Article ID: 1003-6326(2005)06-1394-07
Removal of cadmium from cadmium-contaminated red soils using electrokinetic soil processing
LIU Yun-guo(刘云国), LI Cheng-feng(李程峰), ZENG Guang-ming(曾光明),
YUE Xiu(岳 秀), LI Xin(李 欣), XU Wei-hua(徐卫华),
TANG Chun-fang(汤春芳), YUAN Xing-zhong(袁兴中)
(Department of Environmental Science and Engineering, Hunan University,
Changsha 410082, China)
Abstract:
To investigate the feasibility of electrokinetic soil processing on the removal of Cd from Cd-contaminated red soils, a laboratory experiment was conducted. A constant direct current density of 0.5mA/cm2 was applied. The result shows that the Cd-removal efficiency is remarkably pH-dependent, which is caused by the change of Cd retention capacity of the red soils under different pH conditions. The initial Cd concentration is 1.490g/kg and over 79% of it is removed from the red soils after treatment for 96h. The energy expenditure per unit volume at the end of experiment is about 77.6kW·h/m3 and the capital consumed by the whole experiment is 42.6RMB Yuan/m3, which suggests that the electrokinetic soil processing is a promising technology for remedying Cd-contaminated red soils due to its high removal efficiency and low energy consumption.
Key words:
cadmium; electrokinetic soil processing; red soil; electroosmosis CLC;
number: X131.3 Document code: A
1 INTRODUCTION
Electrokinetic soil processing(ESP) is one of the newly developed techniques for the remediation of soils. It use low-level direct current of cross-sectional area between the electrodes placed in soils to be treated[1]. Three transport phenomena, electrophoresis, electroosmosis and electromigration, will occur in soils when an electric field is applied to a soil system[2]. Contaminants in fine-grained soils can be transported toward the anode or cathode by means of the electromigration and/or electroosmosis and thus can be removed from soils. Previous studies showed that relatively high removal efficiency of heavy metals could be obtained in fine-grained soils by using ESP[2-5].
pH is a major factor affecting the removal efficiency of heavy metals adsorbed by soils being treated. In order to control the soil pH and enhance the removal efficiency of heavy metals, many methods such as conductive solutions[2, 6], conditioning fluids[7-11], ion selective membrane[8, 12, 13], electrolyte circulation[14], and a porous layer inserted in soils and continuously flushed with conditioning fluids[15], have been used. Since ESP has great potentials in removing heavy metals in fine-grained soils, more and more scientists are now devoting to the studies relating to it. However, in China, only a few people are studying on ESP[3, 5, 10, 11, 16]. Consequently, it is necessary for researchers inland to develop the ESP.
Cd in soils can be classified into soluble and insoluble Cd. The pH value is a major factor affecting the speciation of Cd in soils. Cd in soils may leach into water, especially under acidic conditions. Transformation processes for Cd in soil are affected by the equilibrium of sorption/desorption and dissolve/precipitation of Cd. People exposed to low levels of Cd over time may incur kidney damage as well as lung, bone, cardiovascular system, liver and reproductive system damage. Due to its high toxicity, the addition of Cd to soils has the tendency to create environmental pollution and human health problems if it is allowed to enter the food chain. The content of Cd in soils has been increasing continuously in China up to now due to the use of phosphatic fertilizers and additions of sewage sludges, which is threatening to soil quality and human health. Therefore, remediation of soils contaminated by Cd is one of the environmental problems that should be resolved urgently for the moment. The objectives of this paper are to obtain the basic data and to investigate the feasibility of electrokinetic soil processing on the removal of Cd from Cd-contaminated red soils.
2 EXPERIMENTAL
2.1 Apparatus
A schematic diagram of the experimental apparatus is shown in Fig.1. Inner dimensions of the soil cell are 15cm×10cm×10cm. Two sheets of filter paper are placed at each side of the soil cell. Graphite electrodes are used at the anode and the cathode. The pole clearance is approximately 30cm. 600mL anolyte and 900mL catholyte are presented in electrode compartments in order to avoid sudden variations of electrolytes. Meanwhile, the electrolytes are recirculated in electrode compartments by peristaltic pump for the purpose of compensating the loss of electrolytes caused by electrolytic reactions during experiment. The graduated cylinder is used to collecte the electrolytes overflowing from electrode compartments. Therefore, the volume of electroosmotic flow can be obtained from the changes of volume of electrolytes in graduated cylinder.
