Effects of rare earth addition on sintering process and dielectric property of cordierite based glass-ceramics
CHEN Guo-hua(陈国华)1, 2, LIU Xin-yu(刘心宇)1, 2
(1. Department of Materials Science and Engineering,Guilin University of Electronic Technology, Guilin 541004, China;
2. School of Materials Science and Engineering,Central South University, Changsha 410083, China)
Abstract:
The effects of rare earth oxide on the sintering and dielectric property of cordierite-based glass-ceramics with non-stoichiometric composition prepared by quenching of molten droplets were investigated. The results show that the addition of rare earth oxide can lower the sintering temperature of cordierite glass-ceramics, improve the densification process and obviously reduce sintering activation energy. It is found that the densification of cordierite-based glass-ceramics is a liquid phase sintering process. The dielectric constant of the sintered compacts enhances with the increase of the density. When the sintering temperature is identical, the rare earth addition is found to have a noticeable effect on the dielectric loss of glass-ceramics. The properties of the glass-ceramics containing rare earth oxide appear to be correct for low firing temperature substrates.
Key words:
sintering; rare earth; cordierite glass-ceramics; dielectric property CLC number: TG171;
Document code: A
1 INTRODUCTION
In the past, alumina has been extensively used as substrate material because of its good thermal and mechanical properties. However, its high sintering temperature(>1500℃) restricts the associated conductor materials to high melting, costly and generally high-resistivity metals, and its high dielectric constant (about 9) introduces significant signal propagation delay. Therefore, alternative materials, i.e. low firing temperature substrate with low sintering temperature (〈1000℃) and low dielectric constant (about 5), are required[1-3].
Low sintering temperature enables copper-based (or silver-based) conductor materials to be employed as substrates. If a copper-based (or silver-based) conductor is used, it can fulfill the high signal propagation speed[4] and wiring density in electronic devices because copper (or silver) has low resistivity. It is expected that a substrate having a low dielectric constant will also diminish signal propagation delay.
Cordierite (2MgO·2Al2O3·5SiO2) based glass-ceramics are attractive materials for preparing low firing temperature substrates due to their low dielectric constant and their matching thermal expansion coefficient of single crystal silicon[5-7]. It is difficult to obtain dense glass-ceramics below 1000℃ because the cordierite-based glasses have high viscosity and narrow sintering temperature range[6]. Because glass powder sintering proceeds by viscous flow[8], the decreasing glass viscosity shows good effect on its sinterability. In order to fabricate dense glass-ceramics, it may be a critical factor to select adequate glass composition and flux which reduces glass viscosity. Because alkaline ions included in the substrate result in the deterioration of the properties, such as large thermal expansion and higher dielectric constant and dielectric loss, alkali oxides which are generally a good flux, cannot be used.
Zdanieski et al[9] added CeO2 and TiO2 as a nucleating agent to the SiO2-rich cordierite-based glass, and studied the effects of CeO2 on bulk crystallization in bulk glasses. The effect of CeO2 on the glass sintering process, however, has not been reported. In 2001, Sohn et al[10] investigated the crystallization behavior in the glass system MgO-Al2O3-SiO2 containing CeO2, and found that CeO2 can not only reduce the glass transition temperature, which implied that the addition of CeO2 can decrease the glass viscosity, and but also has a little influence on the thermal expansion coefficient. SHI et al[11] pointed that rare earth oxide played an important role in the preparation of cordierite ceramic by sol-gel method and lowered the sintering temperature to the level of about 1000℃. ZHANG et al[12] reported that the addition of rare earth promoted the sintering, microstructure stabilization, and improved the properties of MoSi2 materials. However, there is little information about the effect of rare earth as an addition on sintering process and dielectric properties of cordierite glass-ceramics.
Hence, in the present work, we aimed at understanding the effect of rare earth on sintering and dielectric properties of non-stoichiometric cordierite-based glass-ceramics.
