
Inhibition mechanism of aspartic acid on crystal growth of hydroxyapatite
HUANG Su-ping(黄苏萍), ZHOU Ke-chao(周科朝), LI Zhi-you(李志友)
State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China
Received 25 July 2006; accepted 8 February 2007
Abstract:
The effects of aspartic acid on the crystal growth, morphology of hydroxyapatite(HAP) crystal were investigated, and the inhibition mechanism of aspartic acid on the crystal growth of hydroxyapatite was studied. The results show that the crystal growth rate of HAP decreases with the increase of the aspartic acid concentration, and the HAP crystal is thinner significantly compared with that without amino acid, which is mainly due to the ![]() surface of HAP crystal being inhibited by the aspartic acids. The calculation analysis indicates that the crystal growth mechanism of HAP, following surface diffusion controlled mechanism, is not changed due to the presence of aspartic acid. AFM result shows that the front of terrace on vicinal growth hillocks is pinned, which suggests that the aspartic acid is adsorbed onto the
surface of HAP crystal being inhibited by the aspartic acids. The calculation analysis indicates that the crystal growth mechanism of HAP, following surface diffusion controlled mechanism, is not changed due to the presence of aspartic acid. AFM result shows that the front of terrace on vicinal growth hillocks is pinned, which suggests that the aspartic acid is adsorbed onto the ![]() surface of HAP and interacts with the Ca2+ ions of HAP surface, so as to block the growth active sites and result in retarding of the growth of HAP crystal.
surface of HAP and interacts with the Ca2+ ions of HAP surface, so as to block the growth active sites and result in retarding of the growth of HAP crystal.
Key words:
crystallization growth; hydroxyapatite; inhibition mechanism; aspartic acid;
1 Introduction
Hydroxyapatite [Ca5(PO4)3OH, HAP], is the most stable calcium phosphate salt among the calcium phosphate phases. And it is also the model compound for the inorganic component of bone and teeth[1-5]. In the biomineralization process of bone and teeth, the amino acids took part in and played important role in controlling the crystal growth of HAP[6-8]. On the other hand, calcification is the most frequent reason of the clinical failure of cardiac valve bioprostheses fabricated from porcine aortic valves[9]. The mineral deposits of the human atherosclerotic aorta consisted mainly of calcium apatite (71%), carbonate (9%) and contained high percentage of protein (15%)[5]. The average molar ratio of Ca to P is about 1.7 in mature atherosclerotic plaque biomineral and mature sceletal biomineral, of which there is approximate HAP in composition. Treatment with amino acids appears to prevent the calcification of cardiac bioprostheses[10-13].
Amino acids are compounds of major importance for living organisms to move freely in blood circulation after digestion of proteins. Also, it has been proved that they enter into the cell environment by simple diffusion [14], thus their concentration is controlled by physiological mechanisms. It is obvious that the studies of such molecules of biological relevance on hydroxyapatite crystal growth can be related directly to important process of desirable or pathological calcification.
In order to assess the effect of amino acids on crystal growth of HAP and elucidate the mechanism of calcification, the role of amino acids in the crystal growth of HAP has been investigated[15-20]. It is found that the crystal growth of HAP decreases in the presence of amino acids compared with that without amino acids, and conferred that the main reason for inhibiting the crystal growth of HAP is the adsorption of amino acid on HAP. However, these results come from speculation and are lack of real insight into the biological calcification process. In this work, the kinetics of HAP crystal growth and the morphology of HAP crystal in the presence of aspartic acid were investigated. And the inhibition mechanism of aspartic acid on the crystal growth of hydroxyapatite was discussed.
2 Experimental
2.1 Materials
High purity aspartic acid was obtained from Sigma Chemical Company(>99%). Hydroxyapatite powder was synthesized by our groups, and its preparation procedure and characterization of HAP were reported in Ref.[21]. HAP was used as adsorbent and seed material. The specific surface area(SSA) of HAP was 320 m2/g, as determined by N2 gas adsorption (Micromeritics, Gemini) using a multiple point BET method.
