Trans. Nonferrous Met. Soc. China 26(2016) 1714-1720
Effects of electrolyte recycling on desulfurization from bauxite water slurry electrolysis
Ai-jing  1,2, Yi-qi SHEN1, Xu-zhong GONG1, Zhi WANG1, Yu-hua WANG1, Ming-yong WANG1
1,2, Yi-qi SHEN1, Xu-zhong GONG1, Zhi WANG1, Yu-hua WANG1, Ming-yong WANG1
1. National Engineering Laboratory for Hydrometallurgical Cleaner Production Technology, Key Laboratory of Green Process and Engineering, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China;
2. College of Environmental Science and Engineering, Liaoning Technical University, Fuxin 123000, China
Received 29 July 2015; accepted 11 January 2016
Abstract:
To lower the cost of bauxite electrolysis desulfurization using NaOH solution as the supporting electrolyte, effects of electrolyte recycling on bauxite electrolysis desulfurization were investigated. The results indicate that electrode corrosion, cell voltage, the desulfurization rate and the pH value of the electrolyte have no obvious changes with the increase of cycle times. Additionally, there were some transitive valence S-containing ions in electrolyte after the electrolysis, such as 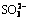 ,
, 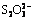 . However, most of the sulfur in bauxite was eventually oxidized into
. However, most of the sulfur in bauxite was eventually oxidized into 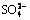 into the electrolyte, and these S-containing ions did not affect the recycling utilization for electrolyte.
into the electrolyte, and these S-containing ions did not affect the recycling utilization for electrolyte.
Key words:
high sulfur bauxite; electrolysis; desulfurization; electrolyte; recycling;
1 Introduction
During alumina production process, S-containing phase is transformed into sulfates (Na2SO4) [1], resulting in many problems, such as the decline of dissolution rate of Al2O3, the corrosion of equipment, the reduction of alumina production yield and quality [2]. Once the Na2SO4 scale is generated, the operation efficiency of the whole production system and heat transfer coefficient of the equipment will be lowered, and thus increasing the energy consumption [2]. At present, there are many methods for bauxite desulfurization, including calcination [3], flotation [3,4], wet desulfurization [5] and sintering desulfurization with adding reducing agent and so on [6]. But these methods have many limitations, such as low desulfurization rate, harsh reaction condition and complex process, especially for SO2 emission due to the calcination.
Pyrite, the main S-containing phase in high sulfur bauxite [3], is the same as the S-containing phase in coal. The process of electrolysis desulfurization relies mainly on the electrochemical oxidation. Pyrite could be oxidized into sulfate by oxidant produced from water electrolysis on anode, achieving the desulfurization through solid-liquid separation. Electrolysis desulfuriza- tion has many advantages, including low temperature, normal pressure and high desulfurization ratio [7]. It is noted that electrolysis could also remove simultaneously both organic and inorganic sulfur in minerals, such as coal desulfurization [8]. Additionally, based on the previous results [9-12], the electrolysis desulfurization is more suitable to inorganic ore compared with coal water slurry (CWS) electrolysis [13-16], since anode is covered by organic products as organic ore is electrolyzed.
In our previous work, the feasibility of bauxite electrolysis desulfurization had been verified [17]. To lower the cost of bauxite electrolysis, electrolyte recycling was proposed in this work. However, the process of electrolysis desulfurization might be affected by electrolyte recycling. With the increase of the electrolyte cycle times, the S-containing anions in electrolyte are accumulated gradually. It might bring about bad effects to the desulfurization reaction, and finally affecting the desulfurization efficiency. However, if there are no effects of electrolyte recycling on electrolysis desulfurization, electrolyte recycling could be applied to the desulfurization of bauxite electrolysis, which not only could lower the cost of production, but also could lower the environmental pollutant risk. Thus, it is very essential to study the effects of electrolyte recycling on bauxite electrolysis desulfurization.
In this work, the optimal experiment was not done for the ore sample, and thus the best experimental conditions were not obtained. However, this research was focused on the feasibility of electrolyte recycling. It did not affect the experimental results. Grinded bauxite was prepared into slurry with NaOH solution, and the effects of electrolyte recycling on desulfurization from bauxite electrolysis were studied through a variety of characterization methods. Meanwhile, the desulfurization mechanism of bauxite electrolysis in NaOH solution and the conversion of sulfur state in the filtrate were examined by the analysis of the bauxite and the filtrate before and after electrolysis.
