
Adsorption-extraction mechanism of heavy rare earth by
Cyanex272-P507 impregnated resin
LIAO Chun-fa(廖春发), JIAO Yun-fen(焦芸芬), LIANG Yong(梁 勇),
JIANG Ping-guo(姜平国), NIE Hua-ping(聂华平)
School of Materials and Chemistry Engineering,
Jiangxi University of Science and Technology, Ganzhou 341000, China
Received 11 September 2009; accepted 11 January 2010
Abstract:
Instrument of IR spectrometer and methods of saturation, equimolar series change and slope were applied to study the extraction mechanism of Cyanex272-P507 impregnated resin for heavy rare earths from hydrochloric acid solution. The results show that the molar ratio of Cyanex272-P507 to rare earth in the extraction complex is 3. Chlorine ions do not participate in coordination.
The extraction reaction can be expressed as ![]() =
=![]() (where HA represents
(where HA represents
Cyanex272 and HL represents P507). The synergic extractant formed in extraction chromatography is in a form of monopolymer and with a chemical structure of REA3/2L3/2.
Key words:
Cyanex272; P507; impregnated resin; heavy rare earth elements; adsorption-extraction mechanism;
1 Introduction
The method of extraction chromatography combines advantages of high selectivity in extraction and simple equipment, strong separation capability in ion exchange, and has become one of the important methods in preparation of high purity rare earth products[1-3]. In this method, the common stationary phase is P507 impregnated or levextrel resin. However, with this common stationary phase, the method needs high acid medium and the separation effect is poor. These problems are even more serious in separation of heavy rare earths, which surely brings about troubles in real production. Cyanex272 is a new type of synthetic organic phosphonic extractant made in 1980s by American Cyanamid Company. As one Cyanex272 has one more alkyl and one less alkoxy than one P507 and its pKa value is much higher than that of P507, it has been widely used in separation of non-ferrous metals[4-5], especially in the separation and purification of heavy rare earth. Compared with P507 impregnated resin, Cynex272
impregnated one needs low acid medium, but its extraction capacity is lower than that of the former (equals 1/2 that of the former)[6-7]. As to the separation coefficient, there is no obvious difference between these two impregnated resins.
Since the discovery of synergy in 1950s, metallurgists have carried out a series of research work on synergistic extraction system with phosphine (phosphorus) type extractants[8-17]. They have reported that some synergistic extractants can improve extraction effect, which has been called positive synergistic extraction. LIAO et al[18-19] reported that Cyanex272-P507 impregnated resin is this kind of extraction system which can increase the distribution ratio and can improve the saturated extraction capacity.
In order to have a comprehensive understanding on this synergistic system, in this work, the authors have chosen Tm-Yb-Lu heavy rare-earths’ enrichment (HREE) as experimental material to be extracted, utilized IR spectrometer to detect related structures, and applied methods of saturation, equimolar series and slope as well to analyze the extraction mechanism.
2 Experimental
2.1 Major reagent and materials
P507 and commercial organic phosphonic Cyanex272 were in industrial purity, others were in analytical purity, and macroporous adsorption resin (this resin is a kind of cross-linked polymer without ion exchange groups, which type is HPD800) produced in Cangzhou Chemical Co., Ltd. were used to prepare the impregnated resin through the method presented in Ref.[19]. The experimental material of HREE provided by a factory in Guangzhou was rich in thulium, ytterbium and lutetium elements, and was composed of 11.51% Tm2O3, 72.71% Yb2O3 and 14.75% Lu2O3. Its average relative molecular mass is 196.81.
2.2 Experimental
2.2.1 Concentration determination of rare earth
The concentration of rare earth is titrated according to the following procedure. Getting an accurate volume (1 mL) of rare earth filtrate with pipette and putting in conical flask (Capacity of 300 mL), adding a little ascorbic acid and diluting to about 20 mL with distilled water, shaking until ascorbic acid dispersed evenly and adding an appropriate amount of hexamethylene tetramine buffer solution. Adding two drops of arsenazo (Ⅲ) indicator, until the solution becomes blue, titration with EDTA solution to make the blue into a purple-red, that is
![]() (1)
(1)
where c1 is the molar concentration (mol/L) of EDTA; V1 and V are the volumes of EDTA and liquid (mL), respectively.
