Trans. Nonferrous Met. Soc. China 25(2015) 2095-2102
Electrosynthesis of Al/Pb/α-PbO2 composite inert anode materials
Gang HU1,2, Rui-dong XU1,2, Shi-wei HE2, Bu-ming CHEN2, Hai-tao YANG3, Bo-hao YU2, Qiang LIU2
1. State Key Laboratory of Complex Nonferrous Metal Resources Clean Utilization, Kunming University of Science and Technology, Kunming 650093, China;
2. Faculty of Metallurgical and Energy Engineering, Kunming University of Science and Technology, Kunming 650093, China;
3. State Key Laboratory of Multi-Phase Complex Systems, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China
Received 16 August 2014; accepted 10 January 2015
Abstract:
The α-PbO2 deposition layers were prepared on the surface of Al/Pb substrates by constant current electrosynthesis from an alkaline bath, and Al/Pb/α-PbO2 composite inert anode materials were obtained. The effects of the bath composition and bath temperature on the electrosynthesis of α-PbO2 were investigated by means of anodic polarization method, the phase structures and surface microstructures of Al/Pb and α-PbO2 deposition layers were tested by means of X-ray diffraction (XRD) and scanning electron microscopy (SEM), respectively. The experimental data have shown that the process of α-PbO2 formation have several stages. The appropriate conditions can effectively improve the formation rate of α-PbO2 and avoid the occurrence of oxygen evolution reaction. The α-PbO2 deposition layer obtained in alkaline bath possesses rhombic structure, and it is composed of well developed spherical unit cells.
Key words:
Al/Pb substrate; α-PbO2; electrosynthesis; anodic polarization;
1 Introduction
The anode material is one of the critical components in the process of zinc electrowinning. The direction and kinetics of electrode process, structure of electrolytic cell and power consumption are closely related to not only the electrolyte composition but also the property of the electrode materials. Only some inert materials such as Pb and Pb-based alloys, PbO2, Fe3O4, Pt and Pt group metal oxides can exist steadily in the electrowinning process which mostly happened in the sulfuric acid medium, and its main anode reaction is the oxygen evolution. In addition, the corrosivity of sulfuric acid and the oxidizing ability of new generated oxygen are strong. PbO2 has attracted extensive attention due to its good electrochemical performance and stability in the acidic medium. At present, a new type of metal substrate/ α(β)-PbO2 composite inert anode materials is the research hotspot of hydrometallurgy. The thermal decomposition coating is served for the underlayer, such as silver or lead silver alloys, palladium oxides, tin-antimony oxides and titanium-tantalum composite oxides. The α-PbO2 is used for the interlayer between the Ti substrate and β-PbO2. In such way, the stability and corrosion resistance of Ti/α(β)-PbO2 composite inert anode materials can be effectively improved as the Ti substrate passivation can be inhibited and the stress between the coatings can be reduced [1-4]. However, the preparation of Ti/α(β)-PbO2 composite inert anode materials is complicated with high cost. Moreover, the passivation of Ti substrate cannot be solved fundamentally. So, the application of Ti/α(β)-PbO2 in zinc electrowinning is unsuitable [5,6]. Some hydrometallurgy researchers have put forward to Al/Pb/α(β)-PbO2 composite inert anode materials in recent years [7,8].
The α-PbO2 working as the interlayer has remarkable impacts on the performance of Al/Pb/α(β)- PbO2 composite inert anode materials. Therefore, it is significant to study the electrosynthesis of α-PbO2 on Al/Pb. The study of Al/Pb/α-PbO2 shows that there is PbO impurity phase existing in α-PbO2, and the higher the current density of α-PbO2 electrochemical synthesis is, the greater the roughness and porosity of the deposition layer are [9]. Furthermore, the factors of the Pb(II) concentration, bath temperature and electrosynthesis time can also influence the electrosynthesis of α-PbO2. The α-PbO2 deposition layers prepared at room temperature usually present loose structure of clusters, but the compact layers can be obtained at 40 °C [9,10]. When α-PbO2 is electrochemically synthesized in alkaline bath, tender stirring can prevent the generation of reddish brown and loosely bound PbO. And the α-PbO2 synthesized may partly transform into reddish brown PbO when Pb(II) is insufficient in bath [11]. The PbO2 synthesized through constant current presents more compact structure and more excellent performance than that synthesized through constant voltage, as the former method can control the PbO2 electrosynthesis rate by adjusting the current density [12,13].