Table 1 Composition of bauxite sample (mass fraction, %)
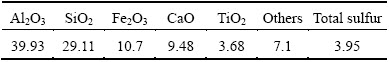
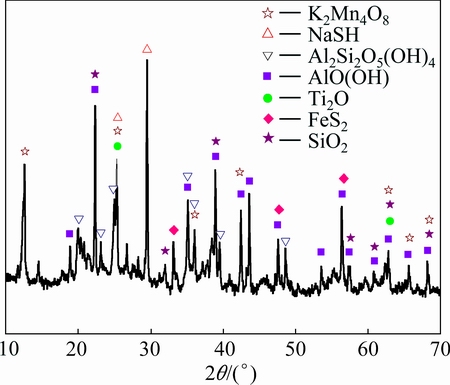
Fig. 1 XRD pattern of bauxite
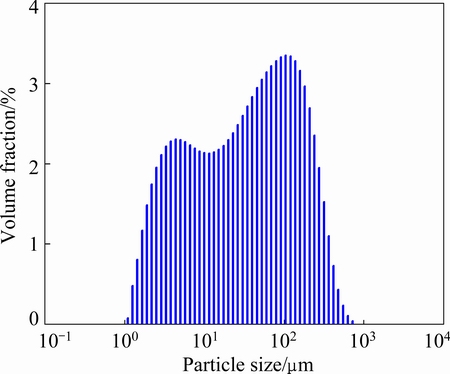
Fig. 2 Particle size distribution of bauxite
2 Experimental
2.1 Samples
A type of high sulfur bauxite from Guizhou province of China was selected as sample. Bauxite composition was analyzed by X-ray fluorescence (XRF, AXIOS, Holland), as listed in Table 1, and ore phase was characterized by X-ray diffraction (XRD, X’ Pert PRO MPD, Holland), as shown in Fig. 1. Figure 2 shows the particle distribution of bauxite. As can be seen from the data, the aluminum in bauxite mainly exists as the form of diaspore, and the sulfur mainly exists as the form of pyrite. Additionally, there are also potassium manganate and other impurities. The total sulfur content in bauxite is 3.95%.
All chemical compounds used in this work were listed as follows: NaOH solution (1.0 mol/L), dilute hydrochloric acid solution (1.0 mol/L, 3 mol/L), BaCl2 solution (10%), Pb(Ac)2 solution (1 mol/L), HNO3 solution (2 mol/L), AgNO3 solution (0.5 mol/L), ethanol (95%), Na2CO3 solution (100 g/L), ZnSO4 solution ((ZnSO4×7H2O) 100 g/L), glacial acetic acid (1 + 10), iodine standard solution (0.1 mol/L), starch indicator (5 g/L) and deionized water.
2.2 Experiment
The experimental apparatus is shown in Fig. 3, including DC-3006 circulating water bath and WYK-3030S DC power supply. The active area of the nickel electrode was 4 cm × 4 cm. Electrolysis conditions were as follows: the current of 2.4 A, the electrolysis temperature of 90 °C, the electrolyte volume of 400 mL, the slurry concentration of 25 g/L, the stirring speed of 500 r/min, the concentration of NaOH of 1.0 mol/L and the electrolysis time of 4 h. After electrolysis, the filtrate was retained by suction filtration. 1.0 mol/L NaOH was added to the filtrate in each experiment until 400 mL, and then it was reused as the electrolyte. The filter residue was washed repeatedly until there is no white precipitate generated by adding the BaCl2 solution acidified with hydrochloric acid, and then it was dried. Changes of the electrolysis voltage under the cycle constant current and the pH of the electrolyte before and after the reaction were recorded. Bauxite phase before and after electrolysis was analyzed by XRD, and the sulfur contents in bauxite before and after electrolysis were analyzed by C-S analyzer (LECO CS-344, USA). The sulfur states in the filtrate were tested by titration, and the crystal of the last filtrate was analyzed by XRD.
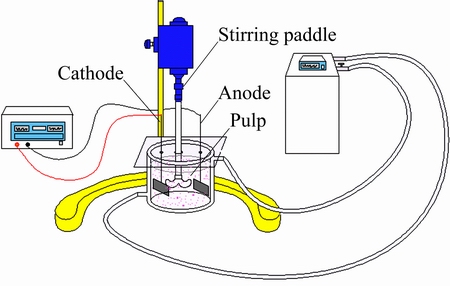
Fig. 3 Schematic diagram of electrolytic device
2.3 Desulfurization rate calculation
The method was focused on the sulfur content in the bauxite after electrolysis [17]. If considering the mass variation of bauxite, the desulfurization rate was high. Whereas the sulfur content in the bauxite after electrolysis was still high, thus the computing method was as follows:
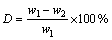 (1)
(1)
where D is the desulfurization rate; w1 and w2 are the mass fractions of sulfur in bauxite before and after electrolysis, respectively.