2 EXPERIMENTAL
Melts were prepared from reagent-grade batches of SiO2, Al2O3, MgO, H3BO3, NH4H2PO4, and rare earth oxide (abbreviated as RE). The glasses were based on 95% (mass fraction) of the non-stoichiometric cordierite composition, which is equivalent to 16.0%MgO+26.0%Al2O3+53.0%SiO2, plus a total of 5% B2O3 and P2O5, doped with 4% RE and without RE, respectively. B2O3 and P2O5 are chosen for lowering the melting temperature and achieving homogenized melt. A glass batch of homogeneous mixture was prepared by ball milling, melted in alumina crucibles at 1560℃ for 4h in an electric furnace and then quenched in distilled water to form frits, which were crushed to pass through a 50 mesh sieve and milled in distilled water with agate balls for 50h. The mean particle size was about 3μm. The glass powders were dried and granulated by passing through a 100 mesh sieve. The samples were shaped with a pressure of 110MPa into 4mm×5mm×40mm for the evaluation of the density and 18mm diameter and about 1.5mm thickness for the evaluation of the dielectric properties respectively, and then sintered at different temperatures. The microstructure for fractured surface of glass-ceramics was observed with scanning electron microscope (Model: JSM-5610LV). Density and apparent porosity of the samples were measured by water displacement techniques (Archimedess principle). The dielectric property was measured with an impedance analyzer (HP-4278A, Agilent).
3 RESULTS
3.1 Crystallization behavior
Fig.1 shows DTA traces for the glasses investigated, with a heating rate of 5K/min. Only one exothermic peak appear between 960-1020℃ for the glasses with or without RE. The glass transition temperature (tg=864℃) of the glass with RE is lower than that of the glass without RE(tg=908℃ ), which can improve the sinter-ability of glass powder because viscous flow occurs at lower temperature. As a whole, both the crystallization onset temperature and the maximum point of the exothermic peak shift slightly towards higher temperatures with the increase of RE addition. This shift may enhance sinter-ability because crystallization suppresses densification during glass powder sintering.
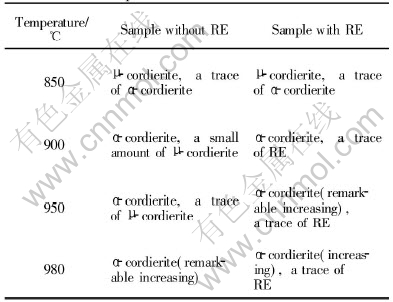
The XRD patterns of the samples sintered at different temperatures are shown in Fig.2. The crystal phase components of two samples sintered at different temperatures are shown in Table 1.
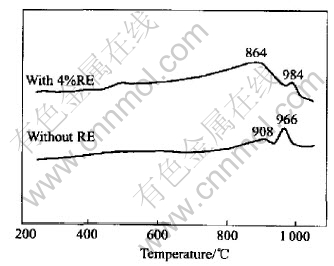
Fig.1 DTA traces of glasses with RE and without RE at 5K/min
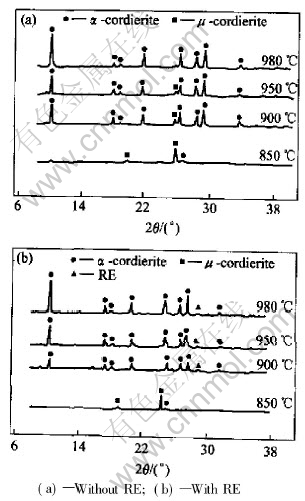
Fig.2 XRD patterns of samples sintered at different temperatures for 2h
3.2 Density and porosity of sintered compacts
The relative density of the sample doped with RE is obviously higher than that of the sample
Table 1 Crystal phase components of samples with and without RE
without RE when sintered at the temperature of 800-980℃, as shown in Fig.3. At 900℃, its relative density is above 97.5%, and is slightly rising with the increase of sintering temperature. This implies that the densification temperature of the sample doped with RE is about 900℃. Because the sintering temperature for the sample without RE is often above 1000℃[13], the addition of RE remarkably decreases the sintering temperature of cordierite-based glass ceramics.
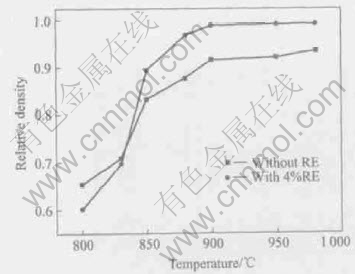
Fig.3 Relative densities of compacts sintered at different temperatures
To calculate the sintering apparent activation energy, the porosity of two samples sintered at different temperature and time is shown in Table 2 and Table 3, respectively.