Four pieces of electrokinetic apparatus were used in this study (Fig.1). Each of them was removed in turn at a special time for the analysis on the soil pH and the amount of target contaminant remain-ing in the studied soils.
2.2 Materials
The samples of the red soils were collected from Yuelu Mountain located in Changsha, Hunan Province, China. Some physical and chemical properties of the soil are listed in Table 1. The soils were air dried, and the agglomerates were broken by hand and by using a wooden mallet. Particles larger than 2mm were removed by sieving. All further tests were performed on the soils with size of less than 2mm.
About 1500mg/L Cd(Ⅱ) solution was prepared by dissolving Cd(NO3)2 as required in 1L of deionized water. Each 2kg sample of air-dried soils was mixed with 1L prepared solution. The slurry was stirred mechanically for 3h with an electric stirrer and then settled down for more than 7d to attain uniform distribution of contaminant and to complete adsorption. After 7d, four samples with mass of 5g, two for the determination of initial soil pH and the other for the determination of initial concentration of contaminant, were then taken from the prepared Cd-contaminated soils. Anode purging solution of 0.0025mol/L H2SO4 and cathode electrolyte solution of 0.25mol/L H2SO4 were used over a 90h experimental period.
2.3 Methods
Constant current was used to keep the net rates of the electrolysis reactions constant and to minimize the complicated current-boundary conditions. Four pieces of ESP apparatus with the same size are connected in series in the experiment (Fig.1).
During the experiment, the overall voltage drop between the ends of the soils, the electroconductivity variations of anolyte and catholyte and the volume of electroosmotic flow were measured. Three of the four pieces of ESP apparatus were removed in turn after 24, 48 and 72h. After removal, soils in each one were sectioned into 5 segments (3cm in length) for the analysis on the amount of contaminant and soil pH. The soil pH measurement was based on a sample having a soil (dried at 110℃)/water ratio of 1∶5. pH meter was used to measure the suspensions pH value. In addition, 50mL of 0.1mol/L HCl solution was added into 10g of soil (dried at 110℃) and then the mixture was oscillated in a constant temperature oscillator at 30℃ for 1h (150r/min). After 1h, the mixture was filtered. The amount of Cd remaining in each soil segment was obtained by analyzing the filter liquor with AAS after filtration. The parameters in the experiment are summarized in Table 2.
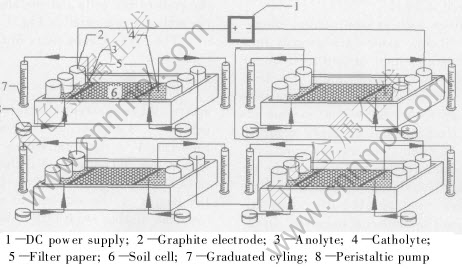
Fig.1 Schematic diagram of experimental apparatus
Table 1 Chemical and physical properties of red soils

Table 2 Parameters of ESP in experiment

3 RESULTS AND DISCUSSION
3.1 Soil pH
Changes of pH values in soil segments bed are shown in Fig.2. Generally, the soil pH values are less than the initial value of 4.17 after experiment. Under an electric field, hydrogen ions generated at the anode by the electrolysis of water migrate from anode to cathode, resulting in the decrease of the overall soil pH. It is obvious that the pH values in the soil segments near the anode are much lower than those in the vicinity of the cathode. This can be explained by the facts below: firstly, the ionic mobility of hydrogen ions is twice as high as that of the hydroxyl ions generated at the cathode[1]. As a consequence, the length that hydrogen ions transporting through soils is longer than that of hydroxyl ions when they meet. Secondly, in this study, 0.25mol/L H2SO4, which can neutralize the hydroxyl ions generated at the cathode and decrease the amount of hydroxyl ions migrating toward the anode, was used as catholyte. Lastly, as shown in Table 1, the carbonate content and the concentration of CEC of the red soils used in this study are relatively low (0.24% and 9.11×10-2mol/kg, respectively). Therefore, the soils pH buffering capacity is low, which facilitates the decrease of the overall soil pH.