3 Results and discussion
3.1 Effects of electrolyte recycling on corrosion and cell voltage
Figure 4 shows the effects of electrolyte recycling on electrode. From Fig. 4, certain amount of the dark matter is deposited on cathode, and there is a little change of electrode with the increase of cycle times. There is red matter on anode, but there is no obvious corrosion on anode. As far as the corrosion of the electrode is concerned, the electrolyte can be recycled. During the bauxite electrolysis process, the anode lost electrons, and the metal ions entered into electrolyte. Additionally, FeS2 was oxidized into Fe3+ and  by oxidants on the surface of anode. Most of the Fe3+ was generated Fe(OH)3, which would become Fe2O3 further, because of the instability of Fe(OH)3 [18-20]. Then, the red oxidation film, Fe2O3, was generated covering on the surface of the anode. A small quantity of Fe3+ or Ni2+ was reduced on cathode, and forming metallic iron or nickel (alloy).
by oxidants on the surface of anode. Most of the Fe3+ was generated Fe(OH)3, which would become Fe2O3 further, because of the instability of Fe(OH)3 [18-20]. Then, the red oxidation film, Fe2O3, was generated covering on the surface of the anode. A small quantity of Fe3+ or Ni2+ was reduced on cathode, and forming metallic iron or nickel (alloy).
Figure 5 shows the changes in cell voltage with cycle times. The cell voltage has a fluctuation after the rapid decline, subsequently, being steady with time.
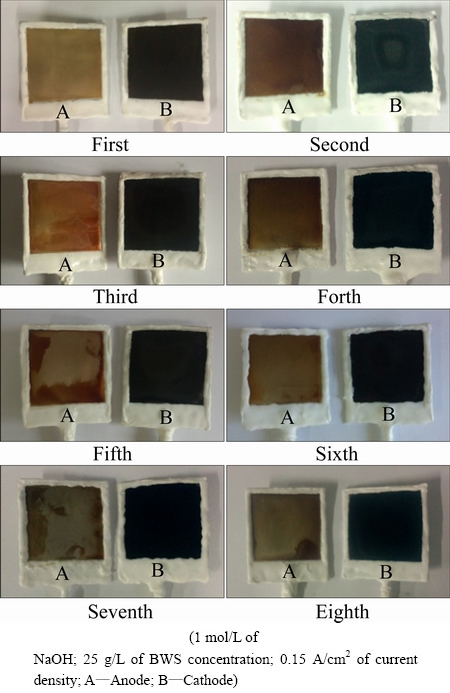
Fig. 4 Effects of electrolyte used times on electrode
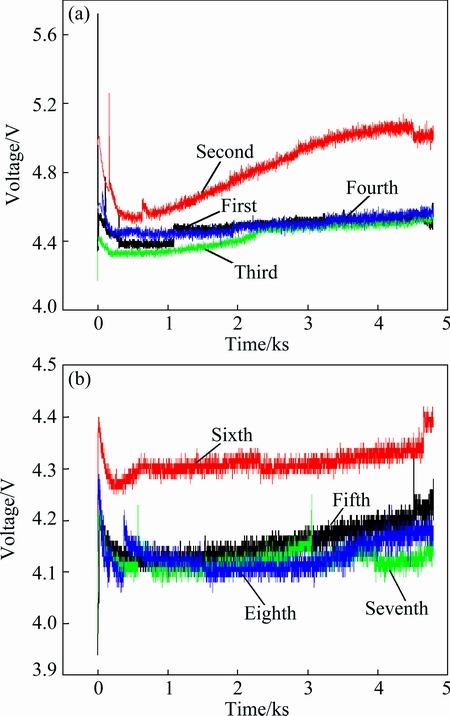
Fig. 5 Effects of electrolyte used times on cell voltage
In NaOH solution, the polarization process of nickel electrode resulted in rapid decrease in cell voltage [21]. From Fig. 5, with the increase of electrolyte cycle times, the cell voltage varies between 4 and 5 V, and there is no obvious increase or decrease. The change in the average voltage with electrolyte recycling is shown in Fig. 6. As can be seen from Fig. 6, the average voltage shows a slight downward trend with the increase of the electrolyte cycle times, resulting from the increase in ion concentration with electrolyte recycling, such as  . The lower the voltage is, the smaller the power energy consumption is. Therefore, the electrolyte recycling is beneficial to saving power energy for bauxite electrolysis.
. The lower the voltage is, the smaller the power energy consumption is. Therefore, the electrolyte recycling is beneficial to saving power energy for bauxite electrolysis.