2.2.2 Equimolar series change method
In this method, seven parts of impregnated resins in different mass were mixed with the corresponding part of rare earth solution in different volumes to ensure that the molar ratio of rare earth to extractant should vary within a certain range, while the total amount of them was invariable (1.2 mmol) and pH was 3 in all solutions. Then, the mixing systems were placed in an oscillation (40 °C) for 50 min, respectively. For every system, the adsorption amount Q (mg/g) can be calculated according to the following equation:
![]() (2)
(2)
where D is the distribution ratio; ρ0 and ρe are the initial and equilibrium concentrations (mg/L) of rare-earth in aqueous phase, respectively; V is the solution volume (mL); and m is the mass (g) of dry resin.
2.2.3 Slope method
In this method, 10 mL of rare earth solutions with same ion concentration (0.002 2 mol/L) but different pH values were added into 6 g of impregnated resin, respectively. Whereafter, all the extraction systems were placed in oscillations (40 °C) for 50 min. For every system, according to Eq.(1), the distribution ratio D can be calculated.
3.1 Extracting mechanism of Cyanex272-P507 impregnated resin
3.1.1 Influence of chloride ion on extraction process
In the view of the complexity of the synergistic extraction, it is very essential to make sure whether Cl- ligands have been participated in coordination. If Cl- did not coordinate, the following mechanism study will be simplified, while even if Cl- has participated in coordination, the following mechanism study can still confirm at least that the chloride ion exists in coordination adduct. So, in the experiment, the distribution ratios between organic and aqueous phases under different Cl- concentrations have been studied, and the relationship between lgD and lg[Cl-] has been obtained (Figure omitted). The results indicate that the slope of lgD to lg[Cl-] is near to zero, which means that Cl- has not participated in coordination in the extraction process.
3.1.2 Saturation method for determining extraction ratio
According to Ref.[18], the saturated adsorption of Cyanex272-P507 impregnated resin for heavy rare-earth is 25.37 mg/g, that is, 0.128 9 mmol/g, as the average relative molecular mass of the raw material is 196.81.
Elemental analysis shows that phosphorus content in Cyanex272-P507 impregnated resin is 1.24%, that is, 0.40 mmol/g. So, the content of general extractant (whether Cyanex27 or P507) is 0.40 mmol/g, too, for 1 mol Cyanex272 or P507 contains 1 mol phosphorus.
These corresponded data and some other elemental results are shown in Table 1. From Table 1, it can be seen that the molar ratio of rare earth to extractant is 1?3.10, close to 1?3. This means that the saturation method can be used to identify the molar ratio of rare earth to extractant, however, it cannot be used to solve the problems about the detailed structure of the extraction complex. The reason is that different relative amount of the substance between Cyanex272 and P507 would result in different chemical structures for the complex. Here, four kinds of extraction complexes with different structures are listed in Table 2.
Table1 Extraction ratio determined by saturation method

Table 2 Four kinds of extraction complexes with different chemical structures

Because chloride ions do not participate in coordination, the extraction reaction can be expressed as follows:
![]() =
=
![]() (1)
(1)
where HA represents Cyanex272, and HL represents P507.
From reaction (1), it can be known that to study the extraction mechanism further, the key is to determine the specific value of x. In order to solve this problem, methods of slope and equimolar series changes have been applied and the results will be discussed in the following sections.
3.1.3 Equimolar series change method for determining extraction ratio
In this part, experiments have been carried out in accordance with section 2.2.2, and the results are shown in Fig.1, where the longitudinal coordinate represents the adsorption capacity, Q(mg/g), and the abscissa represents the continuous changes of molar ratio of (HA + HL) to RE. In this figure, it can be seen that the abscissa point which corresponds the maximal point of the adsorption capacity shows the extraction ratio (HA+HL:RE) is close to 1?3, which is in consistent with the above result obtained by the method of saturation.
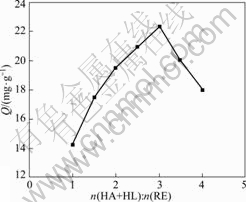
Fig.1 Adsorption capacity Q changed with extraction ratio of HA+HL to RE
In order to identify the value of x in reaction (1), another group of experiments with this method has been carried out on the condition that the mole number of P507 was kept invariable as 0.3 mmol, the total mole number of RE and Cyanex272 was accordingly maintained as 0.9 mmol, while the mole ratio of rare earth ions to Cyanex272 was controlled to change within a certain range. The corresponded results are shown in Fig.2, where the longitudinal coordinate still represents the adsorption capacity, Q(mg/g), and the abscissa represents the continuous changes of molar ratio of HA to RE.