2.2 Precipitation experiments
All experiments were performed at (37±0.13)℃ in a thermo-stated double walled. Solid reagent-grade (Merck) Ca(NO3)2, NH4H2PO4, NaCl and triply distilled CO2-free water were used in the preparation of the supersaturated solutions. The molar ratio of Ca to P was kept at 5?3. The ionic strength of the solutions was adjusted to 0.15 mol/L by the addition of NaCl. NH4OH and HCl were used to adjust the pH at 7.4. Followed by the pH adjustment, the crystal growth process was initiated by the addition of 20 mg well characterized HAP seed crystals. The aspartic acid was dissolved in the supersaturated solution before the crystallization process. Throughout the crystallization process water-saturated purified nitrogen was bubbled through the solution in order to preclude atmospheric carbon dioxide from dissolving into the solution. A pH-meter (Metrohm 691) was used for measuring pH value. Connection of the pH-meter was modified so as to accommodate two burettes, mechanically coupled and mounted onto the shaft of the piston burette, through the simultaneous addition of exactly equal volumes of reagents, to achieve invariability of all species in solution.
2.3 Morphology investigation
The HAP nanoparticles were put into alcohol and dispersed by the ultrasonic, then the suspend solution was dropped onto the bronze net and bomb load, respectively. The morphology and selected area diffrac- tion (SAD) were investigated on Jeol 2000FXⅡTEM. The surface morphology was observed on AFM.
3 Results
The crystal growth rates of HAP in the absence and presence of aspartic acid are listed in Table 1. From it we can see that the crystal growth rate of HAP decreases in the presence of the aspartic acid compared with that without amino acids, and the inhibitory activity of aspartic acid increases with increasing concentration of aspartic acid. Experiments at different amounts of seed crystals (10, 15, 20 and 40 mg) show the same initial crystal growth rates. Changes in the stirring rate between 100 and 800 r/min have no effect on the growth rate.
Table 1 Crystal growth rate of HAP under presence of aspartic acid
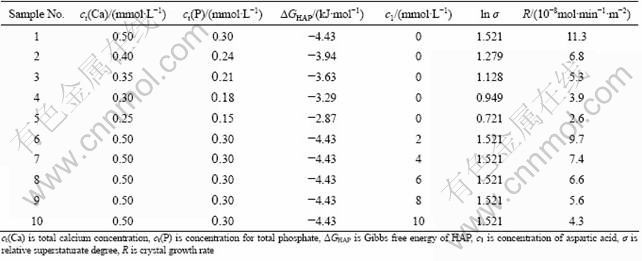
Fig.1 shows the TEM images of HAP in the absence and presence of aspartic acid, respectively. From it we can see that the HAP crystal in the presence of aspartic acid has silk-like shape. And HAP crystal is thinner significantly compared with that without amino acid. The SAD pattern of HAP crystal (see Fig.1(b)) shows that the long axis of HAP crystal is parallel to the (0001) surface of HAP, which indicates that the (0001) surface of HAP is unchanged, namely, the ![]() surface of HAP is inhibited.
surface of HAP is inhibited.
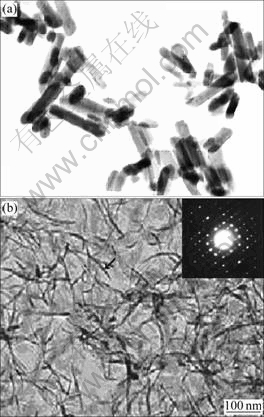
Fig.1 TEM images and SAD pattern of HAP in absence (a) and in presence (b) of aspartic acid
4 Discussion
The crystal growth rate of HAP (R) is proportional to the relative supersaturation, which can be defined as [15-20]
R=kSσn (1)
where k is the rate constant; S is a function of the active growth site on the crystal surface; n is the apparent order of the crystallization, σ is the relative supersaturate degree degree that can be expressed as[15-20]
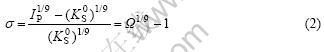
where ![]() is the thermodynamic solubility product of materials at 37 ℃,
is the thermodynamic solubility product of materials at 37 ℃, ![]() 2.35×10-59(mol?L-1)-9; IP the ionic product of the precipitating salt, which is calculated as[15-20]
2.35×10-59(mol?L-1)-9; IP the ionic product of the precipitating salt, which is calculated as[15-20]
![]()
According to Eqn.(1), logarithmic plots yield straight lines in all cases. From the slope of the linear plots, the apparent order of the crystallization n can be obtained. The ionic product of the supersaturated solution is calculated by taking into account of all equilibriums.