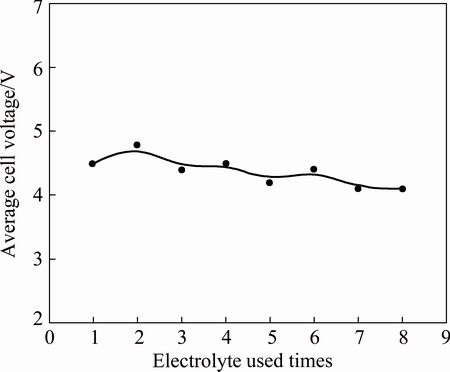
Fig. 6 Effects of electrolyte used times on average cell voltage
3.2 Changes of desulfurization rate and pH
Desulfurization rate is an important indicator for evaluating the effect of electrolyte recycling. The increase of electrolyte cycle times could effectively save the production cost under the premise of good desulfurization effects. From Fig. 7, the desulfurization rate has some changes with the increase of the electrolyte cycle times after it drops to 10% from 20% for the first time. The electrolyte recycling not only saves NaOH solution, but also lowers environmental pollutant. Because the electrolysis condition is not the best, the desulfurization rate is low. However, the above experimental conditions do not influence the research on electrolyte recycling.
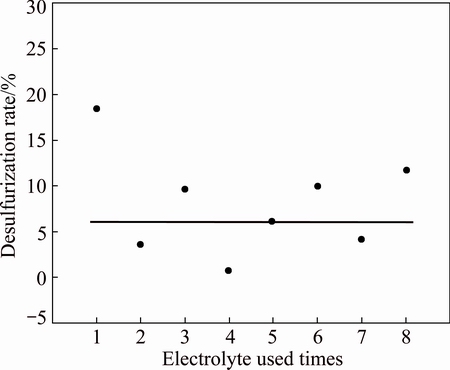
Fig. 7 Effects of electrolyte used times on desulfurization rate
Figure 8 shows the changes in the pH value with electrolyte cycle times. As we know, the pH value of 1.0 mol/L NaOH was 14. The pH value of electrolyte dropped to 13.21 after the first electrolysis, indicating that part of NaOH was consumed. The pH value of the electrolyte should be slightly less than 14, because 1.0 mol/L of NaOH was added into the electrolyte until 400 mL after each reaction. The change of pH value of the electrolyte after reaction is not obvious with the increase of the electrolyte cycle times. This indicates that part of NaOH solution has been consumed during bauxite electrolysis process. For NaOH system, FeS2 was oxidized into Fe3+ and  . But, there were not lots of Fe3+ in NaOH solution, thus OH- in solution reacted with Fe3+, generating Fe(OH)3 sediment, and resulting in the consumption of OH-.
. But, there were not lots of Fe3+ in NaOH solution, thus OH- in solution reacted with Fe3+, generating Fe(OH)3 sediment, and resulting in the consumption of OH-.
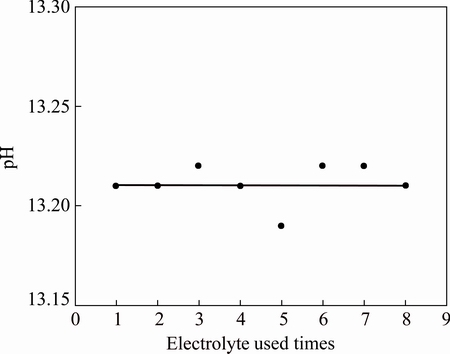
Fig. 8 Effects of electrolyte used times on pH
3.3 Phase change and sulfur form in electrolyte
Figure 9 shows the XRD patterns of bauxite after the first and the eighth electrolysis. From Fig. 9, there are mainly AlO(OH) peaks and impurities peaks containing SiO2 and TiO2 in bauxite after electrolysis. Moreover, there are Fe(OH)3 peaks which are the product of the electrolysis oxidation process of FeS2. The result indicates that the method of electrolysis oxidation could effectively remove S-containing phase in bauxite.
Figure 10 shows the XRD patterns of electrolyte crystal after the first and the eighth electrolysis. There are NaOH peaks in the electrolyte crystal after the first electrolysis. The main component of crystals is NaOH, but there is hydrate of NaOH peaks in the crystals due to the solid of NaOH absorbing water soon and becoming damp in the air. Meanwhile, there are sulfate peaks, such as K2SO4 and Na2SO4, also thiosulfate peaks, such as Na2S2O3(H2O)5. This indicates that the oxidation process of FeS2 underwent complex changes during bauxite electrolysis process. The conversion of FeS2 was not directly converted from solid phase sulfur into liquid phase sulfur ( ). There are also NaOH peaks in electrolyte crystal after the eighth electrolysis, and the main component of crystals is still NaOH. The sulfate was found, such as K2SO4, in the electrolyte crystals, indicating that FeS2 eventually could be oxidized into
). There are also NaOH peaks in electrolyte crystal after the eighth electrolysis, and the main component of crystals is still NaOH. The sulfate was found, such as K2SO4, in the electrolyte crystals, indicating that FeS2 eventually could be oxidized into  for bauxite electrolysis.
for bauxite electrolysis.