In this research, the α-PbO2 deposition layers were prepared on the surface of Al/Pb substrates by constant current electrosynthesis from an alkaline bath, and Al/Pb/α-PbO2 composite inert anode materials were obtained. The effects of the NaOH concentration, the addition amount of PbO and the bath temperature on the electrosynthesis of α-PbO2 were investigated by means of anodic polarization method, the phase structures and surface microstructures of Al/Pb substrates and α-PbO2 deposition layers were tested by means of X-ray diffraction (XRD) and scanning electron microscopy (SEM), respectively. The research results are helpful to provide theoretical basis and technical support for the preparation of Al/Pb/α-PbO2 composite inert anode materials.
2 Experimental
The Al sheets with dimensions of 20 mm × 40 mm × 2 mm were used as the substrates of the inert anode materials. The pretreatment of Al substrates was performed by the procedures such as polishing, sand blasting, chemical degreasing, and zinc galvanizing twice. Then, the Al/Pb was obtained by cathodic electrochemical synthesizing Pb underlayer on Al with double-pulse method. Finally, the Al/Pb/α-PbO2 was obtained by anodic electrochemical synthesizing α-PbO2 on Al/Pb at constant current.
The bath components of cathodic electrochemical synthesis Pb underlayer were as follows: 180 g/L Pb(AC)2, 220 mL/L HBF4, 20 g/L H3BO3, 1 g/L gelatin, 0.2 g/L thiourea, 5 mL/L polyethylene glycol. The parameters of the double-pulse were as follows: the forward and reverse duty ratios of pulse were 10% and 30%, respectively; the average current densities of forward and reverse pulse were 4 A/dm2 and 0.4 A/dm2, respectively; the forward and reverse working time of pulse were 200 ms and 20 ms, respectively. The bath temperature was 35 °C and the electrosynthesis time was 0.5 h with the pH smaller than 1. The Pb sheet was used as the anode material and the Al sheet was used as the cathodic material.
The electrosynthesis of α-PbO2 on the surface of Al/Pb was performed on a DDZ-20A/12V rectifier. The bath components of α-PbO2 electrosynthesis contained 20-50 g/L PbO and 120-160 g/L NaOH, and the bath temperature was 25-45 °C. The Al/Pb was used as the anode material and the stainless steel sheet was used as the cathodic material. In addition, the red sediments generated during the preparation of α-PbO2 baths were removed by filtration.
All the electrochemical tests of anodic polarization, galvanostatic polarization and quasi-steady state polarization were performed on a PARSTAT2273 electrochemical workstation with three-electrode system. The working electrode, whose effective area was 1 cm2, was dealt with wax-sealing. The reference electrode was saturated calomel electrode (SCE). The counter electrode was graphite electrode. The reference electrode and the working electrode were linked by luggin capillary filled with KCl and agar. In addition, the distance between the capillary and the working electrode surface was about 2d (d is the diameter of the capillary) to minish the solution resistance and avoid shielding the electrode surface to the greatest extent. The quasi-steady state polarization curve was obtained under the condition that the current density at 0.1-0.8 V was stable for more than 2 min. All the potentials shown in the figures were against the SCE.
The concentration of Pb(II) in α-PbO2 bath was tested by a Z-2310 atomic absorption spectrometer. The phase composition and surface microstructure characteristics of the Pb underlayers and α-PbO2 deposition layers were tested by a D/Max-2200 X-ray diffractometer (XRD) and a VEGA3SBH scanning electron microscope (SEM), respectively.
3 Results and discussion
3.1 Effect of NaOH concentration on α-PbO2 electrosynthesis
The anodic polarization curves of the electrosynthesis of α-PbO2 on Al/Pb at 25 °C are shown in Fig. 1.
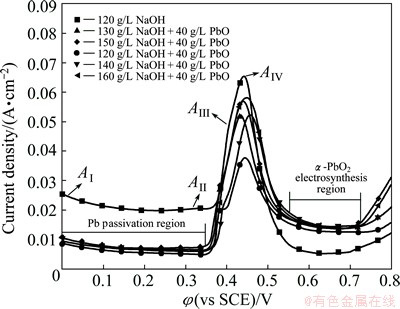
Fig. 1 Anodic polarization curves of Al/Pb in alkaline baths at 25 °C (Scan rate: 10 mV/s)
As can be seen from Fig. 1, the anodic polarization curve of Al/Pb in 120 g/L NaOH pure solution shows four peaks. Peak AI appears at the beginning, and then the current density gradually decreases and reaches a steady value as the Pb underlayer can be oxidized to form a PbO passivating film in alkaline bath. The Pb passivation can be shown as Reaction (1) [14,15]. The PbO film may break during its growth process, thus promoting the Pb oxidation again. Therefore, the current density increases slightly and peak AII appears at about 0.32 V.
