J. Cent. South Univ. (2017) 24: 1941-1952
DOI: https://doi.org/10.1007/s11771-017-3602-x
Removal of Cd(II) and Pb(II) from soil through desorption using citric acid: Kinetic and equilibrium studies
TANG Qiang(唐强)1, 2, 3, ZHOU Ting(周婷)1, GU Fan(顾凡)4, WANG Yan(王艳)5, CHU Jia-ming(褚嘉明)1
1. School of Rail Transportation, Soochow University, Suzhou 215000, China;
2. Key Laboratory for Geomechanics and Embankment Engineering, Ministry of Education,Hohai University, Nanjing 210098, China;
3. Jiangsu Research Center for Geotechnical Engineering Technology, Hohai University, Nanjing 210098, China;
4. Texas A&M Transportation Institute, Texas A&M University, College Station, TX 77843, USA;
5. Architectural, Civil Engineering and Environment College, Ningbo University, Ningbo 315211, China
 Central South University Press and Springer-Verlag GmbH Germany 2017
Central South University Press and Springer-Verlag GmbH Germany 2017
Abstract:
The desorption test was conducted to evaluate the desorption behavior of Pb(II) and Cd(II) using citric acid. The influential factors that were considered included initial Pb(II), Cd(II) contamination levels in soil, concentration of citric acid, reaction time, soil pH value and ionic strength. The test results indicated that the desorption was a rapid reaction (less than 6 h), and the removal percentages of Cd(II) and Pb(II) increased with the increasing contamination levels, concentration of citric acid and the addition of Na+, Ca2+, Cl-. However, the desorption of Pb(II) and Cd(II) decreased with the addition of SO42– because of the precipitation in the form of MSO4(s). The high pH condition indicated a negative effect on Pb(II) desorption. The removal percentage decreased from 71.39% to 10.9% as pH increased from 2 to 10.8. The desorption behavior predicted by Visual MINTEQ was in good agreement with the experimental testing result. The results of X-ray diffraction (XRD), X-ray fluorescence (XRF) and N2-BET adsorption test demonstrated that the desorption behavior of heavy metals (i.e., Pb(II) and Cd(II)) was controlled by the affinity of sorption sites for heavy metals, the competition of H+, Ca2+, Na+, Cl– and the chelating of organic ligands.
Key words:
Cd(II) and Pb(II); desorption; citric acid; mechanism; simulation;
1 Introduction
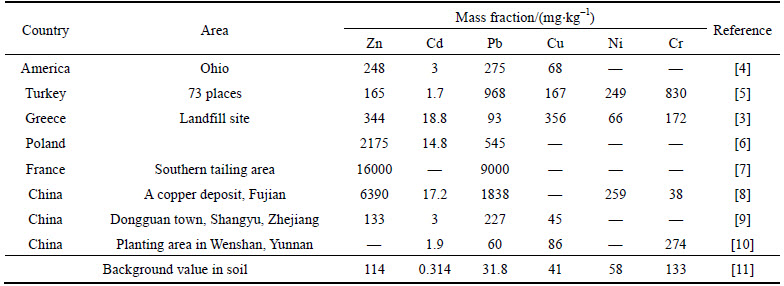
With the development of industrialization and urbanization, heavy metal contamination in soil causes a worldwide concern [1]. For example, approximately 20 million hectare of arable soils in China were polluted in the last decade [2]. In northern Greece, the heavy metals diffused from a closed unlined landfill were detected at 16 m underground due to the migration nature of the contaminant [3]. Table 1 summarized some selected cases of heavy metal pollution in soils in various countries. It was demonstrated that the concentration of some heavy metals in the polluted soil is more than 100 times higher than that of the natural soil. Distinct from the biodegraded contaminants, heavy metals are prone to keep in a stable status in the form of oxide or salt, which resulted in the pollution of soil or groundwater [12, 13]. Furthermore, heavy metals have the tendency to accumulate and cause great harm to human health [14-16].
Among these heavy metals, Pb(II) is a non-essential element to human body. Excessive intake of Pb(II) can damage the nervous, skeletal, circulatory, enzymatic, endocrine, and immune systems [17]; Cd(II) is another heavy metal, which can cause lung cancer, pulmonary adenocarcinomas, prostatic proliferative lesions, bone fractures, kidney dysfunction hypertension [18]. Since the chronic exposure to Cd(II) which caused the “itai-itai” disease, more than 100 local residents died in Japan from 1922 to 1965 [19]. Therefore, the upper limit values of Cd(II) and Pb(II) in drinking water have been restricted in many countries and organizations. For instance, the upper limit concentration of Cd(II) in Japan is 0.01 mg/L [20], and in the US, EU, Canada and China, this value is 0.005 mg/L [21-23]. According to the standard of World Health Organization (WHO), the upper limit concentration of Cd(II) is only 0.003 mg/L [24]; The upper limit concentration of Pb(II) is 0.01 mg/L in most countries and organizations, while this value in US is a little high around 0.015 mg/L [20-23]. The concentration of heavy metals in groundwater, to a great extent, depends on the amount of heavy metals in the soil. Thus, to avoid the potential threaten to the human health, it is important to remove the heavy metals from the contaminated soil.
Table 1 Heavy metal pollution in soils in various countries and districts
Chemical leaching and flushing/washing is proven to be effective in remediating the heavy metal- contaminated soil. The conventional reagents include acid, alkali, and salt inorganic compounds due to the low cost and effective characterization. TAMPOURIS and PAPASSIOPI [25] selected HCl and CaCl2 solution as the leaching agent for the soil column experiment. They found that the removal percentages of Cd(II) and Pb(II) reached 70% and 94%, respectively; however, the leaching agent caused a negative effect on the surrounding soil environment. According to PICHTEL and PICHTEL [26], 2%-8% HCl can remove most of the heavy metals; however, it resulted in the dissolution of half of the soil matrix. YANG et al [27] conducted the desorption tests using different acids. They pointed out that the desorption capacity of acetic acid at high concentration (>10-3 mol/L) was higher than that of malic acid, and the desorption capacity of malic acid was higher than that of oxalic acid. Compared to strong acid, WANG and BRUSSEAU [28] found that the weak organic acids such as citric acid can not only leach out the heavy metal ions, but also improve the soil structure.