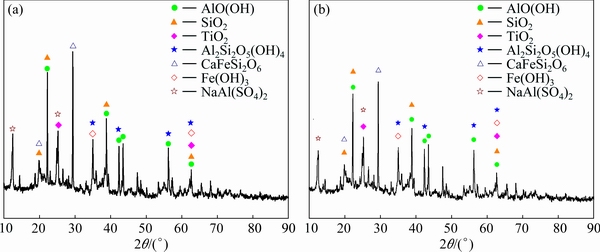
Fig. 9 XRD patterns of bauxite after the first (a) and the eighth (b) electrolysis
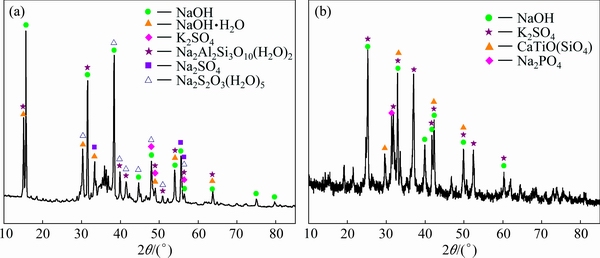
Fig. 10 XRD patterns of electrolyte crystal after the first (a) and eighth (b) electrolysis
The last (eighth) filtrate was tested by titration, and the results are shown in Table 2. The white precipitate was generated by adding the BaCl2 solution acidified with hydrochloric acid. This proves that there was  in the electrolyte solution and FeS2 eventually could be oxidized into
in the electrolyte solution and FeS2 eventually could be oxidized into  by oxidants generated from water electrolysis. There was little change after adding the Pb(Ac)2 solution dropwise into the filtrate. There was no black precipitate generated, so there was no S2- in the filtrate. There were no significant changes in the filtrate after adding the AgNO3 solution nitrated dropwise, so there was no
by oxidants generated from water electrolysis. There was little change after adding the Pb(Ac)2 solution dropwise into the filtrate. There was no black precipitate generated, so there was no S2- in the filtrate. There were no significant changes in the filtrate after adding the AgNO3 solution nitrated dropwise, so there was no 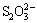 in the filtrate. The
in the filtrate. The 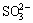 in the filtrate was titrated by GB 10500-2009 [22]. A drop of iodine standard solution was consumed until the test solution turned blue, indicating that there was almost no
in the filtrate was titrated by GB 10500-2009 [22]. A drop of iodine standard solution was consumed until the test solution turned blue, indicating that there was almost no 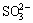 in the filtrate.
in the filtrate. 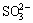 would easily be oxidized to
would easily be oxidized to  by air, so it could not be detected from the filtrate.
by air, so it could not be detected from the filtrate.
Table 2 Sulfur states in last electrolyte

GU et al [23] found that the oxidation reaction of pyrite was divided into two steps, including the oxidation of pyrite to S0 and the oxidation of S0 to  . According to the results above, it can be inferred that the oxidation process from S- of solid phase in bauxite to
. According to the results above, it can be inferred that the oxidation process from S- of solid phase in bauxite to  of liquid phase is a chain reaction process [24,25]. There were some S-containing ions from S- oxidation, such as S0,
of liquid phase is a chain reaction process [24,25]. There were some S-containing ions from S- oxidation, such as S0, 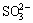 and
and 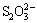 [26]. Finally, they were oxidized into
[26]. Finally, they were oxidized into  further.
further. 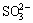 was easy to be oxidized into
was easy to be oxidized into  or
or 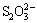 by reacting with the elemental sulfur (S0) in the solution. In the initial stage, due to the high concentration of S- in the solution, the speeds of
by reacting with the elemental sulfur (S0) in the solution. In the initial stage, due to the high concentration of S- in the solution, the speeds of 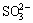 generated and consumed were both faster in the solution. The reactions could keep dynamic balance, and the concentration of
generated and consumed were both faster in the solution. The reactions could keep dynamic balance, and the concentration of 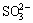 remained relatively unchanged. However, the concentration of S- was continuously reduced in the later stage. The speed of
remained relatively unchanged. However, the concentration of S- was continuously reduced in the later stage. The speed of 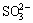 generated was reduced, so its concentration decreased with time. The concentration of
generated was reduced, so its concentration decreased with time. The concentration of 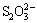 rapidly increased with time until reaching the maximum concentration, and then gradually decreased. But the production process of
rapidly increased with time until reaching the maximum concentration, and then gradually decreased. But the production process of 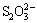 was a fast reaction process, the
was a fast reaction process, the 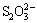 concentration remained at a high level. While the reaction process which was further oxidized into
concentration remained at a high level. While the reaction process which was further oxidized into  was slow. Thus, the main product was
was slow. Thus, the main product was  in the first filtrate after a long time reaction, followed
in the first filtrate after a long time reaction, followed 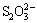 , almost no
, almost no 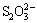 . When the cycle times increased, the
. When the cycle times increased, the 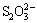 was eventually oxidized into
was eventually oxidized into  through the complex changes after the eighth time.
through the complex changes after the eighth time.