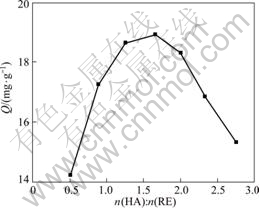
Fig.2 Adsortion capacity Q changed with extraction ratio of HA to RE with mole number of HL fixed
In Fig.2, the abscissa point which corresponds the maximal point of Q shows the extraction ratio on this condition is 1?1.5, which indicates the mole ratio of rare earth to Cyanex272 in the complex is 1?1.5. As the mole ratio of rare earth to total extractants is 1?3, the mole ratio of rare earth to P507, then, must be 1?1.5. Therefore, the value of x in reaction (1) can be deduced as 1.5, and reaction (1) can be expressed as follows:
![]() =
=
![]() (2)
(2)
In reaction (2), extractants Cyanex272 and P507 participate in coordination in a molar ratio of 1?1, which can explain well why under this condition the maximum distribution ratio can be obtained.
3.1.4 Slope method for determining extraction ratio in complex
As we know, the distribution ratio D equals the sum of distribution ratio D1 with single P507 extractant, D2 with single Cyanex272 and D12 (synergistic distribution ratio) under the same extraction conditions. That is, D= D1+ D2+D12, and D12=D-D1-D2.
In accordance with the procedures described in section 2.2.3, a group of experiments has been carried out with pH values in aqueous phase changing, consequently a series of D and the corresponding D12 values have been obtained.
Fig.3 shows the relationship between lgD12 and PH. From Fig.3 it can be found that the slope of the lgD12—pH curve is 3.02, approximated to 3. This suggests when Cyanex272-P507 impregnates resin adsorbed one rare earth ion, three H + ions are released, which coincides with the reaction (2).

Fig.3 Relationship curve of lgD12 changed with pH value
In order to further study the extraction mechanism, another two groups of experiments have been carried out with this method. In each of them, content of one extractant changed successively while content of the other extractant was fixed as 0.1 mmol/g. And all the experiments were exerted at the same PH value, that is, 3. Then, another two series of D and D12 values have been obtained, respectively. Choosing lgD12 to lg[HA](r) (or to lg[HL](r)) to make their respective graph, the results are shown in Fig.4 and Fig.5, respectively.
In these two graphs, the slopes of the curves are 1.52 and 1.53, respectively, both regressed to 1.50. This result also coincides with reaction (2).
3.2 Infrared spectrum analysis
3.2.1 Cyanex272-P507 solvent extracting rare earth
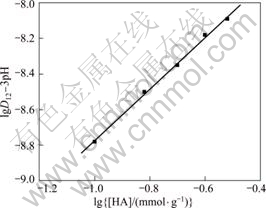
Fig.4 Relationship curve of lgD12 to lg[HA] with pH value fixed at 3
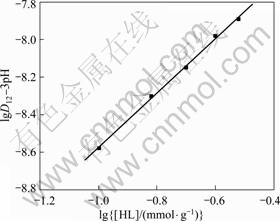
Fig.5 lgD12 changed with lg[HL] with pH value fixed at 3
By comparing the IR spectra of Cyanex272-P507 (Fig.6(a)) and single Cyanex272 (Fig.7(a)) solvents, it can be found that in Fig.6(a), there is a characteristic absorption peak of O—H bond at wavenumber of 3 733.9 cm-1, however, in Fig.7(a) it dose not appear. It is not difficult to find that this peak is formed by the O—H bond in P507. Thus, we can deduce that Cyanex272 and P507 have formed a binary similar synergistic extraction system. In Fig.6(a), it also can be found that the O—H stretching vibration band has moved to the lower wavenumber of 2 720-2 310 cm-1, which was caused by the hydrogen bonds in dimer of the synergistic extraction system; similarly, the P=O band in Cyanex272-P507 has moved forward by about 5.7 cm-1 compared with that in Cyanex272, which was resulted from the weakening of alkoxies (electron-withdrawing) in P507.