Citric acid was widely used in many industry fields, which yielded a large amount of citric acid-containing- wastewater. Considering all of above, citric acid was selected for the evaluation of the desorption behavior towards Pb(II) and Cd(II) contaminated soils. This study takes into account the following influential factors: heavy metal contamination level in the soil, concentration of the citric acid, reaction time, soil pH and ionic strength. The experimental condition was simulated by Software Visual MINTEQ to calculate the ionic form and the distribution through out the desorption process. Combined the experimental and numerical results, the mechanisms were discussed in accordance with the X-ray diffraction (XRD) patterns, X-ray fluorescence (XRF) results and N2-BET adsorption data.
2 Materials and methods
2.1 Soils and solutions
The soils used in this study were colleted from suburb in Suzhou, China (N31°32′, E120°56′), where the strata were formed in the Quaternary Period, consisting of loose deposits. To eliminate the interference of human activities, all the soil was collected from underground at a depth of 3-4 m, which was categorized to the typical lacustrine sediment soil. Firstly, the soil sample was dried in the oven at 105 °C for 24 h (101-A, Leao, China) after the tree roots and rocks were removed. Then, the soil samples were pulverized (YB-1000A, Sufeng Industrial, China), screened through a 0.075 mm opening sieve, homogenized and eventually stored for later use. N2-BET adsorption tests were conducted to analyze various parameters of the soil, including the specific surface area (SSA), total pore volume and average pore size (NOVA2000e, Quantachrome, US). Natural moisture content was measured following JIS A 1203. Swelling index of the soil was measured following ASTM D 5890-06. In the aspect of the liquid limit and plastic limit, they were determined according to GB/T50123-1999. Besides, grain size distribution of the soil was tested according to GB/T50123-1999. pH and EC of the sample were measured by pH/EC meter (PH-2603, Lohand, China) following JGS 0211 and JGS 0212, respectively. Chemical composition of the soil was analyzed by XRF (JSX-3400R, JEOL, Japan).
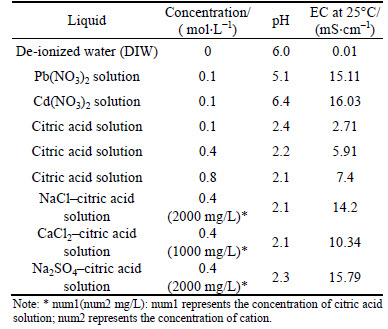
Table 2 presents the types of solution used in this study. De-ionized water (DIW) was prepared from tap water by using a water distillation apparatus (RFD240NA, Advantec, Japan). Pb(NO3)2 and Cd(NO3)2 solids (Analytical reagent, Sinopharm, China) were dissolved in the DIW to prepare standard solutions, and then diluted into a target concentration of 0.1 mol/L. Citric acid solid (Analytical reagent, Sinopharm, China) was dissolved in DIW, following by dilution to obtain the target concentrations of 0.1, 0.4 and 0.8 mol/L. Certain amount of NaCl, CaCl2 and Na2SO4 solid (Analytical reagent, Sinopharm, China) was dissolved in 0.4 mol/L citric acid to reach the target concentrations of 2000 mg/L (Na) and 1000 mg/L (Ca), respectively.
Table 2 Chemical properties of solutions
2.2 Preparation of Pb(II), Cd(II) contaminated soil
Firstly, 5 g soil was weighed (AR2130, Ohaus Corp, China) and added into a 50 mL centrifuge tube. To reach the target contamination level of Pb(II), Cd(II) in the soil (1, 5, 10 mg/g), the amount of Pb(II), Cd(II) (5, 25, 50 mg) was introduced by mixing the soil with certain amount of diluted Cd(NO3)2 or Pb(NO3)2 solutions. According to TANG et al [29], the Pb(II) adsorption percentage onto soil reached 90% after 90 h. Thus the mixtures in this work were shaken for 96 h using a shaking bed (HY-4A, Xinrui, China). Then, all of the samples were centrifuged (TDZ4-WS, Xiangyi, China) at 3000 r/min for 10 min. The volume of supernatant was measured directly and the concentration of the heavy metal was measured through inductive coupled plasma emission spectrometer (ICPE-9820, SHIMADZU, Japan). The contamination level of the heavy metal on the soil was determined by Eq.(1).
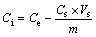 (1)
(1)
where Ci is the actual concentration of Pb(II) or Cd(II) in the soil (mg/g); Ce is the expectant initial concentration of Pb(II) or Cd(II) in the soil (mg/g); Cs is the concentration of Pb(II) or Cd(II) in supernatant (mg/L); Vs represents the volume of supernatant (L); and m is the mass of soil (g). All of the samples were conducted in duplicate for averaging the results.
2.3 Desorption test
The experimental program used in this study is shown in Table 3. The experimental program was separated into 5 groups for evaluating the different influential factors. 5 g soil was taken for use in every group. For group 1, the contamination level of heavy metal in the soil was adjusted at 1, 5 and 10 mg/g. After being blended with 40 mL of 0.4 mol/L citric acid solutions, the soil mixtures were shaken for 6 h. For group 2, 40 mL of 0.1, 0.4, 0.8 mol/L citric acid solutions were blended with soil (contamination level, 5 mg/g), before the soil mixtures were shaken for 6 h. For group 3, 40 mL of 0.4 mol/L citric acid solution were blended with the soil which contamination levels fixed at 5 mg/g; then the soil mixtures were shaken for 2, 6 and 12 h, respectively. For group 4, to discover the effect of soil pH on desorption, HCl and NaOH solution were utilized to adjust the pH environment of soil mixtures to acidity or alkalinity, while the contamination levels of heavy metals in soils were still fixed at 5 mg/g. 40 mL of 0.4 mol/L citric acid solutions were added and the centrifuge tubes were shaken for 6 h. For group 5, 40 mL of 0.4 mol/L citric acid-salt solutions (NaCl-citric acid solution, CaCl2-citric acid solution and Na2SO4 citric acid solution) were blended with soils (contamination level, 5 mg/g); then the soil mixtures were shaken for 6 h. After shaking, the samples were centrifuged at 3000 r/min for 10 min. The volume of the supernatants was measured directly, while the concentration of heavy metals was measured by ICPE. All of the samples were conducted in duplicate for averaging the results.
