389. DOI:10.1179/030192301678244.
[22] KUMAR M, NATH S, PATEL S K. Studies on the reduction–swelling behaviors of hematite iron ore pellets with noncokingcoal [J]. Mineral Processing and Extractive Metallurgy Review, 2010, 31(4): 256-268. DOI: 10.1080/ 08827508.2010.508826.
[23] SINGH M, BJORKMAN B. Effect of reduction conditions on the swelling behaviour of cement-bonded briquettes [J]. ISIJ International, 2004, 44(2): 294-303. DOI: 10.2355/ isijinternational.44.294.
[24] XU Bin, HOU Tong, CHEN Xu-ling, LI Qian, JIANG Tao, LI Peng. Effect of dolomite on reduction swelling property of iron ore pellets [J]. Journal of Central South University, 2013, 20(10): 2806-2810. DOI: 10.1007/s11771-013- 1800-8.
[25] WANG Jian. Slag atlas [M]. 2nd ed. Verlag Stahleisen, GmbH, 1995: 63.
[26] LI Wei, WANG Nan, FU Gui-qin, CHU Man-sheng, ZHU Miao-yong. Influence of TiO2 addition on the oxidation induration and reduction behavior of Hongge vanadium titanomagnetite pellets with simulated shaft furnace gases [J]. Powder Technology, 2018, 326: 137-145. DOI: 10.1016/ j.powtec.2017.12.050.
[27] LI Wei, WANG Nan, FU Gui-qin, CHU Man-sheng, ZHU Miao-yong. Influence of roasting characteristics on gas-based direct reduction behavior of Hongge vanadium titanomagnetite pellet with simulated shaft furnace gases [J]. Powder Technology, 2017, 310: 343-350. DOI: 10.1016/ j.powtec.2017.01.062.
[28] SESEN M K.The influence of CaO on the precipitation behaviour of iron in the reduction of iron oxide [J]. Scand J Metall, 2001, 30: 1-7.
[29] ZHANG Han-quan, LU Man-man, FU Jin-tao. Oxidation and roasting characteristics of artificial magnetite pellets [J]. Journal of Central South University, 2016, 23(11): 2999-3005. DOI: 10.1007/s11771-016-3363-y.
(Edited by HE Yun-bin)
中文导读
MgO 添加剂对熔剂性球团矿冶金性能的影响
摘要:作为高炉炼铁重要原料,球团矿在炼铁过程中起着重要作用。然而,与烧结矿相比,传统的酸性球团矿存在不可避免缺点,如还原膨胀、熔融温度低等。因此,熔剂型球团矿已经在高炉中广泛应用,尤其是MgO熔剂型球团矿。本文研究了MgO添加剂种类和含量对球团矿抗压强度(CS)、还原膨胀指数(RSI)、还原粉化指数(RDI)和熔滴性能的影响;采用了X射线衍射(XRD)、压汞法和扫描电镜(SEM)研究了MgO熔剂型球团矿矿物的组成、孔分布和微观结构。结果表明:对于熔剂型球团矿,轻烧菱镁石(LBM)是合适的含MgO添加剂。随着LBM含量由0%增加到2.0%,CS由3066 N下降到2689 N,RSI由16.43%下降到9.97%,RDI由19.2%下降到12.99%。基于主成分分析(PCA),熔剂型球团矿中最合适的MgO添加剂含量为2.0%。
关键词:球团矿;MgO添加剂;气孔率;膨胀性;炼铁;主成分分析
Foundation item: Projects(51874080,51604069) supported by the National Natural Science Foundation of China; Project(N162504004) supported by the Fundamental Research Funds for the Central Universities, China
Received date: 2018-08-14; Accepted date: 2018-12-28
Corresponding author: JIANG Xin, PhD, Associate Professor; Tel: +86-18904015206; E-mail: jiangx@smm.neu.edu.cn; ORCID: 0000-0003-3455-6229
Abstract: As a main charging burden of blast furnace (BF) ironmaking process, pellets play an important role in ironmaking process. However, compared with sinters, there are some inevitable disadvantages for traditional acid pellets, e.g., reduction swell, low melting temperature. Therefore, the fluxed-pellets have been applied in BF, especially MgO-fluxed pellets. In the present study, the effects of category and content of MgO bearing additive on the compressive strength (CS), reduction swelling index (RSI), reduction disintegration index (RDI) and melting-dripping properties of the pellets were investigated. Minerals composition, pore distribution and microstructure of MgO-flux pellets were studied by X-ray powder diffraction (XRD), mercury intrusion method and scanning electron microscopy (SEM), respectively. The results show that the light burned magnesite (LBM) is more suitable MgO bearing additive for fluxed-pellets. With increasing LBM content from 0 to 2.0%, the CS decreases from 3066 to 2689 N, RSI decreases from 16.43% to 9.97% and RDI decreases from 19.2% to 12.99%. The most appropriate MgO bearing additive content in the fluxed- pellets is 2.0% according to principal component analysis (PCA).