After extraction, the O—H characteristic absorption peak disappears (in Fig.6(b)), and the P=O vibration peak moves from 1 174.6 cm-1 to 1 162.2 cm-1, indicating “red shift” occurred. This also suggests that the synergistic extractant formed by Cyanex272 and P507 exists in the solution in a form of dimer. When this extractant extracts rare earth, the O—H bonds break to
form O—RE bonds, and RE3+ bonds with the lone-pair electron in P=O ligand.
3.2.2 Cyanex272-P507 impregnated resin extracting rare-earth
Fig.8 shows the IR spectra of Cyanex272-P507 inpregnated resin before and after extracting rare earth. Different from Fig.6, there is no characteristic absorption peak of O—H hydroxyl around 2 750-2 600 cm-1 (in Fig.8(a)), indicating that the extractant in Cyanex272- P507 impregnated resin is in a form of monomer.
Comparing with spectra in Fig.8, we can see a slight shift of O—H stretching vibration from 2 365.3 cm-1 in Fig.8(a) to 2 360.8 cm-1 in Fig.8(b), while there is no shift occurred for P=O band. This infers that rare earth ions have replaced H+ in O—H hydroxyl but did not coordinate with P=O ligand, which indicates H+ ions in synergistic extractant have all been replaced by RE3+.
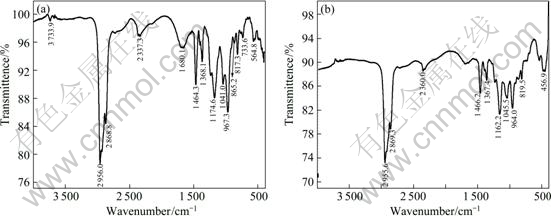
Fig.6 IR spectra of Cyanex272-P507 solvent before (a) and after (b) extracting heavy rare earths
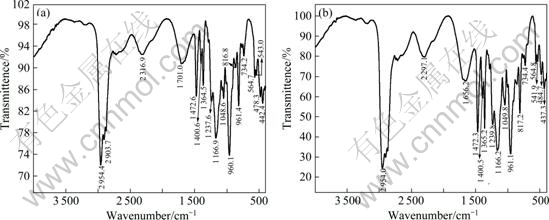
Fig.7 IR spectra of Cyanex272 solvent before (a) and after (b) extracting heavy rare earths

Fig.8 IR spectra of Cyanex272-P507 impregnated resin before (a) and after (b) extracting rare earths
4 Conclusions
1) When Cyanex272-P507 impregnated resin extracts HREE, the molar ratio between Cyanex272-P507 and rare-earth is close to 3, and the chloride ions do not participate in coordination.
2) When Cyanex272-P507 impregnated resin extracts HREE, extractants of Cyanex272 and P507 participate in coordination in a ratio of 1:1, and the molar ratio of rare earth to Cyanex272 or to P507 in the complex both is 1:1.5.
3) The extracting reaction of Cyanex272-P507 impregnated resin for HREE can be expressed as ![]() =
=![]()
![]() . The essence of the reaction is a cation exchange process between the extractant and the heavy rare earth ions.
. The essence of the reaction is a cation exchange process between the extractant and the heavy rare earth ions.
References
[1] LIAO Chun-fa, NIE Hua-ping, JIAO Yun-fen, LIANG Yong. Progress and prospect in separation and purification of rare earth metals with extraction- chromatography [J]. The Chinese Journal of Process Engineering, 2006, 6(z1): 128-132.(in Chinese)
[2] TU Xing, LIAO Lie-wen, YANG Shao-hua, WANG Chun-xiao. The separation technology of the rare earth elements [J]. Hebei Chemical Engineering and Industry, 2003(4): 13-15. (in Chinese)
[3] LI Hong-gui. Hydrometallurgy [M]. Changsha: Central South University Press, 2002: 434-440. (in Chinese)
[4] ZHANG Rui-hua. The property, synthesis, purification and analysis of Cyanex272 [J]. Jiangxi Science, 2001, 19(4): 238-243. (in Chinese)
[5] LIAO Chun-fa, JIAO Yun-fen. Review on research and application of cyanex phosphinic extractants in extractive separation of nonferrous metals [J]. Nonferrous Metals, 2005, 57(4): 76-80. (in Chinese)
[6] MURALIDHARAN S, CAI R, FREISER H. Improved separation of closely related metal-ions by centrifugal partion chromatogaphy [J]. Liquid Chromatogaphy, 1990, 13 (18): 3651-3672.