3 Results and discussion
3.1 Characterization of soil sample
The engineering properties of the soil measured in this study are detailed in Table 4. The natural moisture content of the soil was 23.78%; the swelling index was 2.0 mL/2g-solid, which indicated the soils contained little expansive content such as montmorillonite [30]. The liquid limit and plastic limit of the soil were 41.54% and 16.57%, respectively. According to GB 50021–2001, it was concluded that the soil used in the study was categorized to hard plastic state. The pH of soil was 6.58. Compared to the pH value of Heilongjiang soil (i.e., pH=7.35) used by QIN et al [31], the soil used in this study was slightly acid, which probably was due to the acid rain in Suzhou region. The EC of the soil was 57.68 mS/cm. The particle size distribution of the soil is presented in Fig. 1. The uniformity coefficient and coefficient of curvature were 120 and 7.5, respectively. It was obvious that the particle size distribution was non-uniform. As shown in Fig. 1, the particle size distribution curve was steep when the particle size ranged from 0.1 to 1 mm, which indicated that the particle size distribution was dense within this range.
Table 3 Experimental program
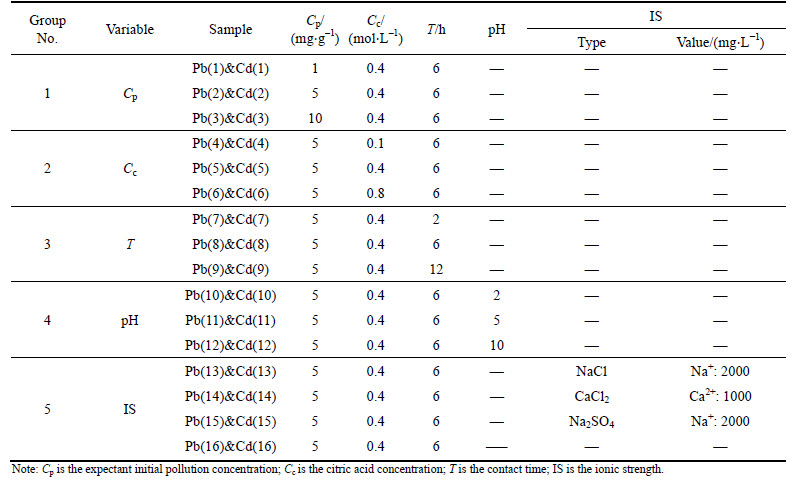
Table 4 Selected properties of utilized soil
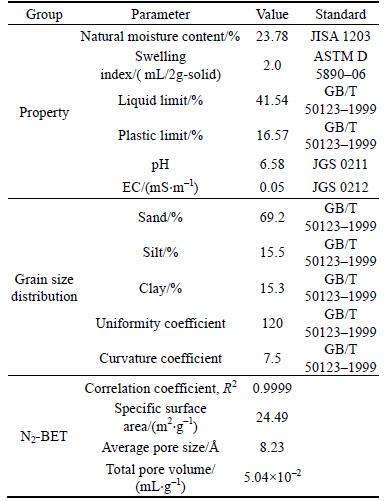
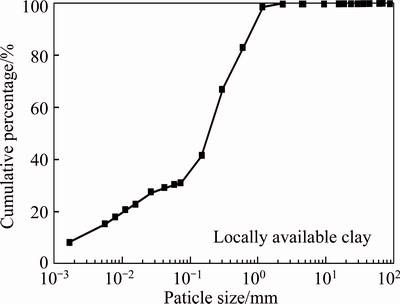
Fig. 1 Particle size distribution curve of soil
The specific surface area (SSA) of the soil used in this experiment was 24.49 m2/g. Comparing the value of SSA to that of the bentonite (i.e., SSA=731 m2/g) used by GLATSTEIN and FRANCISCA [32], the quantity of montmorillonite in the soil was low. While compared to the SSA of Heilongjiang soil (i.e., SSA=4.64 m2/g) used by QIN et al [31], the soil used in this experiment had a much higher SSA since the relative small particle size was utilized in this study. According to BHATTACHARYYA and GUPTA [33], a higher SSA provided more contacts between heavy metals and sorption sites on soil surface, which yielded to a higher adsorption capacity. The average pore size of the soil was 8.23  and the total pore volume was 5.04×10–2 mL/g.
and the total pore volume was 5.04×10–2 mL/g.
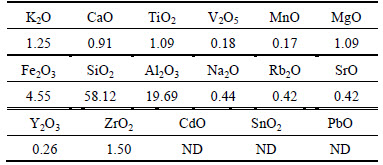
Figure 2 presents the XRD pattern of the soils, and the results of XRF analysis of the soil are shown in Table 5. Based on the XRD pattern shown in Fig. 2, quartz (SiO2) was observed at 2θ=20.76°, 26.51°, 45.69°, 54.77°, 73.41°, 75.60°, 79.90° and 90.83°, while the existence of albite (NaAlSi3O8) was also detected since its characteristic peak appeared at 2θ=27.87°. The observation of these two substances was consistent with the XRF analysis results as shown in Table 5, in which SiO2 accounted for 58.12% of the total content. Based on the above findings, the Suzhou clay used in study presented a similar chemical composition as Fukakusa clay in TANG et al [30], in which both quartz and albite were observed and SiO2 accounts for 49.3% of the total content. According to the XRF results, the soil contained 19.69% Al2O3 and other oxides, such as Fe2O3 (4.55%), ZrO2 (1.5%), K2O (1.25%), MgO (1.09%), TiO2 (1.09%), CaO (0.91%), Na2O (0.44%), Rb2O (0.42%), SrO (0.42%), Y2O3 (0.26%), V2O5 (0.18%) and MnO (0.17%). The observation of Fe2O3, CaO and MgO listed in XRF results was in accordance with the presence of phosphosiderite (Fe+3PO4·2H2O), calcite (CaCO3), monticellite (CaMgSiO4), antigorite (Mg6[Si4O10](OH)8) and magnesite(MgCO3), whose characteristic peaks were found around 2θ=42.35°, 39.34°, 50.06°, 40.16° and 59.83° as shown in Fig. 2. The magnesite was also found in natural kaolin [24], which was attributed to the heavy metal adsorption process.