3.3 Dielectric properties of sintered compacts
The relations of dielectric constant of two samples to sintering temperature are shown in Fig.4. The relations of dielectric loss of two samples to sintering temperature are shown in Fig.5. When the two kinds of materials are sintered at 800-980℃, the dielectric constant of the sample doped
Table 2 Porosity of sample without RE at different temperatures and times
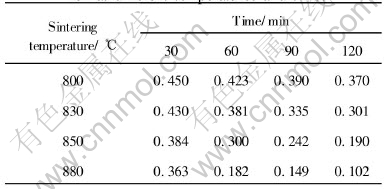
Table 3 Porosity of sample with RE at different temperatures and times
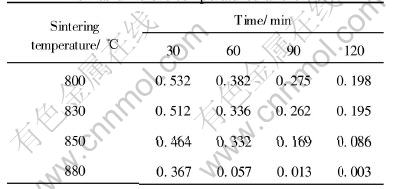
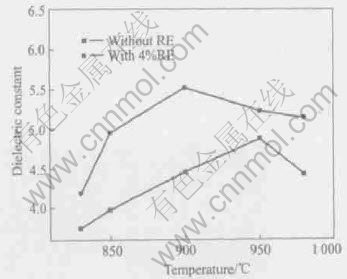
Fig.4 Dielectric constant of samples sintered at different temperatures

Fig.5 Dielectric loss of samples sintered at different temperatures
with RE is obviously higher than that of the sample without RE, but the dielectric loss of the sample doped with RE is obviously lower than that of the sample without RE. The maximum dielectric constant of the sample with RE was obtained when sintered at 900℃, but that of the sample without RE at 950℃.
4 DISCUSION
4.1 Sintering mechanism and sintering apparent activation energy
Fig.6 shows the SEM micrographs of two samples sintered at different temperatures, respectively. The coalescence of glass powder particles is easily seen in Fig.6(a) for the sample without RE. For the sample with RE, pore elimination and the enhancement of densification are promoted by the addition of RE as shown in Fig.6(b). With the increase of sintering temperature, the viscous flow of liquid phase occurs, the coalescence of glass particles and pore elimination are more easily seen in Figs.6(c) and (d). However, the densification of the sample with RE is much more apparent and fewer pores (close pores) are recognized.
Because the viscosity of cordierite-based glass is determined by the amounts of added RE and heat treatment temperature and the viscosity is inversely proportional to the sintering temperature, higher sintering temperature results in easier densification. From SEM micrographs of fractured surfaces of samples and above analysis, Newtonian viscosity flow is found to be the predominant sintering mechanism in the present work[14].
According to the mechanism of viscous flow, the kinetic equation for the sintering process of glass powder can be approximately expressed as[15]
dP/dt=-3γP/ (2rη)(1)
It can be simplified to
-lnP=3γt/(2rη)+B(2)
-lnP=Kt+B(3)
K=3γ/(2rη)(4)
Moreover, the viscosity of glass is dependent on the sintering temperature, the relationship between the viscosity and the sintering temperature corresponds to the Arrhenius equation[15]:
η=A·eE/RT(5)
From Eqn.(4), Eqn.(5) can be simplified to
lnK=C- E/ RT(6)
where P is the porosity of the compacts sintered at different temperatures, γ is the surface tension of the glass phase, r is the average size of the glass powder particles, η is the viscosity of the glass, B is the intercept of the straight line, t is sintering time, K is the sintering kinetic constant representing the linear slope, which can be determined by drawing the plot (as shown in Fig.7(a) and 7(b)), R is the gas constant, T is the absolute temperature, and E is the sintering apparent activation energy. Although the surface tension γ is a
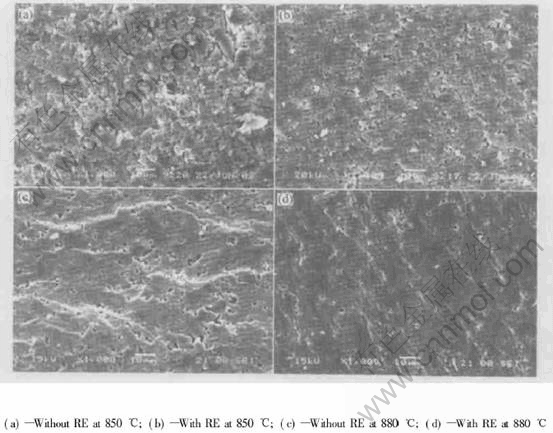
Fig.6 SEM photographs of samples sintered at different temperatures