[7] CUI Da-li. Separation of Tb3+, Dy3+, Ho3+, Er3+ with solvent impregnated resins containing Di-(2, 4, 4-trimethylpentyl) phosphinic acid [J]. Journal of the Chinese Rare Earth Society, 2000, 18(3): 265-267. (in Chinese)
[8] WANG X L, LI W, MENG S L, LI D Q. The extraction of rare earths using mixtures of acidic phosphorus-based reagents or their thio-analogues [J]. Journal of Chemical Technology & Biotechnology, 2006, 81(5): 761-766.
[9] JIA Qiong, LI De-qian, NIU Chun-ji. Synergistic extraction of LaB by mixtures of (1-phenyl-3-methyl-4-benzoyl)-(pyrazolone-5) and neutral organphosphorus extractants [J]. Chinese Journal of Analytical Chemistry, 2004, 32(11): 1421-1425. (in Chinese)
[10] XU Qi-chun, ZHANG Li-xing, YANG Yu-sheng. Extraction of Am3+ and rare earths by DEHDTP combined with TOPO, DPPHEN and TOPS [J]. Nuclear Techniques, 2004, 27(7): 547-550. (in Chinese)
[11] HE Di-ping, HAN Wei-he, WANG Yu. Synergistic extraction of lanthanide by bis (1′-phenyl-3′-methyl-5′-pyrazolone-4′-yl) phthaldione and 8-hydroxyquinoline [J]. Chemical research and application, 2003, (6): 826-828. (in Chinese)
[12] LIU Ying, DENG Zou-guo, XU Ting-hua. Solvent extraction and separation of heavy rare earths with mixed Cyanex272 and HEHEHP [J]. Chinese Journal of Rare Metals, 2000, 24(5): 394-397. (in Chinese)
[13] WANG Zhen-hua, LI Jia-ling, YU Chang-gen. Performance of extractant C272 and C274 for separating rare earths [J]. Chinese Rare Earths, 1999, 20(1): 15-18. (in Chinese)
[14] LIU Ming, ZHOU Yong-mao. Removal of Fe(Ⅲ) from sulphate solutions by synergistic extraction using N235-TBP mixed solvent systems [J]. The Chinese Journal of Nonferrous Metals, 2005, 15(10): 1648-1654. (in Chinese)
[15] NAYAK D, LAHIRI S, DAS N R. Synergistic extraction of Neodymium and carrier-free ion-exchange by the mixture of HDEHP and PC88A [J]. Radioanalytical and Nuclear Chemistry, 1999, 240(2): 555-560.
[16] SALEH M I, BARI M F, SAAD B. Solvent extraction of lanthanum from acidic nitrae-acetato medium by Cyanex272 in toluene [J]. Hydrometallurgy, 2002, 63(1): 75-84.
[17] MURALI M S, MATHUR J N. Use of a mixture of TRPO and TBP for the partitioning of actinides from high-level waste solutions of purex origin and its comparison with CMPO and other phosphous-based extractants [J]. Solvent Extr Ion Exch, 2001, 19(1): 61-77.
[18] LIAO Chun-fa, JIAO Yun-fen, QIU Ding-fan. Characteristics of adsorbing heavy rare earth elements with phosphinic(phosphoric) impregnated resin [J]. The Chinese Journal of Process Engineering, 2007, 7(2): 268-272. (in Chinese)
[19] LIAO Chun-fa, JIAO Yun-fen, QIU Ding-fan, XUE Ji-lai. Separating of thulium, ytterbium, lutetium by cooperative extraction chromatography with Cyanex272-P507 impregnated resin [J]. Journal of the Chinese Rare Earth Society, 2007, 25(2): 249-252. (in Chinese)
Foundation item: Project(50764003) supported by the National Natural Science Foundation of China; Project(0450065) supported by the Natural Science Foundation of JiangXi Province, China
Corresponding author: LIAO Chun-fa; Tel:+86-797-8312243; E-mail: Liaochfa@163.com
DOI: 10.1016/S1003-6326(09)60330-7