AHLBERG et al [9] suggested that the pyrite oxidation mainly generated sulfate sulfur in alkaline solution. According to the study on the mineral phase evolution of BWS electrolysis [27] and the results above, the electrode reaction for BWS electrolysis in alkaline solution is shown in Fig. 11.
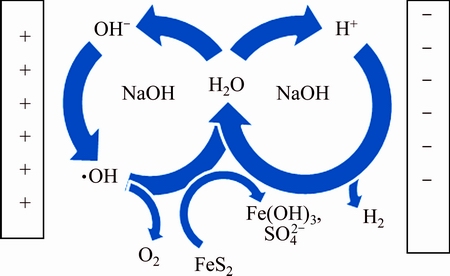
Fig. 11 Desulfurization mechanism from BWS electrolysis in alkaline solution
The main reactions of bauxite electrolysis desulfurization in alkaline solution are as follows:
Cathode: 4H2O+4e→2H2+4OH- (2)
Anode: 4OH-→O2+4e+2H2O (3)
Liquid:
4FeS2+16OH-+15O2→4Fe(OH)3+8 +2H2O (4)
+2H2O (4)
The conversion of sulfur in the liquid phase is as follows:
4S-+O2+2H2O→4S+4OH- (5)
S+O2+2OH-→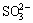 +H2O (6)
+H2O (6)
S+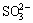 →
→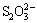 (7)
(7)
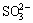 +O2→
+O2→ (8)
(8)
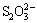 +2O2+2OH-→2
+2O2+2OH-→2 +H2O (9)
+H2O (9)
Some researches suggested that electrochemical oxidation could produce hydroxyl radical (HO×) through Fenton reaction [28-30]. In the electrochemical reaction, some active intermediates could be generated on the surface of electrode, such as HO× and HO2× [31]. These intermediates involved in oxidation reaction [32], espeically for hydroxyl radical (HO×) [33]. Thus, the reaction mechanism of desulfurization from bauxite electrolysis in alkaline solution could be speculated. In alkaline solution, a number of strong oxidants were generated on anode from water electrolysis [31], such as HO× and HO2×. Some oxidants formed oxygen gas due to their reaction, and others reacted with pyrite producing Fe(OH)3. Finally, most of the sulfur in bauxite was oxidized into  through the complicated change in valence.
through the complicated change in valence.
4 Conclusions
1) There is no obvious corrosion on the surface of electrode with electrolyte recycling. The change in pH of the electrolyte is not obvious with the increase of the cycle times of the electrolyte.
2) The recycling of electrolyte hardly influences desulfurization from BWS electrolysis. However, the electrolyte should be supplemented after each cycle due to NaOH consumption.
3) There are transitive valence S-containing ions in the electrolyte after recycling. Finally, they are oxidized into  .
.
References
[1] LI Xiao-bin, LI Chong-yang, PENG Zhi-hong, LIU Gui-hua, ZHOU Qiu-sheng, QI Tian-gui. Interaction of sulfur with iron compounds in sodium aluminate solution [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(2): 608-614.
[2] WANG Peng, WEI De-zhou. Desulfurization technology of high sulfur bauxite [J]. Metal Mine, 2012, 41(1): 108-110. (in Chinese)
[3] HU Xiao-lian, CHEN Wen-mi, XIE Qiao-ling. Sulfur phase and sulfur removal in high sulfur-containing bauxite [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(7): 1641-1647.
[4] LUO Zhao-jun, WANG Yu-hua, HU Yu-hua, QIU Guan-zhou. Optimization of grinding in reverse flotation for bauxite [J]. Transactions of Nonferrous Metals Society of China, 2001, 11(3): 444-446.
[5] HU Xiao-lian, CHEN Wen-mi. Desulfurization from sodium aluminate solution by wet oxidation [J]. Journal of Central South University: Science and Technology, 2011, 42(10): 2911-2916. (in Chinese)
[6] XIONG Dao-ling, MA Zhi-min, PENG Jian-cheng, CHEN Xiang-qing, LI Ying. Present situation of research on desulfurization of high sulfur bauxite [J]. Conservation and Utilization of Material Resources, 2012(5): 53-58. (in Chinese)
[7] LI Deng-xin, GAO Jin-sheng, YUE Guang-xi. Catalytic oxidation and kinetics of oxidation of coal-derived pyrite by electrolysis [J]. Fuel Processing Technology, 2003, 82(1): 75-85.