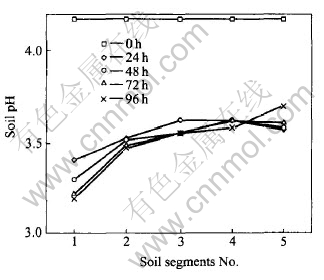
Fig.2 Changes of soil pH values in soil segments after different times
When impurities such as iron oxides, aluminum oxides and calcium oxides are present in a soil, or the carbonate content or/and CEC of the soils being treated is/are relatively high, it is difficult to make the soil pH decrease due to its high pH buffering capacity. We know that cations adsorbed on soil surface can not be easily desorbed under high pH conditions. However, ionic contaminants must be desorbed before they can be removed from soils. Therefore, soils such as illitic and bentonitic clays with high pH buffering capacity, are more difficult to be decontaminated. In order to lower the soil pH and enhance the removal efficiency of heavy metals in these soils, enhancement techniques such as injecting strong anionic complexing agents into cathode[17], conditioning technique combined with ion selective membrane[8], and adding an porous layer in soils which was continuously flushed with conditioning fluids, should be applied[15].
3.2 Electric potential difference and electroconductivity in soils
The total electric potential differences between the ends of the soils and the electroconductivity in the soils are presented in Fig.3.
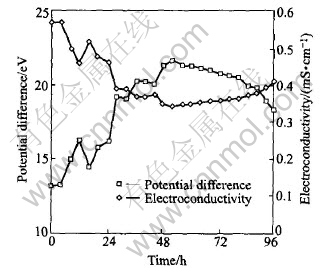
Fig.3 Total electric potential difference andelectroconductivity at different times
The electroconductivity in the soils can be calculated by[6]
![]()
where Ka is apparent electroconductivity of soils, mS/cm; I is current, mA; L is the length of soil cell, cm; V is the total voltage drop between the ends of the soil cell, V; A is the cross-sectional area of soil cell, cm2. Since the current density and the length of the soil cell in the experiment are constant in this study, the electroconductivity is inversely proportional to the voltage drop between the ends of the soils (Fig.3)
In general, the curve of total electric potential difference vs time shows three regions, the total electric potential difference increasing quickly at the initial stage of experiment, then increasing slowly with time, and decreasing gradually at the last stage of experiment. This phenomenon is remarkably related to the migration of ions in the soils, namely, it is bounded up with the chemical reactions in soils during ESP. Generally, a typical surface functional group, S(OH)m, exists on soil surface and it can accept protons at low pH and dissociate at high pH [18].
![]()
where S(OH2)m+m is the protonized S(OH)m, (SOm)m- is the product of S(OH)m after its dissociation. Metal ions in soils can hydrolyze and form hydroxyl complexes. These hydroxyl complexes can be adsorbed by soils. The adsorption reaction is given by
![]()
where Mn+L is the metal ions existing as the dissociated state, (SOm—ML)(m-n)- is the metal ions adsorbed by soils. Combining Eqns.(3) with (4) yields
![]()
At the initial stage of experiment, some hydrogen ions generated at anode are adsorbed by the red soils during its migration toward the cathode (Eqn.(2)), resulting in the decrease of the amount of ionic conductor. For this reason, the voltage drop in soils increases and the electroconductivity of soils decreases. As time passes, the concentration of hydrogen ions increases gradually caused by electrolysis of water, resulting in the equilibrium in Eqn.(5) moving toward left. Therefore, the Cd(Ⅱ) adsorbed by the red soils is gradually desorbed from soil surface and thus the amount of ionic conductor in soils increases constantly, resulting in the decrease of the total electric potential difference and the increase of the electroconductivity in the soils.
The distributions of electric potential differences in the soils are shown in Fig.4. At first, potential gradients have no big changes. However, the electric potential change fast and become space-related after many hours.