Pb+2OH--2e=PbO+H2O (1)
The current density increases rapidly and peak AIII appears at about 0.4 V when the potential reaches 0.36 V. Peak AIII may be the formation peak of Pb3O4 by the oxidation of PbO according to Reaction (2). The appearance of subsequent peak AIV (about at 0.43 V) reflects the formation of α-PbO2 [16,17], which can be expressed by Reactions (3) and (4). Then, the current density drops rapidly and achieves stability (about 5 mA/cm2) as the potential is greater than 0.43 V. This is because the electrosynthesis rate of α-PbO2 may slow down when it reaches the maximum, and the reaction nearly stops after an α-PbO2 film generates on the Pb surface. However, the α-PbO2 film is loose and falls off easily. Thus, the Pb or Pb oxides with intermediate state near the cracks could be continuously oxidized to form α-PbO2 slowly. When the potential is greater than 0.7 V, the current density rises exponentially caused by oxygen evolution. The corresponding reaction is shown as Reaction (5).
3PbO+2OH--2e=Pb3O4+H2O (2)
Pb3O4+4OH--4e=3α-PbO2+2H2O (3)
PbO+2OH--2e=α-PbO2+H2O (4)
4OH--4e=O2+2H2O (5)
The anodic polarization curves of Al/Pb in different α-PbO2 baths (40 g/L PbO, 120-160 g/L NaOH) show that, the current densities of Pb passivation and peak AIV (the formation peak of α-PbO2) decrease significantly, but the initial oxygen evolution potential increases. This is because, the Pb(II) (HPbO2-) exists in α-PbO2 baths and the concentration of OH- ions in them is lower than that in 120 g/L NaOH pure solution. These can be understood by Reactions (6) and (7). Therefore, the Pb oxidation rate decreases and the oxygen generation reaction becomes more difficult. In addition, the current density after peak AIV drops slowly due to the electrosynthesis of α-PbO2, and the current density at 0.55-0.72 V is obviously higher than that in 120 g/L NaOH pure solution. Particularly, the peak AII becomes not so obvious, which may indicate that the primary cause of the fracture of PbO film is the high concentration of OH- ions.
The electrosynthesis of α-PbO2 can be expressed as follows [18]:
PbO+OH-=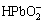 (6)
(6)
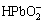 +OH--2e=α-PbO2+H2O (7)
+OH--2e=α-PbO2+H2O (7)
The above analysis shows that, the anodic polarization curves of Al/Pb in α-PbO2 baths can be roughly divided into four parts. They are as follows: 0-0.35 V is the Pb underlayer passivation region, 0.35-0.55 V is the mixed region that the Pb passivation proceeds simultaneously with the α-PbO2 synthesis, 0.55-0.72 V is the α-PbO2 electrosynthesis region, and 0.72-0.80 V is the mixed region that the α-PbO2 electrosynthesis occurs simultaneously with oxygen evolution (oxygen evolution region for short). It can be deduced that when electrosynthesis of α-PbO2 on Al/Pb takes place in the bath, the surface of Pb underlayer firstly is passivated to form a poor conductive PbO film. Then, the electrosynthesis of α-PbO2 proceeds simultaneously with step by step oxidation of PbO (PbO→Pb3O4→α-PbO2 or PbO→α-PbO2). The continual α-PbO2 deposition layer is mainly formed by the oxidation of Pb(II) in the bath after the Pb surface is covered by an α-PbO2 film.
It can also be seen from Fig. 1 that, the current densities of Pb underlayer passivation (0-0.35 V), α-PbO2 electrosynthesis (0.55-0.72 V) and peak AIV increase with increasing the NaOH concentration in different α-PbO2 baths. And the initial oxygen evolution potential decreases from about 0.76 to 0.72 V. It is mainly because the increase of NaOH concentration is beneficial to Reaction (6), thus increasing the Pb(II) concentration. And the concentration of OH- ions also increases with increasing the NaOH concentration. Therefore, the rates of Pb passivation and α-PbO2 electrosynthesis increase, and the oxygen evolution becomes easier. This can be confirmed by the testing of Pb(II) concentration in every α-PbO2 bath using atomic absorption spectrophotometer. The Pb(II) concentration increases with increasing the NaOH concentration, as shown in Table 1.