Fig.2 shows the relationship between ln σ and ln R in absence and in presence of aspartic acid. It can be seen that the apparent order of the crystallization is 2.0±0.1 and 1.9±0.2, respectively. Experiments at different amounts of seed crystals (10, 15, 20 and 40 mg) show the same initial crystal growth rates normalized per unit surface area of the crystal substrate, which indicates that the crystallization process happens without being accompanied by either spontaneous or secondary precipitation. Changes in the stirring rate between 100 and 800 r/min have no effect on the growth rate, suggesting that the growth rate determining step of the process is not bulk diffusion from the bulk solution to the crystal surface, which indicates that the crystal growth mechanism of HAP in the presence of aspartic acid follows surface diffusion controlled mechanism. Furthermore it is proved that the crystal growth mechanism has not changed due to the presence of aspartic acid.
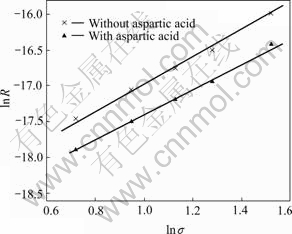
Fig.2 Relationship between ln σ and ln R
The inhibitory activity of aspartic acid on HAP may be related to: 1) formation of ion pairs in the solution, thereby decreasing the drive force, i.e. the degree of supersaturation for crystal growth; 2) blocking of crystal growth sites by adsorption.
In the presence of aspartic acids, the Gibbs free energy does not change appreciably even at the highest concentration level of the additive used, which illustrates the fact that changes in the crystal growth rate cannot be attributed to the reduction of the solution supersaturation due to ion-pair formation of the solute aspartic acid with one or more of the reactants. The data are corrected for changes in surface area, which is reduced during the crystal growth process. Calculations were done by introducing in the computer code HYDRAQL. The formation equilibrium of the 1?1 Ca-aspartic ion pair with its stability constant shows that in the case of the maximum concentration of aspartic (0.010 mol/L) only 0.6% of the total calcium contributes in the formation of the aforementioned ion pair. It is therefore evident that the presence of aspartic acid in the concentration range investigated does not affect the concentration of free Ca2+ ions to any significant extent and the degree of the solution supersaturation with respect to HAP. Consequently, the inhibitory effect of aspartic acid may be ascribed to the adsorption onto HAP and subsequent blocking of the active growth sites.
Fig.3(a) shows the AFM image of the ![]() surface morphology of HAP crystal. It can be seen from the figure that the front of terrace on vicinal growth hillocks is pinned, which suggests that the foreign molecules (aspartic acid) are adsorbed onto the
surface morphology of HAP crystal. It can be seen from the figure that the front of terrace on vicinal growth hillocks is pinned, which suggests that the foreign molecules (aspartic acid) are adsorbed onto the ![]() surface of HAP, and subsequently block the active growth sites of
surface of HAP, and subsequently block the active growth sites of ![]() surface. The adsorption model sketch map of aspartic acid on the
surface. The adsorption model sketch map of aspartic acid on the ![]() surface morphology of HAP is shown in Fig.3(b).
surface morphology of HAP is shown in Fig.3(b).

Fig.3 AFM image (a) and model sketch map (b) of ![]() surface morphology of HAP crystal
surface morphology of HAP crystal
The presence of a foreign compound in the supersaturated solution by that the HAP-growth process takes place, results in an interaction of the solute species with the precipitate solid. The solute species have functional groups such as carboxyl and/or amino groups, which may be adsorbed reversibly onto the HAP crystal surface and interact with the positively and negatively charged ions of HAP crystal surface. From the above result, it is clear that the aspartic acid has a stronger retarding effect on HAP crystal growth rate, which seems to interact with Ca2+ ions of the crystal surface exclusively with their side terminal carboxyl groups. Aspartic acid has a short side group, and can also rotate freely around an axis vertical to the crystal surface when adsorbed onto the crystal surface, and thus the volume of adsorption can be described by a cone. For aspartic acid the projection of the cone onto HAP surface is a circle with radius of 0.332 nm[8]. So, it can be concluded that the aspartic acid has a stronger affinity constant and results in inhibiting activity against HAP crystal growth.