[8] VINCENT L, LI G C, SONG C J, CHEN J W, CRAIG F, ROB H, ZHANG J J. A review of electrochemical desulfurization technologies for fossil fuels [J]. Fuel Processing Technology, 2012, 98(6): 30-38.
[9] AHLBERG E, FORSSBERG K S E, WANG X. The surface oxidation of pyrite in alkaline solution [J]. Journal of Applied Electrochemistry, 1990, 20(6): 1033-1039.
[10] MARINI S, SALVI P, NELLI P, PESENTI R, VILLA M, BERRETTONI M, ZANGARI G, KIROS Y. Advanced alkaline water electrolysis [J]. Electrochimica Acta, 2012, 82(21): 384-391.
[11] ARSLAN F, DUBY P F. Electro-oxidation of pyrite in sodium chloride solutions [J]. Hydrometallurgy, 1997(46): 157-169.
[12] ZHAO Wei, ZHU Hong, ZONG Zhi-min, XIA Jian-hua, WEI Xian-yong. Electrochemical reduction of pyrite in aqueous NaCl solution [J]. Fuel, 2005, 84(2): 235-238.
[13] OSHINOWO T, OFI O. Kinetics of chemical desulphurization of coal in aqueous ferric chloride [J]. The Canadian Journal of Chemical Engineering, 1987, 65(3): 481-486.
[14] LALVANI S B. Coal desulfurization [M]. New York: Marcel Pekker, 1979.
[15] LALVANI S B, PATA M, COUGHLIN R W. Sulphur removal from coal by electrolysis [J]. Fuel, 1983, 62(4): 427-437.
[16] LALVANI S B, NAND S, COUGHLIN R W. Electrolytic pretreatment of Illinois No. 6 coal [J]. Fuel Processing Technology, 1985, 11(1): 25-36.
[17] GE Lan, GONG Xu-zhong, WANG Zhi, ZHAO Li-xin, WANG Yu-hua, WANG Ming-yong. Sulfur removal from bauxite water slurry (BWS) electrolysis intensified by ultrasonic [J]. Ultrasonics Sonochemistry, 2015, 26: 142-148.
[18] KONINCK M D,  D. The electrochemical generation of ferrate at pressed iron powder electrode: Comparison with a foil electrode [J]. Electrochimica Acta, 2003, 48(10): 1435-1442.
D. The electrochemical generation of ferrate at pressed iron powder electrode: Comparison with a foil electrode [J]. Electrochimica Acta, 2003, 48(10): 1435-1442.
[19] BOUZEK K,  I, TAYLOR M A. Influence of anode material on current yield during ferrate (VI) production by anodic iron dissolution. Part II: Current efficiency during anodic dissolution of pure iron to ferrate (VI) in concentrated alkali hydroxide solutions [J]. Journal of Applied Electrochemistry, 1996, 26(9): 925-931.
I, TAYLOR M A. Influence of anode material on current yield during ferrate (VI) production by anodic iron dissolution. Part II: Current efficiency during anodic dissolution of pure iron to ferrate (VI) in concentrated alkali hydroxide solutions [J]. Journal of Applied Electrochemistry, 1996, 26(9): 925-931.
[20] HE Wei-chun, WANG Jian-ming, YANG Chang-chun, ZHANG Jian-qing. The rapid electrochemical preparation of dissolved ferrate (VI): Effects of various operating parameters [J]. Electrochimica Acta, 2006, 51(10): 1967-1973.
[21] LI Di. Electrochemical principle [M]. Beijing: Beijing University of Aeronautics and Astronautics Press, 2008. (in Chinese)
[22] GB 10500-2009. Industrial sodium sulfide [S]. Beijing: China Standard Publishing House, 2009. (in Chinese)
[23] GU Guo-hua, SUN Xiao-jun, HU Ke-ting, LI Jian-hua, QIU Guan-zhou. Electrochemical oxidation behavior of pyrite bioleaching by Acidthiobacillus ferrooxidans [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(5): 1250-1254.
[24] YUAN Shao-jun, LIANG Bin, ZHAO Yu, PEHKONEN S O. Surface chemistry and corrosion behaviour of 304 stainless steel in simulated seawater containing inorganic sulphide and sulphate-reducing bacteria [J]. Corrosion Science, 2013, 74(3): 353-366.
[25] AWE S A, SUNDKVIST J E,  Formation of sulphur oxyanions and their influence on antimony electrowinning from sulphide electrolytes [J]. Minerals Engineering, 2013, 53(10): 39-47.