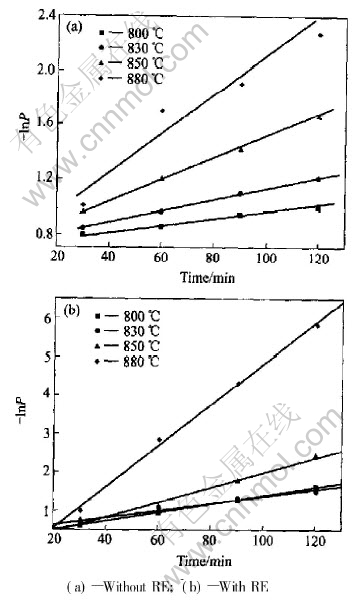
Fig.7 Relation between -lnP and time at different temperatures
function of temperature, the change of surface tension is very small and can essentially be considered as a constant. For the case where the sintering time and the radius of particle are essentially the same, C can be considered as a constant.
From Fig.7, the sintering kinetic constant K of two samples at different temperatures is obtained respectively. The sintering apparent activation energy E of the sample without RE and with RE sintered at 800-900℃ can be determined to be 228.5kJ/mol and 184.0kJ/mol, respectively, as shown in Fig.8. The results also correspond to the changes of the relative density of samples as shown in Fig.3.
4.2 Effect of addition of RE on dielectric properties of glass-ceramics
Generally, ionic polarization and electronic polarization are the two major polarization mechanisms of glass ceramics. Electronic polarization has the most significant effect at high frequency, while ionic polarization plays an important role at low frequency. According to the Clausius-Mossotti equation[16], the dielectric constant is a function of the product of the number of polarizable atoms per
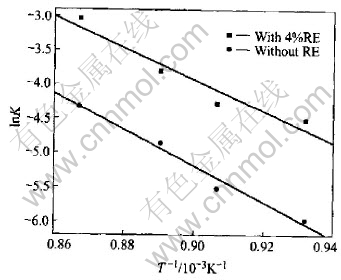
Fig.8 Relation between lnK and 1/T
unit volume and the polarizability of the atoms. Moreover, it has been found that the determination of the dielectric constant in cordierite glass ceramics is the number of polarizable atoms per unit volume. The number of polarizable atoms per unit volume is proportional to the density of the sample. In other words, the dielectric constant of the sample strongly depends on its density[16]. For sintered samples, the higher the density, the higher the dielectric constant. Besides the density of samples, another factor affecting the dielectric constant of the sintered samples is the content of α-cordierite. With the content increase of α-cordierite, the sintered sample has a lower dielectric constant[17].
The dielectric loss of sintered samples is strongly determined by porosity[18, 19]. In other words, it is proportional to the magnitude of porosity.
Based on the above analysis, it is known that the addition of RE increases the density of samples at the same sintering temperature, which means the porosity is reduced. This is why the dielectric constant of the sample with RE is higher than that of the sample without RE and the dielectric loss of the sample with RE is much lower than that of the sample without RE. However, the dielectric constant of two samples at above 900℃ and 950℃ is decreasing because the two samples have much amount of α-cordierite by X-ray analysis ( as shown in Fig.2 ), which has a lower dielectric constant.
5 CONCLUSIONS
1) Adding rare earth oxide can obviously reduce sintering activation energy of cordierite-based glass-ceramics and accelerate its sintering process. It decreases the sintering temperature of glass-ceramics to the level of 900-950℃.
2) The densification for cordierite-based glass powders in the present work is mostly the liquid sintering process.
3) The addition of rare earth oxide increases the density of sintered compacts and slightly elevates its dielectric constant. When the sintering temperature is identical, the rare earth oxide is found to have a noticeable effect on the dielectric loss of glass-ceramics.