Table 1 Pb(II) concentration in α-PbO2 baths with 40 g/L PbO and different NaOH concentrations

Furthermore, the remaining amount of the red sediments produced during the preparation of α-PbO2 bath decreases with increasing the NaOH concentration. This indicates that all the baths are saturated with PbO, and the PbO amount of dissolved is the maximum when the NaOH concentration is 160 g/L. However, the current density increments of peak AIV and α-PbO2 electrosynthesis obtained in 160 g/L NaOH are not obvious compared with those in 150 g/L NaOH. Therefore, the continual increase of NaOH concentration in the bath has no obvious significance to the increase of Pb(II) concentration, which will even cause severe oxygen evolution due to the unsaturation of PbO when NaOH reaches 160 g/L. Therefore, it is suitable to keep the NaOH concentration in the bath at 160 g/L.
3.2 Effect of PbO addition amount on α-PbO2 electrosynthesis
The anodic polarization curves tested in the bath with 160 g/L NaOH and 20-50 g/L PbO are shown in Fig. 2. The current densities of Pb passivation and peak AIV obtained in the bath with 20 g/L PbO are obviously higher than the others. However, the α-PbO2 electrosynthesis current density and the initial oxygen evolution potential (about 0.68 V) in such bath are the lowest. It is mainly because the concentrations of Pb(II) and OH- ions are the lowest and highest in the bath containing 20 g/L PbO, respectively. This can also be confirmed by the complete solution of PbO, and there is no red sediment remaining during the preparation of such bath, i.e., the Pb(II) does not reach saturation. Therefore, the Pb passivation rate is the fastest, but the α-PbO2 synthesis rate is the lowest. When the PbO addition amount is 30-50 g/L, the current density magnitude of each curve has no obvious difference except that the α-PbO2 electrosynthesis current density obtained in the bath with 30 g/L PbO is lightly lower than that obtained in the baths with 40 and 50 g/L PbO. In addition, there is little red sediment remaining during the preparation of the bath containing 30 g/L PbO. However, a few red sediments remain when the PbO addition amount is 40 g/L and more red sediments remain as the PbO addition amount reaches 50 g/L. It can be concluded that the Pb(II) concentration in the bath with 30 g/L PbO is near saturated, and that in the baths with 40 and 50 g/L PbO is fully saturated. In consequence, it is suitable to keep the PbO addition amount in the bath at 40 g/L.
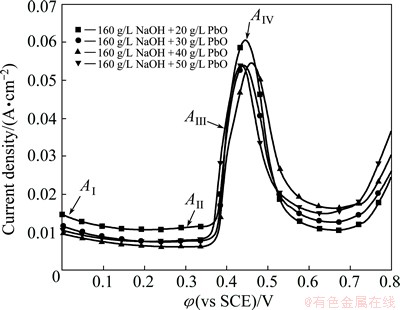
Fig. 2 Anodic polarization curves of Al/Pb in baths at 25 °C (Scan rate: 10 mV/s)
3.3 Influence of bath temperature on α-PbO2 electrosynthesis
The anodic polarization curves of Al/Pb obtained in the bath with 160 g/L NaOH and 40 g/L PbO at different temperatures (25-45 °C) are shown in Fig. 3.
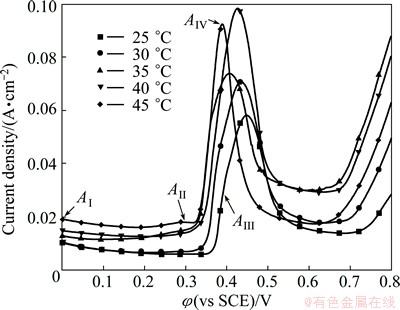
Fig. 3 Anodic polarization curves of Al/Pb at different bath temperatures (Scan rate: 10 mV/s)
With increasing the bath temperature, the current density in the passivation region increases, but the initial oxygen evolution potential decreases gradually for the activity of OH- ions is enhanced in the bath. Thus, the Pb passivation rate increases, and the oxygen evolution reaction becomes easier. In addition, the rupture of the PbO passivition film becomes easier as peak AII becomes marked. Moreover, the current densities of peak AIV and α-PbO2 electrosynthesis gradually increase to the maximum with the temperature increasing to 40 °C. The thermodynamic analysis of α-PbO2 electrosynthesis in alkaline bath shows that the standard electrode potential falls from 0.6392 to 0.5644 V when the bath temperature rises from 25 to 100 °C [20]. Therefore, increasing the bath temperature is beneficial to promoting the α-PbO2 electrosynthesis.