5 Conclusions
1) The crystal growth of HAP is inhibited in the presence of aspartic acid and the inhibitory activity of aspartic acid increases with increasing concentration of aspartic acid. TEM and SAD results show that the morphology of HAP is silk-like, which indicates that the ![]() surface of HAP is inhibited by aspartic acid.
surface of HAP is inhibited by aspartic acid.
2) Calculation indicates that the crystal growth mechanism of HAP in the presence of aspartic acid follows surface diffusion controlled mechanism, which reveals that the crystal growth mechanism is not changed in the presence of aspartic acid.
3) AFM image shows that the inhibitory activity of aspartic acid on HAP is not due to the formation of ion pairs in the solution, but the aspartic acid adsorption onto the ![]() surface of HAP and interaction with the Ca2+ ions on the
surface of HAP and interaction with the Ca2+ ions on the ![]() surface of HAP, which blocks the
surface of HAP, which blocks the ![]() surface growth of HAP.
surface growth of HAP.
References
[1] MANGOOD A, MALKAJ P, DALAS E. Hydroxyapatite crystallization in the presence of acetaminophen [J]. Journal of Crystal Growth, 2006, 290(2): 565-570.
[2] XIN Ren-long, LENG Yang, WANG Ning. In situ TEM examinations of octacalcium phosphate to hydroxyapatite transformation [J]. Journal of Crystal Growth, 2006, 289(1): 339- 344.
[3] SHIH W E, WANG M C, HON M H. Morphology and crystallinity of the nanosized hydroxyapatite synthesized by hydrolysis using cetyltrimethylammonium bromide (CTAB) as a surfactant [J]. Journal of Crystal Growth, 2005, 275(1/2): e2339-e2344.
[4] HUANG Su-ping, ZHOU Ke-chao. Controlled crystallization of hydroxyapatite under hexadecylamine self-assembled monalayer [J]. Trans Nonferrous Met Soc China, 2003, 13: 595-599.
[5] KONG X D, CUI F Z, WANG X M, ZHANG M, ZHANG W. Silk fibroin regulated mineralization of hydroxyapatite nanocrystals [J]. Journal of Crystal Growth, 2004, 270(1/2): 197-202.
[6] KOUTSOPOULOS S, DALAS E. Hydroxyapatite crystallization on sodium cholate [J]. Journal of Crystal Growth, 2001, 222(1/2): 279-286.
[7] ZHAI Y, CUI F Z. Recombinant human-like collagen directed growth of hydroxyapatite nanocrystals [J]. Journal of Crystal Growth, 2006, 291(1): 202-206.
[8] ONUMA K, YAMAGISHI K, OYANE A. Nucleation and growth of hydroxyapatite nanocrystals for nondestructive repair of early caries lesions [J]. Journal of Crystal Growth, 2005, 282(1/2): 199-207.
[9] KOUTSOPOULOS S, BARLOS K, GATOS D, DALAS E. The effect of various Prothymosin α fragments on the crystal growth of hydroxyapatite in aqueous solution [J]. Journal of Crystal Growth, 2004, 267(1/2): 306-311.
[10] CHEN C W, RIMAN R E, TENHUISEN K S, BROWN K. Mechanochemical-hydrothermal synthesis of hydroxyapatite from nonionic surfactant emulsion precursors [J]. Journal of Crystal Growth, 2004, 270(3/4): 615-623.
[11] KURIAKOSE T A, KALKURA S N, PALANICHAMY M, ARIVUOLI D, DIERKS K, BOCELLI G. Synthesis of stoichiometric nanocrystalline hydroxyapatite by ethanol-based sol-gel technique at low temperature [J]. Journal of Crystal Growth, 2004, 263(1/4): 517-523.