REFERENCES
[1]Tummala R R, Kumar A H, McMillan P W. Glass-ceramics structures and sintered multilayer substrates thereof with circuit patterns of gold, silver or copper[P]. US 4301324. 1981.
[2]Shimade Y, Ustumi K, Suzuki M, et al. Low firing temperature multilayer glass-ceramics substrate[J]. IEEE Trans Compon, Hybrids Manuf Technol, 1983, 6: 382-388.
[3]Tummala R R. Ceramic and glass-ceramic packaging in the 1990s [J]. J Am Ceram Soc, 1991, 74(5): 905-908.
[4]Delaney K, Barrett J, Barton, et al. Characterization and performance prediction for integral capacitors in low temperature co-fired ceramic technology [J]. IEEE Trans Advanced Packaging, 1999, 22(1): 68-77.
[5]Bridged Holland D R, McMillan P W. Development of the Alpha-cordierite phase in glass-ceramics for use in electronic devices[J]. Glass Technology, 1985, 26(6): 286-292.
[6]Sarah H K, Ananda H K, Wynn Herron. Cordierite glass-ceramics for multiplayer ceramic packaging[J]. Am Ceram Soc Bull, 1993, 72(1): 90-95.
[7]Okuyama M, Fukui T, Sakurai C. Phase transformation and mechanical properties of B2O3-doped cordierite derived from complex-alkoxide[J]. J Mater Sci, 1993, 28(16): 465-470.
[8]Mei S, Yang J, Ferreira J M F. The densification and morphology of cordierite-based glass-ceramics [J]. Meter Lett, 2001, 47: 205-211.
[9]Zdaniewski W. DTA and X-ray analysis study of nucleation and crystallization of MgO-Al2O3-SiO2 glasses containing ZrO2, TiO2 and CeO2 [J]. J Am Ceram Soc, 1975, 58(5-6): 163-169.
[10]Sohn S B, Choi S Y. Crystallization behavior in the glass system MgO-Al2O3-SiO2; influence of CeO2 addition [J]. J Non-Cryst Solids, 2001, 282: 221-227.
[11]SHI Z M, LIANG M, ZHANG Q, et al. Effect of cerium addition on phase transformation and microstructure of cordierite ceramics prepared by sol-gel method[J]. J Mater Sci, 2001, 36: 5227-5230.
[12]ZHANG Hou-an, LIU Xin-yu, NING Ai-lin, et al. Rare earth actived sintering of MoSi2 and its electric conductivity[J]. Trans Nonferrous Met Soc China, 2001, 11(1): 141-144.
[13]Hu Yi, Tsai H T. Compositional effect on the crystallization of the cordierite-type glasses[J]. J Mater Sci, 2001, 36: 123-129.
[14]Yang C F, Cheng C M. The influence of B2O3 on the sintering of MgO-CaO-Al2O3-SiO2 composite glass powder[J]. Ceramics International, 1999, 25: 383-387.
[15]LI Jin-ping, YANG Fu, GUO Xing-lin. Study on sintering and elastic modulus of porous glass-ceramics [J]. J Chin Ceram Soc, 1999, 27(3): 370-375. (in Chinese)
[16]Chen L S, Fu S L. Densification and dielectric properties of cordierite-lead borosilicate glasses[J]. Jpn J Appl Phys, 1992, 31(12A): 3917-1.
[17]Wu J M, Huang H L. Effect of crystallization on microwave dielectric properties of stoichiometric cordierite glasses containing B2O3 and P2O5 [J]. J Mater Res, 2000, 15(1): 222-227.
[18]Camerucci M A, Urretavizcaya G, Castro M S, et al. Electrical properties and thermal expansion of cordierite and cordierite-mullite materials[J]. J Euro Ceram Soc, 2001, 21: 2917-2923.
[19]Penn S J, Alford N M, Templeton A, et al. Effect of porosity and grain size on the microwave dielectric properties of sintered alumina[J]. J Am Ceram Soc, 1997, 80(7): 1885-1888.
Foundation item: Project (0339066) supported by the Natural Science Foundation of Guangxi Province
Received date: 2004-05-25; Accepted date: 2004-09-17
Correspondence: CHEN Guo-hua, Associate professor; Tel: +86-773-5601434; E-mail: chengh@gliet.edu.cn