However, the current densities of peak AIV and α-PbO2 electrosynthesis decrease obviously when the temperature exceeded 40 °C, and peak AIV becomes sharp. This indicates that the α-PbO2 electrosynthesis rate slows down under this condition. As the electrosynthesis of α-PbO2 is an exothermic reaction, the faster the reaction runs, the larger the amount of heat releases. As a result, the continual increase of the bath temperature will hinder Pb(II) moving to the surface of the working electrode to oxidize and synthesize α-PbO2 [19]. And the peak AII at 45 °C is more obvious than others, indicating that the PbO passivation film can break down easily, which may weaken the bond between the α-PbO2 deposition layer and the Pb underlayer. In consequence, it is advisable to keep the bath temperature at 40 °C.
3.4 Influence of current density on α-PbO2 electrosynthesis
At the beginning of α-PbO2 electrosynthesis on Al/Pb, the high current density is necessary to promote the passivation of Pb underlayer and the nucleation of α-PbO2, otherwise the Pb underlayer will be dissolved and the α-PbO2 electrosynthesis will be impossible [18,20]. The galvanostatic polarization curves obtained in the bath with 160 g/L NaOH and 40 g/L PbO at 40 °C and different current densities are shown in Fig. 4.
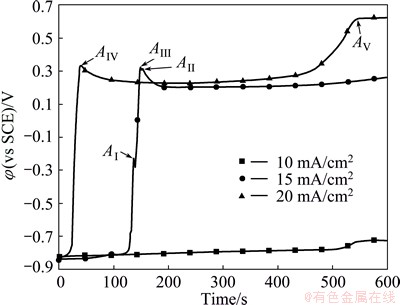
Fig. 4 Galvanostatic polarization curves of Al/Pb at different current densities
As shown in Fig. 4, when the current density is 10 mA/cm2, the working electrode potential almost keeps at about -0.8 V, just a little rise after 500 s. The open circuit potential of Al/Pb under such condition is about -0.82 V (vs SCE), indicating that the Pb underlayer is difficult to be passivated at 10 mA/cm2. Of course, it is impossible to synthesize α-PbO2 on Al/Pb. When the current density is 15 mA/cm2, the working electrode potential is about -0.8 V during the first 130 s, then sharply rises to 0.35 V and gradually decreases to a stable value (about 0.2 V). In detail, the process presents three peaks (AI, AII and AIII). This indicates that the Pb surface is passivated to form a PbO film with poor conductivity in first 130 s, then further oxidized into α-PbO2. Peak AI may reflect that the current density is not high enough to promote PbO transform into α-PbO2 smoothly. Peak AII reflects that the α-PbO2 formed by the self-oxidation of PbO film, whereas the peak AIII reflects that the α-PbO2 is formed by the oxidation of Pb(II) in the bath. In addition, the potential difference between AII, AIII (about 0.35 V) and the stable value (about 0.2 V) reflects that the nucleation of α-PbO2 is difficult, and it requires a certain magnitude of overpotential to decrease the activation energy of the α-PbO2 electrosynthesis. The electrode potential begins to slightly increase at about 500 s due to the concentration polarization.
When the current density is 20 mA/cm2, the potential rapidly increases from -0.8 V to 0.35 V in first 50 s and peak AIV appears but peak AI disappears. This indicates that the Pb underlayer can be rapidly passivated to form PbO, and then successfully oxidized into α-PbO2 at such a current density. In addition, it can be thought that peak AIV is overlapped by peaks AII and AIII, i.e., the oxidization of PbO film and Pb(II) to synthesize α-PbO2 occurs simultaneously. Then, the potential is maintained at about 0.25 V, and obviously rises to 0.6 V after 450 s. Peak AV appears at about 550 s, and severe oxygen evolution occurs. Therefore, it is suitable to keep the current density at 20 mA/cm2 to promote the Pb surface passivation and α-PbO2 nucleation for 3 min at the beginning of the α-PbO2 electrosynthesis on Al/Pb. Then, a low current density is chosen to steadily synthesize α-PbO2 and effectively avoid oxygen evolution caused by the concentration polarization.
The current density in the α-PbO2 electrosynthesis region of anodic polarization curve is higher than the real limiting diffusion current density of α-PbO2 electrosynthesis, as the anodic polarization is a transient process and the current density in the α-PbO2 synthesizing region also includes that in the Pb passivation region. Therefore, the small current density should be obtained by quasi-steady state polarization. The quasi-steady state polarization curve of Al/Pb in α-PbO2 bath is shown in Fig. 5. In which, the Al/Pb is passivated at 20 mA/cm2 for 3 min in advance. As Fig. 5 shows, the current density increases with increasing the electrode potential. In detail, it changes little (about 5.5 mA/cm2) within 0.3-0.5 V. This indicates that the α-PbO2 electrosynthesis is controlled by the mass-transfer diffusion step as the potential is above 0.3 V, however, it is controlled by the electrochemical step when the potential is below 0.3 V. Then, the current density increases sharply due to the oxygen evolution when the potential is above 0.5 V. Therefore, the stable electrosynthesis of α-PbO2 can be realized at 5 mA/cm2, and the oxygen evolution reaction can be avoided.