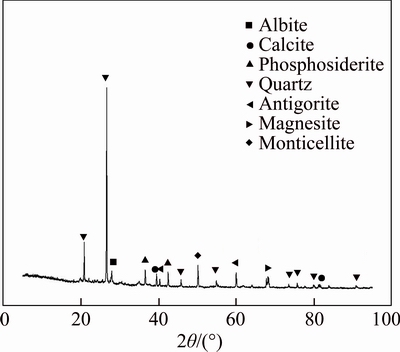
Fig. 2 XRD pattern of soil
Table 5 Chemical compositions of utilized soil (mass fraction, %)
3.2 Results and discussion of adsorption process
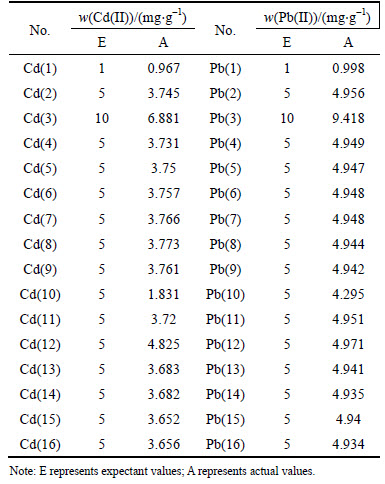
The expected and actual initial heavy metal concentrations of the soils are presented in the Table 6. At a low concentration level, the Pb(II) and Cd(II) adsorption amounts increased accordingly with the increasing heavy metal concentrations. The positive correlation between concentration of heavy metals and adsorption amount was attributed to the increasing occupations of the available binding sites [34, 35]. At a high concentration level, the adsorption amount of Pb(II) still increased with the concentration level. Most of the Pb(II) was found to be adsorbed by the soils. It was also shown that the adsorption amount of Cd(II) remained unchanged, which nearly reached the maximum value (i.e., 6.881 mg/g). The maximum adsorption capacity towards Cd(II) herein was close to 9.37 mg/g [36], which was much lower than that of Pb(II) adsorption on Chinese loess (i.e., 270.26 mg/g) according to LI et al [37]. This was because the affinity of the surface of soil towards Pb(II) was much greater than that towards Cd(II) [38]. According to WANG et al [36] and LI et al [37], the heavy metals might be adsorbed on the clay minerals through the electrostatic force with the permanent negative sorption sites, which was expressed as
Table 6 Expectant and actual contamination level of Cd(II) and Pb(II)
 SO—K+M2++
SO—K+M2++  SO—K→
SO—K→  SO—M—OS
SO—M—OS + K+ (2)
+ K+ (2)
According to TANG et al [39], some function groups, such as hydroxyl group (—OH) and carboxyl group (—COOH), remained stable, even after oven dry process. These two function groups were recognized as the adsorption site, by which the heavy metal ions can be fixed effectively. The adsorption process was expressed as
—COOH+M2+→COOM++H+ (3)
 SOH+M2+→
SOH+M2+→  SOM++H+ (4)
SOM++H+ (4)
Based on Eqs. (3) and (4), the heavy metal complexes were generated. YEE [40] proposed another type of heavy metals fixation that weak protonation and deprotonation reactions occurred at the silanol group (—SiOH) on the surface of quartz. Therefore, Cd(II) and Pb(II) were likely to be absorbed in the form of surface complex in this study, which was illustrated as
—SiOH+M2+→—SiOM++H+ (5)
In addition, as a very reactive component of soil, manganese oxide was also observed that can adsorb the heavy metals. The powerful bind, to some extent, can restrict the release of heavy metals during the desorption process.
3.3 Results and discussion of desorption test
Figures 3(a) and (b) present the effect of Cd(II) and Pb(II) contamination levels on the desorption behavior of the soils, respectively. It was observed that the desorption amount of Cd(II) and Pb(II) increased with the initial pollution concentration, which indicated the effectiveness of citric acid solution to dissolve the fixed heavy metals. With the increasing contamination level, the removal percentage of Cd(II) decreased as shown in Fig. 3(a). This might be because the insufficient amount of organic ligands played a dominant role in the heavy metal desorption [32]. According to ZHAO et al [41], adsorption of heavy metals in soils was divided as a nonspecific adsorption and a specific adsorption. The adsorption process was primarily a specific adsorption at a low contamination level, while was substituted by a nonspecific adsorption at a high contamination level. The nonspecific adsorption was retained on the negatively charged sites on the soil colloids by the electrostatic attractions. It was also attracted by other cations through ion exchange [42]. Thus, it was rational that the removal of Pb(II) at the contamination level of 5 mg/g was more than that of 1 mg/g, as shown in Fig. 3(b). The sequent declined afterwards when the contamination level increased to 10 mg/g due to the lack of the organic ligands during the desorption process.
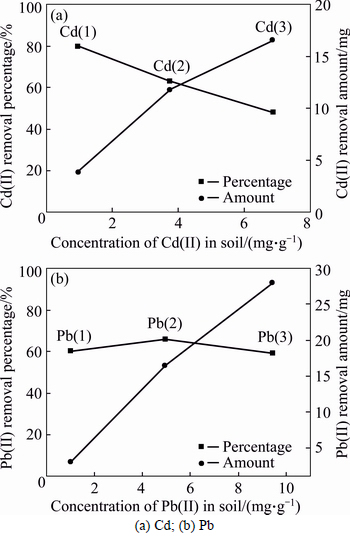
Fig. 3 Desorption behaviors under various contamination level:
The desorption behavior under various concentrations of citric acid is shown in Fig. 4. When the concentration of citric acid increased from 0.1 to 0.8 mol/L, the removal percentage of Cd(II) increased from 58.70% to 71.57%. Meanwhile, the removal percentage of Pb(II) increased from 54.72% to 70.05%. In Fig. 4, the sharp increase of the removal percentages of Cd(II) and Pb(II) was also observed at the citric concentration ranged from 0.1 mol/L to 0.4 mol/L. This might be attributed to the drop of soil pH. On one hand, the pH condition changed the cohesion of soils towards the heavy metals, which was caused by the pH- dependent proton competition, surface potential, and surface-charge density on soil colloids [43]. Such a variation in cohesion resulted in the decrease of heavy metal adsorption amount at lower pH conditions. On the other hand, organic acid provided H+ ions and organic ligands [36]. H+ ions were likely to compete with the heavy metals for the adsorption sites on the soil surface, which led to the increase of the desorption on the heavy metals. This process was expressed as
—COOM++H+→ —COOH+M2+ (6)
 SOM++H+→
SOM++H+→ SOH+M2+ (7)
SOH+M2+ (7)
—SiOM++H+→—SiOH+M2+ (8)
Organic ligands chelated with Pb2+, PbOH+, Cd2+,CdOH+ to form Pb–citrate and Cd–citrate, which yielded smaller amount of Pb2+ or Cd2+ adsorbed onto the soil surface. The organic ligands were not only adsorbed on the external surface, but also incorporated into the structural network of the soil. All of these factors significantly influenced the kinetics of metal adsorption by varying the charge of soils. Moreover, the ratio of organic ligand concentration in solution to the organic ligand adsorbed by the soil increased with the continuously enhancement of organic acid concentrations. An increased competitive ability of organic ligands in the solution for adsorbing sites with metals resulted in the enhanced desorption with the increasing organic acid concentrations beyond a certain level [27]. In addition, the difficulty for desorption at specific adsorption site condition could explain the smooth trend when the concentration of citric acid increased from 0.4 to 0.8 mol/L.