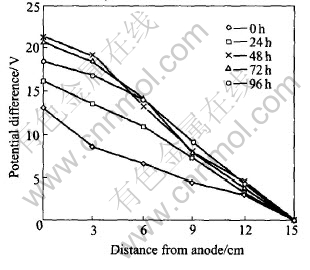
Fig.4 Electric potential difference across soils after different times
The biggest one among the five potential gradients is in the region from 6cm to 9cm, increasing from initial value of 0.7V/cm to 2.03V/cm after 72h treatment. Although it subsequently decreases to 1.7V/cm, it remains the biggest one. The potential gradients in the vicinity of the cathode are the second and those in the regions near the anode are the third. The reasons for this phenomenon may be: 1) The transportation of hydrogen ions from anode to cathode results in that the concentration of hydrogen ions in the regions near the anode is higher than that in the regions close to the cathode. Consequently, the potential gradients in the latter are bigger than those in the former. 2) 0.25mol/L H2SO4 which has relatively high concentration of hydrogen ions, was used as catholyte. Notwithstanding that it can be neutralized by hydroxyl ions produced at the cathode, hydrogen ions concentration difference between catholyte and soil pore water exists yet. As a result, hydrogen ions diffuse freely from cathode to anode due to its high diffusion coefficient, resulting in that the amount of ionic conductor in the vicinity of the cathode is bigger than that in the midmost region. Therefore, the potential gradient in the midmost region of soils is the biggest one.
3.3 Electroconductivity in electrolytes and electroosmotic flow
Variations of the electroconductivity in electrolytes are shown in Fig.5.
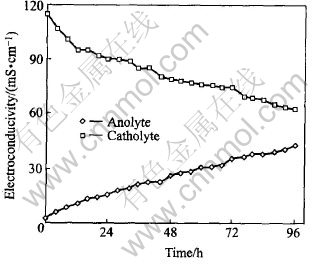
Fig.5 Electroconductivies of anolyte and catholyte at different times
As hydrogen ions were produced by the electrolysis of water, electroconductivity in anolyte increases slowly from 2.20 to 42.6mS/cm. However, the catholyte (sulfuric acid) were buffered by hydroxyl ions produced in the cathode, which result in that the electroconductivity in catholyte decreases gradually from 115 to 62.8mS/cm.
The change of cumulative electroosmotic flow volume with time is shown in Fig.6. The cumulative electroosmosis flow volume transported toward the cathod increases with time in the initial stage of experiment.
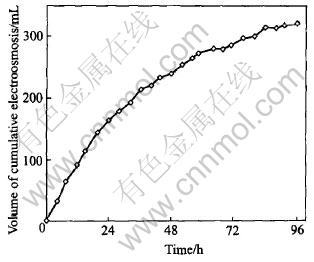
Fig.6 Volume change of electroosmotic flow with time
The main factors affecting the electroosmotic flow in the soil system are soil pH, applied electric field intensity and soil permeability. Since the pH and electroconductivity of the anolyte control the soil pH and the electroconductivity in soils, the anolyte may affect electroosmosis in soils. Electroosmotic velocity on a plane surface (veo), is expressed as[19]
![]()
where ε is permittivity of the pore liquid, C/(V·m); ζ is zeta potential of the soil, V; Ex is electric field (V/m) parallel to direction of electroosmotic flow, and μ is viscosity of the pore liquid (N·s/m2). According to the Eqn.(6), the electroosmotic velocity is significantly affected by the zeta potential. Generally, the zeta potential of most soils is negative because of a negative charge carried by soil surfaces. Therefore, electroosmotic flow is generally toward the cathode. However, when the cationic concentrations in soil pore water increase caused by increase of the cationic concentrations in the anolyte, the cations adsorbed on the surface of the clay particles will increase. The increased cations will result in decrease of the zeta potential and the electroosmotic velocity. Consequently, the decrease of the soil pH and the increase of the electroconductivity of soil pore water will make the electroosmotic velocity decrease. Based on above-mentioned descriptions, the increment of the volume of electroosmotic flow decreases significantly from the middle stage of the experiment (Fig.6). Furthermore, when the zeta potential becomes positive, the electroosmotic flow will reverse, which was observed in a few experiments[15, 17]. The reversal of electroosmosis is helpless to the removal of heavy metals in contaminated soil because it is contrary to the direction of movement of metallic cations. Negative zeta helps the electroosomosis transport toward the cathode. Low pH accelerates the dissolution and desorption of metal contaminants but facilitates the increase of the zeta potential. Therefore, how to simultaneously maintain the negative zeta potential and low pH in soils is the key in the successful implementation of the ESP.