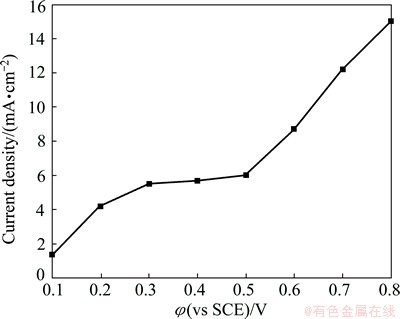
Fig. 5 Quasi-steady state polarization curve of Al/Pb with passivation treatment
3.5 Phase compositions and surface microstructures of Al/Pb and Al/Pb/α-PbO2
The XRD patterns of Al/Pb before and after passivation treatment at 20 mA/cm2 for 3 min, and those of Al/Pb/α-PbO2 synthesized at 5 mA/cm2 for 2 min, 5 min and 2 h, respectively, are shown in Fig. 6. And their SEM images are shown in Fig. 7.
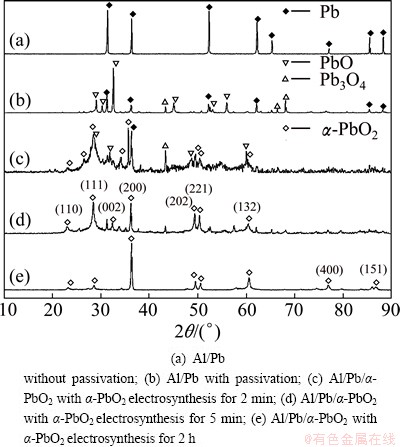
Fig. 6 XRD patterns of Al/Pb and Al/Pb/α-PbO2
As shown in Fig. 6, there are some lead oxides with intermediate state, such as PbO and Pb3O4, appearing in the Pb underlayer after passivating the Al/Pb for 3 min. This indicates that the Pb passivation process is step by step oxidation. However, no α-PbO2 is detected, as the α-PbO2 film formed by the oxidation of Pb surface is too thin and loose to be detected. The α-PbO2 appears after synthesizing α-PbO2 for 2 min, which indicates that the α-PbO2 begins to be synthesized on Al/Pb. The characteristic peaks of PbO and other lead oxides with intermediate state are weakened and even disappear when α-PbO2 electrosynthesis is lasted for for 5 min, for the α-PbO2 deposition layer covers the Al/Pb surface and its thickness gradually increases. The α-PbO2 with rhombic structure completely covers the Al/Pb when synthesizing α-PbO2 for 2 h, and the characteristic peak of lead oxides with intermediate state disappears. Moreover, the α-PbO2 crystalline characteristic peak intensities of (200) and (132) planes increase, but the rest is weakened with different extent.
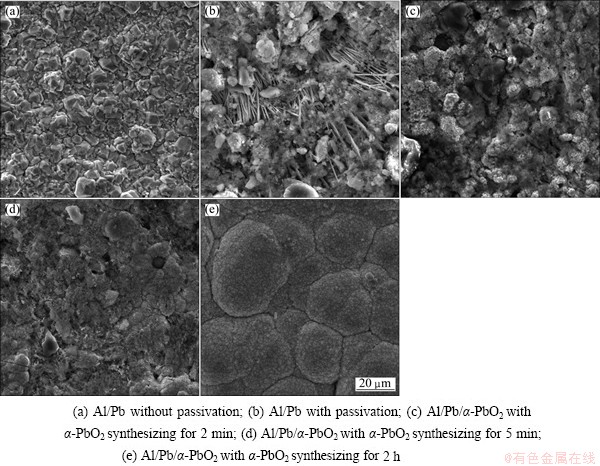
Fig. 7 SEM images of Al/Pb and Al/Pb/α-PbO2
Figure 7 shows the SEM images of Al/Pb and Al/Pb/α-PbO2. As can be seen from Fig. 7(a) that, the grains of Pb underlayer are uniform and arrange compactly. There are some whisker structures after passivating the Pb underlayer for 3 min (Fig. 7(b)). In addition, the grain shapes are various and the surface is loose. The whisker structure disappears and the grains are unevenly distributed on Al/Pb when α-PbO2 is continued to synthesize for 2 min (Fig. 7(c)). It shows that the nucleation locations of α-PbO2 on Al/Pb surface are selective. A uniform α-PbO2 deposition layer covers the Al/Pb with α-PbO2 electrosynthesis for 5 min (Fig. 7(d)). Then the α-PbO2 deposition layer is constituted by well developed spherical unit cells with α-PbO2 electrosynthesis for 2 h (Fig. 7(e)). In consequence, the α-PbO2 electrosynthesis process according to the electrochemical analyses above is further confirmed here.