Fig. 4 Desorption behaviors under various concentration of citric acid:
The effect of contact time on the desorption behaviors of Cd(II) and Pb(II) is shown in Fig. 5. As shown in Fig. 5(a), the removal of Cd(II) desorbed from the soil was not sensitive towards the variation of contact time setting at 2 h, 6 h and 12 h. This was due to the rapid reaction between citric acid and fixed heavy metal ions in a short time (less than 2 h). This phenomenon illustrated that the desorption by citric acid towards Cd(II) contaminated soil was effective. Such a remediation method can save a lot of time when the technology is used in practice. Figure 5(b) showed an increasing tendency to the influence of contact time. The reaction reached equilibrium when the contact time increased from 6 to 12 h, which indicated that 6 h was the optimum contact time for removing Pb(II). Although the reaction duration was slightly longer than that of Cd(II), the method was still not time consuming compared with other literatures as shown in Table 7. Moreover, the total removal percentages of two heavy metals (i.e., Cd(II) and Pb(II)) reached almost 70%.
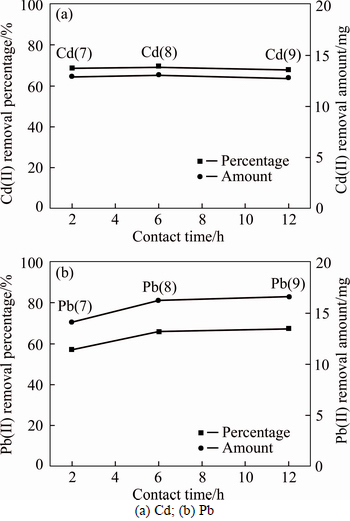
Fig. 5 Desorption behaviors under different contact time:
The effect of pH on the desorption behavior of Cd(II) and Pb(II) is shown in Fig. 6. Figure 6(b) demonstrates that the desorption percentage of Pb(II) decreased from nearly 71.39% to 10.9% in the soils with pH 2-10.8. This indicated that the removal of Pb(II) was significantly dependent on the pH condition. The similar findings were also found in other previous literatures [27, 31]. In general, more heavy metals were likely to be released from the soil at a lower soil pH condition. The increasing pH led to an increase of the heavy metal adsorption [46]. The sensitivity of pH-dependent desorption was mainly demonstrated by the following four points: 1) H+ ion could be absorbed in the bonding sites of mineral surface, which led to the increasing amount of the heavy metal desorption. The reactions were in agreement with Eqs. (6)-(8); 2) Hydroxyl groups tended to fix protons in acidic condition. As a result, the concentration of effective anion in soil surface decreased, meanwhile the adsorption capacity towards heavy metals shown as Eq. (2) was reduced [45]; 3) More hydroxyl groups was dissociated and the zeta potential of soils became more negative, which further increased the soil pH. Such a condition variation yielded that the specific adsorption is enhanced, which stemmed from the existence of surface charge. The reaction was expressed as Eqs. (3)-(4) [47]. This change in affinity led to the decrease of heavy metal desorption with the increase of the pH value; 4) pH changed the ionic forms of heavy metals. With the increase of pH value, the hydrolysis of metal cations increased, whose reaction equation was written as
Pb2++H2O→Pb(OH)++H+ (9)
The hydrolyzed forms had lower solvation energies for surface binding than aquo metal ions, which resulted in less desorption of heavy metals [48]. Furthermore, precipitations of insoluble lead hydroxides and lead carbonates were generated in the form of
Pb(OH)++OH-→Pb(OH)2(s) (10)
Pb(OH)++CaCO3(s)→PbCO3(s)+Ca2++OH- (11)
Based on Eqs. (10) and (11), the desorption became more difficult. Distinct from Pb(II), the results of Cd(II) was explained by different Cd(II) contamination level. It was well recognized that that pH highly affected the adsorption/desorption behavior of heavy metals on soils. Therefore, at a low concentration of Cd(II) during the preparation of the contaminated soil, the adsorption type was dominated by the specific adsorption, which caused a low removal percentage in acidic condition. The pH environment was simulated by Visual MINTEQ, and the calculation results are shown in Fig. 7. When pH increased from 1 to 4, the concentration of citrate-Cd increased from 6.75% to 94.58%; meanwhile the concentration of citrate-Pb increased from 14.93% to 99.04%. On the contrary, the concentration of Cd2+ decreased from 93.25% to 5.42%, and that of Pb2+ decreased from 85.06% to 0.96%. This indicated that the amount of organic ligands increased with the increasing of pH, which resulted in the increase of the desorption of Pb(II) and Cd(II). This also demonstrated that citric acid was effective in desorbing the heavy metals through the formation of the citrate complexes. As the pH increased to above 11, desorption of heavy metals tended to decrease, which was mainly caused by the precipitation generation as described in Eq. (10).