Formation of sulphur oxyanions and their influence on antimony electrowinning from sulphide electrolytes [J]. Minerals Engineering, 2013, 53(10): 39-47.
[26] ZHONG Shi-teng, ZHAO Wei, SHENG Chen, XU Wen-juan, ZONG Zhi-min, WEI Xian-yong. Mechanism for removal of organic sulfur from guiding subbituminous coal by electrolysis [J]. Energy Fuels, 2011, 25(8): 3687-3692.
[27] GONG Xu-zhong, ZHUANG Si-yuan, GE Lan, WANG Zhi, WANG Ming-yong. Desulfurization kinetics and mineral phase evolution of bauxite water slurry (BWS) electrolysis [J]. International Journal of Mineral Processing, 2015, 139: 17-24.
[28]  D R, SHARMA A K, FINNEY W C, LOCKE B R. The role of Fenton’s reaction in aqueous phase pulsed streamer corona reactors [J]. Chemical Engineering Journal, 2001, 82(1-3): 189-207.
D R, SHARMA A K, FINNEY W C, LOCKE B R. The role of Fenton’s reaction in aqueous phase pulsed streamer corona reactors [J]. Chemical Engineering Journal, 2001, 82(1-3): 189-207.
[29] TOMAT R, RIGO A. Electrochemicalied oxidation of toluene promoted by OH radicals [J]. Journal of Applied Electrochemistry, 1983, 14(1): 1-8.
[30] JIANG Cheng-chun, ZHANG Jia-fa. Progress and prospect in electro-Fenton process for wastewater treatment [J]. Journal of Zhejiang University (Science A) , 2007, 8(7): 1118-1125.
[31] OTURAN M A, AARON J J. Advanced oxidation processes in water/wastewater treatment: Principles and Applications—A review [J]. Critical Reviews in Environmental Science and Technology, 2014, 44(23): 2577-2641.
[32] QU Jiu-hui, LIU Hui-juan. The electrochemical principle and technology of water treatment [M]. Beijing: Science Press, 2007. (in Chinese)
[33] POLCARO A M, PALMAS S, RENOLDI F, MASCIA M. On the performance of Ti/SnO2 and Ti/PbO2 anodes in electrochemical degradation of 2-chlorophenol for wastewater treatment [J]. Journal of Applied Electrochemistry, 1999, 29(2): 147-151.
电解液循环对铝土矿电解脱硫的影响
吕艾静1,2,沈羿麒1,公旭中1,王 志1,汪玉华1,王明涌1
1. 中国科学院 过程工程研究所 湿法冶金清洁生产国家工程实验室,中国科学院绿色过程工程实验室,北京 100190;
2. 辽宁工程技术大学 环境科学与工程学院,阜新 123000
摘 要:为了降低电解脱硫成本,采用NaOH溶液作为电解液,研究电解液循环对铝土矿电解过程的影响。研究发现随着电解液循环次数增多,电极腐蚀程度、电解电压、脱硫率及母液pH值均无明显变化。另外,电解后电解液中含有一些过渡态的含硫离子,如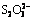 和
和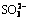 ,最终被氧化为
,最终被氧化为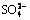 溶于水中,这些离子的生成不影响电解液的回收利用。
溶于水中,这些离子的生成不影响电解液的回收利用。
关键词:高硫铝土矿;电解;脱硫;电解液;循环
(Edited by Xiang-qun LI)
Foundation item: Projects (51004090, 51474198) supported by the National Natural Science Foundation of China; Project (KF13-03) supported by State Key Laboratory of Advanced Metallurgy University of Science and Technology Beijing; Project (2015036) supported by Youth Innovation Promotion Association, Chinese Academy of Sciences
Corresponding author: Xu-zhong GONG; Tel/Fax: +86-10-82544926; E-mail: xzgong@ipe.ac.cn
DOI: 10.1016/S1003-6326(16)64280-2
Abstract: To lower the cost of bauxite electrolysis desulfurization using NaOH solution as the supporting electrolyte, effects of electrolyte recycling on bauxite electrolysis desulfurization were investigated. The results indicate that electrode corrosion, cell voltage, the desulfurization rate and the pH value of the electrolyte have no obvious changes with the increase of cycle times. Additionally, there were some transitive valence S-containing ions in electrolyte after the electrolysis, such as 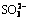 ,
, 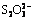 . However, most of the sulfur in bauxite was eventually oxidized into
. However, most of the sulfur in bauxite was eventually oxidized into 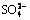 into the electrolyte, and these S-containing ions did not affect the recycling utilization for electrolyte.
into the electrolyte, and these S-containing ions did not affect the recycling utilization for electrolyte.