3.4 Cd Concentration in soils and energy consumption
The distributions of Cd at different experimental stgaes are shown in Fig.7.
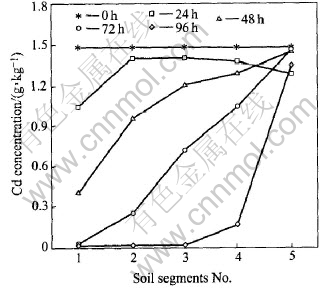
Fig.7 Cd concentrations in red soil segments
Under the electric field, that the hydrogen ions move from the anode toward the cathode makes the Cd desorb from the red soil surface. The desorbed Cd then gradually migrates toward the cathode by electromigration and electroosmosis, resulting in the decrease of the overall amount of Cd in the soils. The actions of hydrogen ions are diplex. On the one hand, the increase of hydrogen ions in the anolyte makes the hygrogen ions in the double layer increase. On the other hand the inner sphere complexes occuring at the interface between the soil particles and soil pore water make the value of the negative surface charge decrease, which is helpless to ESP because it makes the electroosmosis transport toward the cathode decrease.
Fig.8 shows the relationship between soil pH and Cd-removal efficiency. After experiment, the final soil pH and Cd-removal efficiency in segment 1 are 3.18% and 99.42%, respectively while those in segment 5 are 3.69% and 9.4%, respectively. It is obvious that the Cd-removal efficiency is pH-dependent. This pH-dependence may be explained by the change of Cd retention capacity of the red soils with the change of soil pH [20].
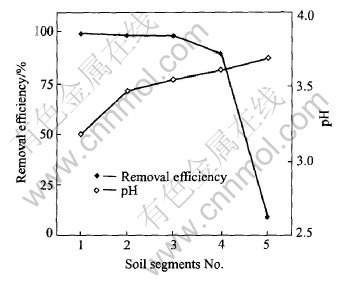
Fig.8 Relationship between soil pH and Cd-removal efficiency
After treatment for 96h, 79.18% of Cd is removed from the red soils. The removal efficiency of Cd, presumably, will be much higher if we prolong the experimental period. With a constant current density of 0.5mA/cm2 and a treatment period of 96h, the calculated energy consumption is 1.164×10-1kW·h. Since the volume of the soil cell is 1500cm3, the energy expenditure per unit volume at the end of experiment is about 77.6kW·h/m3. According to the standard electricity price of 0.549RMB Yuan/(kW·h) for ordinary industry in Changsha, China, the capital consumed by the whole experiment is 42.6RMB Yuan/m3, which indicates that ESP, due to its high removal efficiency and low energy consumption, is a promising remedial technique for the removal of Cd in red soils.
4 CONCLUSIONS
ESP is a promising technique for remedying Cd-contaminated red soils due to its high Cd-removal efficiency and low energy consumption. pH and electroosmosis are the major factors affecting the Cd removal efficiency. Low soil pH accelerates the desorption of adsorbed Cd and thus it helps to remedy the contaminated soils. At the same time, low soil pH will also make the electroosmosis transported toward the cathode decrease, which is helpless to the electrokinetic remediation. Therefore, the key of the successful implementation of ESP is how to control the soil pH so as to make the soils being treated in low pH conditions, while the electroosmosis will not reverse its direction.
REFERENCES
[1]Acar Y B, Alshawabkeh A N. Principles of electrokinetics remediation [J]. Environ Sci Technol, 1993, 27(13): 2638-2647.
[2]LI Z M, YU J W, Neretnieds I. A new approach to electrokinetic remediation of soil polluted by heavy metals [J]. J Contaminant Hydrology, 1996, 22(4): 241-253.
[3]LIU Yun-guo, LI Xin, ZENG Guang-ming, et al. Electrokinetics removal of lead from lead-contaminated red soils [J]. Trans Nonferrous Met Soc China, 2003, 13(6): 1475-1478.