Table 7 Summary of heavy metal removal rate by different types of desorption agents
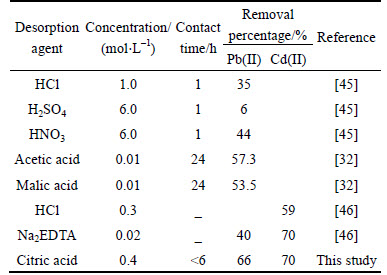
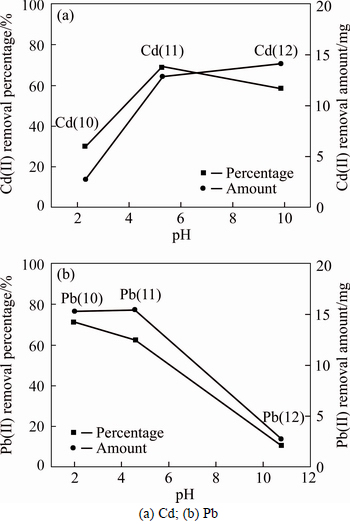
Fig. 6 Desorption behaviors under different pH conditions:
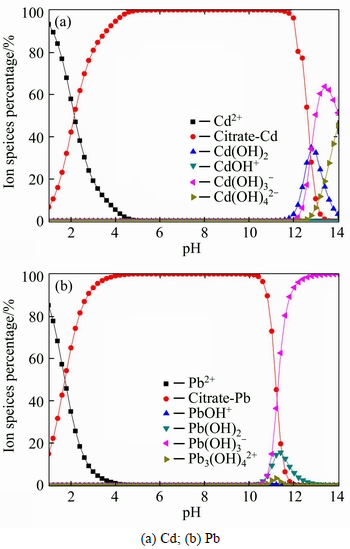
Fig. 7 Calculation results of species distribution under variable pH condition:
The effect of ionic strength on the desorption behaviors of Cd(II) and Pb(II) is shown in Fig. 8. The desorption of Cd(II) and Pb(II) was observed to increase with the addition of Na+, Ca2+, Cl–. This effect was attributed to: 1) the competition of high concentrations of cations in electrolyte with cadmium or lead for the adsorption sites on the soils surface; 2) the decrease of the activities of Cd(II) or Pb(II) due to the increasing ionic strength; 3) the formation of ionic pairs or chelating compounds, which is expressed as [49]
 SO—M—OS
SO—M—OS +Na+→
+Na+→ SONa+M2+ (12)
SONa+M2+ (12)
 SO—M—OS
SO—M—OS +Ca2+→
+Ca2+→ SO—Ca—OS
SO—Ca—OS +M2+ (13)
+M2+ (13)
M2++Cl-→MCl+ (14)
MCl++Cl-→MCl2 (15)
For different cations, it followed the order: CaCl2> NaCl, which was consistent with the ion exchange ability of Ca2+ and Na+ from large to small [25]. Additionally, the presence of salts may compress the electric double layer surrounding the negatively charged surfaces [30], which contributed to the release of adsorbed Cd(II) or Pb(II). However, with SO42- added, the precipitate was formed due to the combination of Pb(II) and Cd(II) with SO42-, which resulted in the decrease of desorption of Pb(II) and Cd(II). That was expressed by the following equation:
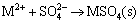 (16)
(16)
The conditions of salt introduction were simulated by Visual MINTEQ. The calculation results are shown from Fig. 9 to Fig. 12. According to Fig. 9(a), with the concentration of Na+ increased from 0 to 0.01 mol/L, the concentration of Cd-citrate decreased from 42.64% to 42.34%, at the same time, the concentration of Cd2+ increased from 57.35% to 57.65%. The similar phenomenon was also found in Fig. 9(b). That might be due to the competition of Na+ with Cd(II) or Pb(II). The competition between Ca2+ and heavy metal ions is observed in Fig. 10. As can be seen from Fig. 11, with the additive amount of Cl- from 0 to 0.05 mol/L, the concentration of Cd(II) and Pb(II) decreased from 57.83% to 29.15% and 34.94% to 29.04%, respectively. Meanwhile, the concentration of CdCl+ and PbCl+ increased from 0 to 45.29% and 0 to 18.11%. This indicated that the formation of ionic pairs decreased the concentration of Cd(II) and Pb(II). The corresponding reactions were presented in Eqs. (14)-(15). Figure 12 shows that with the addition of SO42– from 0 to 0.04 mol/L, the amount of precipitation in the form of CdSO4 and PbSO4 slightly increased, whose reactions were described in Eq.(16).
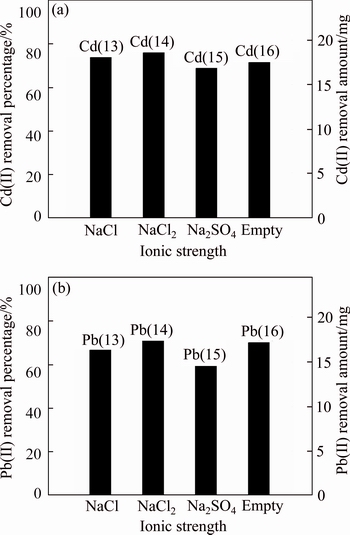
Fig. 8 Desorption behavior under different ionic strength
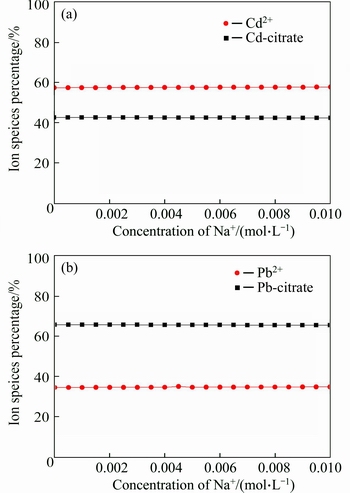
Fig. 9 Calculation results of species distribution under variable Na+ concentration
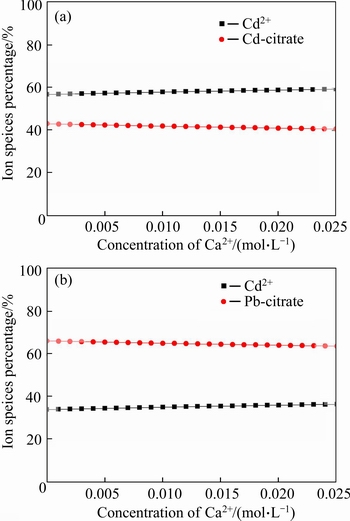
Fig. 10 Calculation results of species distribution under variable Ca2+ concentration
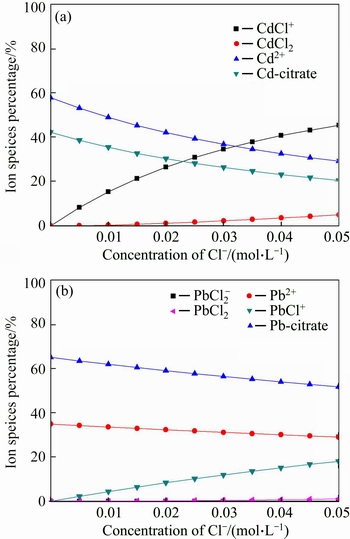
Fig. 11 Calculation results of species distribution under variable Cl- concentration
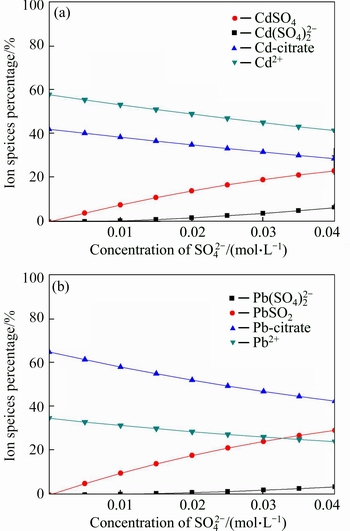
Fig. 12 Calculation results of species distribution under variable SO42- concentration
According to Figs. 3-6 and Fig. 8, it was concluded that Cd(II) was more prone than Pb(II) to be desorbed. This was because Pb(II) had a great affinity for the surface of soil than Cd(II) [30]. The similar finding was also found in Ref. [50], in which Pb(II) was recognized as the most difficult heavy metal for release. Besides, this finding was also consistent with the investigation results shown in Table 1. Table 7 shows the removal efficiencies of the different desorbents under the different conditions. In this study, the reaction duration was less than 6 h. Compared to the strong acid such as HCl, H2SO4, HNO3 whose reaction time is less 1 h, it took a longer time for reaction using citric acid. However, the average values of Pb(II) and Cd(II) removal efficiencies in this paper were 66% and 70% respectively. It was much higher than that of strong acid (less than 50%). Therefore, citric acid had higher efficiency on desorbing heavy metals.