[12] KOUTSOPOULOS S, DALAS E. The effect of acidic amino acids on hydroxyapatite crystallization [J]. Journal of Crystal Growth, 2000, 217(4): 410-415.
[13] KOUTSOPOULOS S, DALAS E. Hydroxyapatite crystallization in the presence of aspartic, tyrosine and hydroxyproline amino acids with polar side groups [J]. Journal of Crystal Growth, 2000, 216(1/4): 443-449.
[14] SPANOS N, KLEPETSANIS P G, KOUTSOUKOS P G. Model studies on the interaction of amino acids with biominerals: The effect of L-serine at the hydroxyapatite-water interface [J]. Journal of Crystal Growth, 2001, 236(1/2): 260-265.
[15] KOUTSOPOULOS S, DALAS E, TZAVELLAS N, KLOURAS N, AMORATIS P. Effect of vanadocene dichlorides on the crystal growth of hydroxyapatite [J]. Journal of Crystal Growth, 1998, 183(1/2): 251-257.
[16] KOUTSOPOULOS S, PIERRI E, DALAS E, TZAVELLAS N, KLOURAS N. Effect of ferricenium salts on the crystal growth of hydroxyapatite in aqueous solution [J]. Journal of Crystal Growth, 2000, 218(2/4): 353-358.
[17] CUISINIER F J G, STEUER P, BRISSON A, VOEGEL J C. High resolution electron microscopy study of crystal growth mechanism in chicken bone composites [J]. Journal of Crystal Growth, 1995, 156(4): 443-453.
[18] NGANKAM P A, SCHAAF D, VOEGEL J C, CUISINIER F J G. Heterogeneous nucleation of calcium phosphate salts at a solid/liquid interface examined by scanning angle reflectometry [J]. Journal of Crystal Growth, 1999, 197(4): 927-938.
[19] GAFNI G, SEPTIER D, GOLDBERG M. Effect of chondroitin sulfate and biglycan on the crystallization of hydroxyapatite under physiological conditions [J]. Journal of Crystal Growth, 1999, 205(4): 618-623.
[20] FORTUN Y, PADRINES M. Effects of fibronectin on hydroxyapatite formation [J]. Inorganic Biochemical, 1999, 73(1/2): 129-136.
[21] ZHU Shai-hong, ZHOU Ke-chao, HUANG Su-ping. The HAP nanoparticles as a novel gene carrier [J]. Journal of Nanoparticle Research, 2004, 6(2): 307-311.
Foundation item: Project(2003AA302210) support by the National High-Tech Research and Development Program of China; Project(05JJ20014) supported by the Natural Science Foundation of Hunan Province, China
Corresponding author: HUANG Su-ping; Tel: +86-731-8836264; Fax: +86-731-8836311; E-mail: huangsp1@mail.csu.edu.cn
Abstract: The effects of aspartic acid on the crystal growth, morphology of hydroxyapatite(HAP) crystal were investigated, and the inhibition mechanism of aspartic acid on the crystal growth of hydroxyapatite was studied. The results show that the crystal growth rate of HAP decreases with the increase of the aspartic acid concentration, and the HAP crystal is thinner significantly compared with that without amino acid, which is mainly due to the ![]() surface of HAP crystal being inhibited by the aspartic acids. The calculation analysis indicates that the crystal growth mechanism of HAP, following surface diffusion controlled mechanism, is not changed due to the presence of aspartic acid. AFM result shows that the front of terrace on vicinal growth hillocks is pinned, which suggests that the aspartic acid is adsorbed onto the
surface of HAP crystal being inhibited by the aspartic acids. The calculation analysis indicates that the crystal growth mechanism of HAP, following surface diffusion controlled mechanism, is not changed due to the presence of aspartic acid. AFM result shows that the front of terrace on vicinal growth hillocks is pinned, which suggests that the aspartic acid is adsorbed onto the ![]() surface of HAP and interacts with the Ca2+ ions of HAP surface, so as to block the growth active sites and result in retarding of the growth of HAP crystal.
surface of HAP and interacts with the Ca2+ ions of HAP surface, so as to block the growth active sites and result in retarding of the growth of HAP crystal.