4 Conclusions
1) At the beginning of the α-PbO2 electrosynthesis on Al/Pb with high current density, the Pb underlayer is firstly passivated to form a poor conductive PbO film. Then, the α-PbO2 electrosynthesis is proceeded simultaneously with step by step oxidation of PbO (PbO→Pb3O4→α-PbO2 or PbO→α-PbO2). When an α-PbO2 film is formed on the Pb underlayer, the continual α-PbO2 deposition layer is mainly formed by the oxidation of Pb(II) in the bath.
2) The suitable plating conditions of α-PbO2 electrosynthesis are 40 g/L PbO and 160 g/L NaOH at 40 °C. At the beginning of the α-PbO2 electrosynthesis, the large current density of 20 mA/cm2 is kept for 3 min to promote Pb passivation and α-PbO2 nucleation. Then, steadily synthesizing α-PbO2 is performed at a small current density of 5 mA/cm2.
3) The α-PbO2 deposition layer obtained in the alkaline bath possesses the rhombic structure, and it is composed of well developed spherical unit cells.
References
[1] ZHAO Jun, ZHU Cheng-zhu, LU Jun, HU Cai-ju, PENG Shu-chuan, CHEN Tian-hu. Electro-catalytic degradation of bisphenol A with modified Co3O4/β-PbO2/Ti electrode [J]. Electrochimica Acta, 2014, 118: 169-175.
[2] YANG Xiao-yong, SI Yun-sen, ZHU Pei-xian, ZHOU Sheng-gang. The current research status of intermediate layer preparation technology of Ti-based lead oxide coating electrode [J]. Materials Review, 2013, 27(21): 31-34.
[3] LI Xiao-lin, LI Xue-ming, YANG Wen-jing, CHEN Xiao-hua, LI Wu-lin, LUO Bin-bin, WANG Kui-long. Preparation of 3D PbO2 nanospheres@SnO2 nanowires/Ti electrode and its application in methyl orange degradation [J]. Electrochimica Acta, 2014, 146: 15-22.
[4] XUE B, ZHANG Y, WANG J Y. Electrochemical oxidation of bisphenol A on Ti/SnO2- Sb2O5/PbO2 anode for waste water treatment [J]. Procedia Environmental Sciences A, 2011, 10: 647-652.
[5] CAO Jian-chun, GUO Zhong-cheng, PAN Jun-yi, ZHOU Xiao-long. Preparation of a novel PbO2/PbO2-CeO2 composite electrode with stainless steel as substrate [J]. Journal of Kunming University of Science and Technology (Natural Science Edition), 2004, 29(5): 38-41. (in Chinese)
[6] JI Yue-fei, WEI Jie, WANG Dong-tian. Preparation of four DSAs and comparison of their electro-catalytic activity [J]. The Chinese Journal of Process Engineering, 2012, 12(2): 345-348. (in Chinese)
[7] ZHU Zi-yu, WU Yu-en, CHEN Qing-song, CAO Mei. Preparation of electrodeposited lead dioxide coatings on aluminum substrate and study of their properties as electrodes [J]. Journal of Materials Protection, 2012, 45(11): 1-3.
[8] CHEN Zhen, YU Qiang, LIAO Deng-hui, GUO Zhong-cheng, WU Jian. Influence of nano-CeO2 on coating structure and properties of electrodeposited Al/α-PbO2/β-PbO2 [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(5): 1382-1389.
[9] CHEN Bu-ming, GUO Zhong-cheng, YANG Xian-wan, CAO Yuan-dong. Morphology of alpha-lead dioxide electrodeposited on aluminum substrate electrode [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(1): 97-103.
[10] INGUANTA R, VERGOTTINI F, FERRARA G, PIAZZA S, SUNSERI C. Effect of temperature on the growth of α-PbO2 nanostructures [J]. Electrochimica Acta, 2010, 55(28): 8556-8562.
[11] MUNICHANDRAIAH N, SATHYANARAYANA S. Insoluble anode of α-lead dioxide coated on titanium for electrosynthesis of sodium perchlorate [J]. Applied Electrochemistry, 1988, 18(2): 314-316.