4 Conclusions
In this study, the desorption behavior of Cd(II) and Pb(II) from Suzhou soils was evaluated. The influential factors that were taken into account included the initial Pb(II), Cd(II) contamination levels in the soil, concentration of citric acid, reaction time, soil pH value and ionic strength. The test results indicated that the citric acid solution was effective in extracting heavy metal from the soil. The removal percentages of two heavy metals Cd(II) and Pb(II) can reach almost 70% in a short period (less than 6 h). The removal efficiency of Cd(II) was higher than that of Pb(II) under the same conditions, because Pb(II) has great affinity for the surface of adsorption than Cd(II). Through changing the affinity of sorption sites for heavy metals, the quantity of H+, organic ligands and the forms of metals, pH significantly affected desorption of heavy metals in soils. As pH increased, the desorption of heavy metal decreased. The desorption of heavy metals increased with the addition of H+, Ca2+, Na+, Cl- due to the competition of cations with cadmium or lead for the adsorption sites on soils surface, the formation of ionic pairs and the chelating of compounds. Because of the formation of precipitation, the efficiency of desorption decreased with the existence of SO42-.
References
[1] SOLGI E, ESMAILI-SARI A, RIYAHI-BAKHTIARI A, HADIPOUR M. Soil contamination of metals in the three industrial estates, Arak, Iran [J]. Bull Environ Contam Toxicol, 2012, 88: 634-638.
[2] WEI C Y, CHEN T B. Hyperaccumulators and phytoremediation of heavy metal contaminated soil: A review of studies in China and abroad [J]. Acta Ecol Sin, 2001, 21: 1196-1203.
[3] KASASSI A, RAKIMBEI P, KARAGIANNIDIS A. Soil contamination by heavy metals: Measurements from a closed unlined landfill [J]. Bioresource Technology, 2008, 99: 8578-8584.
[4] RITTER C, RINEFIERD S M. Natural background and pollution levels of some heavy metals in soils from the area of Dayton, Ohio [J]. Environmental Geology, 1983, 5(2): 73-78.
[5] COSKUN M, STEINNES E, VILADIMIROVNA M. Heavy metal pollution of surface soil in the Thrace region, Turkey [J]. Environmental Monitoring and Assessment, 2006, 119: 545-556.
[6] VERNER J F, RAMSEY M H. Heavy metal contamination of soils around a Pb-Zn smelter in Bukowno [J]. Poland Applied Geochemistry, 1996, 11(1, 2): 11-16.
[7]  C, RABOYEAU S, DOSSANTOS A, GRUBER W, MAREL J C C,
C, RABOYEAU S, DOSSANTOS A, GRUBER W, MAREL J C C,  H, NORET N, MAHIEU S, COLLIN C, VAN OORT F. Heavy metal concentration survey in soils and plants of the Les Malines Mining District (Southern France): Implications for soil restoration [J]. Water, Air, and Soil Pollution, 2011, 216(1-4): 485-504.
H, NORET N, MAHIEU S, COLLIN C, VAN OORT F. Heavy metal concentration survey in soils and plants of the Les Malines Mining District (Southern France): Implications for soil restoration [J]. Water, Air, and Soil Pollution, 2011, 216(1-4): 485-504.
[8] XU Y L, OUYANG T, CHEN J J. Heavy metal contamination in the soil of a copper mine [J]. Environmental Science & Technology, 2009, 32(11): 146-151.
[9] LI J, YU T M, ZHOU J. Assessment of health risk for mined soils based on critical thresholds for lead, zinc, cadmium and copper [J]. Environmental Science & Technology, 2008, 29(8): 2327-2330.
[10] LIN L Y, YU B B, YAN X L, LIAO X Y, ZHANG Y X. Accumulation of soil Cd, Cr, Cu, Pb by Panaxnotoginseng and its associated health risk [J]. Acta Ecologica Sinica, 2014, 34(11): 2868-2875.
[11] CUI X, SUN X L, HU P J, CHENG Y, LUO Y M, WU L H, CHRISTIE P. Concentrations of heavy metals in suburban horticultural soils and their uptake by Artemisia selengensis [J]. Pedosphere, 2015, 25(6): 878-887.
[12] TANG Q, LIU W, WANG H Y, CHENG R, QIAN Y F. Membrane behavior of bentonite-amended Fukakusa clay under K, Na and Ca solutions [J]. Journal of Central South University, 2016, 23: 3122-3131.
[13] TANG Q, KATSUMI T, INUI T, LI Z Z. Influence of pH on the membrane behavior of bentonite amended Fukakusa clay [J]. Separation and Purification Technology, 2015, 141: 132-142.
[14] TANG Q, ZHANG Y, GAO Y F, GU F. Use of cement-chelated solidified MSWI fly ash for pavement material: Mechanical and environmental evaluations [J]. Canadian Geotechnical Journal, 2017, Doi: 10.1139/cgj-2017-0007.