[4]Pascal M, Paul B, Pascal B. Electrokinetic remediation of cadmium-spiked clayey medium: pilot test [J]. Earth & Planetary Sciences, 1999, 328(1): 37-43.
[5]QIAN Shu-qiang, JIN Wei-hua, LIU Zheng. Removal of Cu2+ from clay by electroremediation [J]. J Chem Industry Engineering, 2002, 53(3): 236-240. (in Chinese)
[6]LI Z M, YU J W, Neretnieds I. Removal of Pb(Ⅱ), Cd(Ⅱ) and Cr(Ⅲ) from sand by electromigration [J]. J Hazardous Materials, 1997, 55(3): 295-304.
[7]Mohamed A M O. Remediation of heavy metal contaminated soils via integated electrochemical processes [J]. Waste Management, 1996, 16(8): 741-747.
[8]Puppala S K, Alshawabkeh A N, Acar Y B, et al. Enhanced electrokinetic remediation of high sorption capacity soil [J]. J Hazardous Materials, 1997, 55(2): 203-220.
[9]Reddy K R, Chinthamreddy S. Sequentially enhanced electrokinetic remediation of heavy metals in low buffering clayey soil [J]. J Geotechnical and Geoenvironmental Engineering, 2003, 129(3): 263-277.
[10]ZHOU Dong-mei, Zorn R, Czurda K. Electrochemical remediation of copper contaminated kaolinite by conditioning anolyte and catholyte pH simultaneously [J]. J Environ Sci, 2003, 15(3): 396-400.
[11]ZHOU Dong-mei, DENG Chang-fen, CANG Long. Electrokinetic remediation of a Cu contaminated red soil by conditioning catholyte pH with different enhancing chemical reagents [J]. Chemosphere, 2004, 56(3): 265-273.
[12]LI Z M, YU J W, Neretnieks I. Electroremediation: removal of heavy metals from soils by using cation selective membrane [J]. Environ Sci Technol, 1998, 32(3): 394-397.
[13]Ottosen L M, Hansen H K, Ribeiro A B. Removal of Cu, Pb and Zn in an applied eelctric field in calcareous and non-calcareous soils [J]. J Hazardous Materials B, 2001, 85(3): 291-299.
[14]Lee H, YANG J. A new method to control electrolytes pH by circulation system in electrokinetic soil remediation [J]. H Hazardous Materials B, 2000, 77(2): 227-240.
[15]LI R S, LI L Y, Enhancement of electrokinetic extraction from lead-spiked soils [J]. J Environ Engineering, 2000, 126(9): 849-857.
[16]ZHOU Dong-mei, Alshawabkeh A N, Deng Chang-fen, et al. Electrokinetic removal of chromium and copper from contaminated soils by lactic acid enhancement in the catholyte [J]. J Environ Sci, 2004, 16(4): 529-532.
[17]Yeung A T, Hsu C, Memon R M. EDTA-enhanced electrokinetic extraction of lead [J]. J Geotechnical Engineering, 1996, 122(8): 666-673.
[18]LI Xue-yuan. Soil Chemistry [M]. Beijing: Higher Education Press, 2001. 185- 198. (in Chinese)
[19]Park J S, Kim S O, Kim K W, et al. Numerical analysis for electrokinetic soil processing enhanced by chemical conditions of the electrode reservoirs [J]. J Hazardous Materials B, 2003, 99(1): 71-88.
[20]LIU Yun-Guo, LI Cheng-Feng, ZHAO Xin, et al. Effect of pH on cadmium adsorption by red soil [J]. Trans Nonferrous Met Soc China, 2004, 14(S1): 60-65.
(Edited by LONG Huai-zhong)
Foundation item: Project(04JJ3013) supported by the Natural Science Foundation of Hunan Province, China; Project(2001AA644020) supported by the National Hi-tech Research and Development Program of China
Received date: 2005-04-01; Accepted date: 2005-07-11
Correspondence: LIU Yun-guo, Professor, PhD; Tel: +86-731-8649208; E-mail: liuyunguo@hnu.cn