[12] GHASEMI S, MOUSAVI M F, KARAMI H, SHAMSIPUR M, KAZEMI S H. Energy storage capacity investigation of pulsed current formed nano-structured lead dioxide [J]. Electrochimica Acta, 2006, 52(4): 1596-1602.
[13] DONG Lan-zhou, GAO Li-jun. Effect of electrochemical preparation methods on structure and properties of PbO2 anodic layer [J]. Electrochimica Acta, 2007, 53(4): 2060-2064.
[14] EI WANEES S A, EI AAL E A, EI AAL A A. Inhibition of pitting corrosion of Pb in alkaline chlorate and perchlorate solutions by some inorganic anions [J]. Anti-Corrosion Methods and Materials, 1991, 38 (12): 4-6.
[15] EL REHIM S S A, MOHAMED N F. Passivity breakdown of lead anode in alkaline nitrate solutions [J]. Corrosion Science, 1998, 40(11): 1883-1896.
[16] CHEN Zhen, WU Jian, GUO Zhong-cheng, YU Qiang. Influence of fluoride ion on the electrochemical properties of Al/β-PbO2 electrode [J]. Advanced Materials Research, 2012, 490-495: 3160-3166.
[17] CARR J P, HAMPSON N A. The lead dioxide electrode [J]. Chemical Reviews, 1972, 72(6): 684-685.
[18] JONES P, THIRSK H R, WYNNE JONES W F K. Oxide formation and overvoltage of oxygen on lead and silver anodes in alkaline solution [J]. Transactions of the Faraday Society, 1956, 52: 1003-1011.
[19] CHEN Bu-ming, GUO Zhong-cheng, YANG Xian-wan. The thermodynamic analysis of electrodeposited α-PbO2 and β-PbO2 coatings [J]. China Nonferrous Metallurgy B(2), 2009(2): 54-58. (in Chinese)
[20] PAUL D, MARCEL P, PIERRE V R B. Potential-pH diagram of lead and its applications to the study of lead corrosion and to the lead storage battery [J]. Journal of the Electrochemical Society, 1951, 98(2): 57-64.
Al/Pb/α-PbO2复合惰性阳极材料的电化学合成
胡 钢1,2,徐瑞东1,2,何世伟2,陈步明2,杨海涛3,于伯浩2,刘 强2
1. 昆明理工大学 省部共建复杂有色金属资源清洁利用国家重点实验室,昆明 650093;
2. 昆明理工大学 冶金与能源工程学院,昆明 650093;
3. 中国科学院 过程工程研究所 多相复杂系统国家重点实验室,北京 100190
摘 要:利用恒电流从碱性镀液中在Al/Pb表面电化学合成α-PbO2沉积层,制备出Al/Pb/α-PbO2复合惰性阳极材料。通过阳极极化法考察α-PbO2镀液组成及镀液温度对在Al/Pb表面电化学合成α-PbO2的影响,采用XRD和SEM分别测试Al/Pb基体材料及α-PbO2沉积层的相结构和表面微观组织特征。结果表明:α-PbO2的电化学合成分由几个不同的步骤完成;适宜的条件能有效提高α-PbO2电化学合成速率并避免析氧副反应的发生;从碱性溶液中合成的α-PbO2具有斜方晶型结构,沉积层由发育良好的圆球形晶胞构成。
关键词:Al/Pb基体;α-PbO2;电化学合成;阳极极化
(Edited by Mu-lan QIN)
Foundation item: Project (20125314110011) supported by the Specialized Research Fund for the Doctoral Program of the Ministry of Education of China; Project (2014FA024) supported by the Key Project of Yunnan Province Applied Basic Research Plan of China; Project (51004056) supported by the National Natural Science Foundation of China
Corresponding author: Rui-dong XU; Tel: +86-871-65160072; E-mail: rdxupaper@aliyun.com
DOI: 10.1016/S1003-6326(15)63820-1
Abstract: The α-PbO2 deposition layers were prepared on the surface of Al/Pb substrates by constant current electrosynthesis from an alkaline bath, and Al/Pb/α-PbO2 composite inert anode materials were obtained. The effects of the bath composition and bath temperature on the electrosynthesis of α-PbO2 were investigated by means of anodic polarization method, the phase structures and surface microstructures of Al/Pb and α-PbO2 deposition layers were tested by means of X-ray diffraction (XRD) and scanning electron microscopy (SEM), respectively. The experimental data have shown that the process of α-PbO2 formation have several stages. The appropriate conditions can effectively improve the formation rate of α-PbO2 and avoid the occurrence of oxygen evolution reaction. The α-PbO2 deposition layer obtained in alkaline bath possesses rhombic structure, and it is composed of well developed spherical unit cells.