[15] TANG Q, LIU Y, GU F, ZHOU T. Solidification/stabilization of fly ash from a municipal solid waste incineration facility using Portland cement [J]. Advances in Materials Science and Engineering, 2016: 7101243, doi:10.1155/2016/7101243.
[16] TANG Q, KIM H J, ENDO K, KATSUMI T, INUI T. Size effect on lysimeter test evaluating the properties of construction and demolition waste leachate [J]. Soils and Foundations, 2015, 55(4): 720-736.
[17] ZHANG X W, YANG L S, LI Y H, LI H R, WANG W Y, YE B X. Impacts of lead/zinc mining and smelting on the environment and human health in China [J]. Environ Monit Assess, 2012, 184: 2261-2273.
[18]  J, BIZIUK M. Methodological evaluation of method for dietary heavymetal intake [J]. Journal of Food Science. 2008, 73: R21-R29.
J, BIZIUK M. Methodological evaluation of method for dietary heavymetal intake [J]. Journal of Food Science. 2008, 73: R21-R29.
[19] SUN Y, SUN G, XU Y, WANG L, LIANG X, LIN D. Assessment of sepiolite for immobilization ofcadmium-contaminated soils [J]. Geoderma, 2003, 193-194: 149-155.
[20] Japan. Ministry of Health, Labour and Welfare. Drinking water quality standards [EB/OL][2015]. http://www.mhlw.go.jp/stf/ seisakunitsuite/bunya/topics/bukyoku/kenkou/suido/kijun/kijunchi.html.
[21] USEPA. National Primary Drinking Water Regulations [EB/OL]. [2001]. http://water.epa.gov/drink/contaminants/.
[22] EU. Meeting of the Drinking Water Committee, Drinking Water Regulations 98/83/EC, [EB/OL]. [2013]. http://ec.europa.eu/ environment/water/water-drink/legislation_en.html.
[23] Canada. Guidelines for Canadian Drinking Water Quality [EB/OL]. 2014. http://www.hc-sc.gc.ca/ewh-semt/pubs/water-eau/sum_guide- res_recom/index-eng.php.
[24] WHO. Guidelines for drinking-water quality, fourth edition [EB/OL]. [2011]. http://www.who.int/water_sanitation_health/publications/2011/ dwq_guidelines/en/.
[25] TAMPOURIS S, PAPASSIOPI N I. Removal of contaminant metals from fine grained soil using agglomeration chloride solutions and pile leaching techniques [J]. Journal of Hazard Material, 2001, 84(2): 297-319.
[26] PICHTEL J, PICHTEL T M. Comparison of solvents for ex situ removal of chromium and lead from contaminated soil [J]. Environmental Engineering Science, 1997, 14(2): 97-104.
[27] YANG J Y, YANG X E, HE Z L, LI T Q, SHENTU J L, STOFFELLA P J. Effects of pH, organic acids, and inorganic ions on lead desorption from soils [J]. Environmental Pollution, 2006, 143: 9-15
[28] WANG X J, BRUSSEAU M L. Simultaneous complexation of organic compounds and heavy metals by a modified cyclodextrin [J]. Environmental Science and Technology, 1995, 29(10): 2632-2635.
[29] TANG Q, TANG X W, LI Z Z, CHEN Y M, KOU N Y, SUN Z F. Adsorption and desorption behaviour of Pb(II) on a natural kaolin: Equilibrium, kinetic and thermodynamic studies [J]. Journal of Chemical Technology and Biotechnology, 2009, 84: 1371-1380.
[30] TANG Q, KATSUMI T, INUI T, LI Z Z. Membrane behavior of bentonite-amended compacted clay [J]. Soils and Foundations, 2014, 54(3): 329-344.
[31] QIN F, SHAN X Q, WEI B. Effects of low-molecular-weight organic acids and residence time on desorption of Cu, Cd, and Pb from soils [J]. Chemosphere, 2004, 57: 253-263.
[32] GLATSTEIN D A, FRANCISCA F M. Influence of pH and ionic strength on Cd, Cu and Pb removal from water by adsorption in Na-bentonite [J]. Applied Clay Science, 2015, 118: 61-67.
[33] BHATTACHARYYA K G, GUPTA S S. Removal of Cu(II) by natural and acid-activated clays: An insight of adsorption isotherm, kinetic and thermodynamics [J]. Desalination, 2011, 272 (1): 66-75.
[34] TANG Q, TANG X W, LI Z, WANG Y, HU M M, ZHANG X J, CHEN Y M. Zn(II) removal with activated firmiana simplex leaf: Kinetics and equilibrium studies [J]. Journal of Environmental Engineering, 2012, 138(2): 190-199.
[35] TANG Q, WANG H Y, TANG X W, WANG Y. Removal of aqueous Ni(II) with carbonized leaf powder: Kinetics and equilibrium [J]. J Cent South Univ, 2016, 23: 778-786.
[36] WANG Y, TANG X W, CHEN Y M, ZHAN L T, LI Z Z, TANG Q. Adsorption behavior and mechanism of Cd(II) on loess soil from China [J]. Journal of Hazardous Materials, 2009, 172: 30-37.
[37] LI Z Z, TANG XW, CHEN Y M, WANG Y. Sorption Behavior and Mechanism of Pb(II) on Chinese Loess [J]. Journal of Environmental Engineering, 2009, 135(1): 58-67.
[38] JUWARKAR A A, KIRTI V A N, SINGH S K, DEVOTTA S. Biosurfactant technology for remediation of cadmium and lead contaminatedsoils [J]. Chemosphere, 2007, 68: 1996-2002.
[39] TANG Q, TANG X W, HU M M, LI Z Z, CHEN Y M, LOU P. Removal of Cd(II) from aqueous solution with activated Firmiana Simplex Leaf: Behaviors and affecting factors [J]. Journal of Hazardous Materials, 2010, 179: 95-103.
[40] YEE N. Experimental studies of adsorption in bacteria–water–rock systems: Implications for heavy metal transport in the subsurface [D]. Indiana: University of Notre Dame, 2001.
[41] ZHAO X L, JIANG T, DU B. Effect of organic matter and calcium carbonate on behaviors of cadmium adsorption–desorption on/from purple paddy soils [J]. Chemosphere, 2014, 99: 41-48.
[42] LOGANATHAN P, VIGNESWARAN S, KANDASAMY J, NAIDU R. Cadmium sorptionand desorption in soils: A review [J]. Critical Reviews in Environmental Science and Technology, 2012, 42(5): 489-533.
[43] NAIDU R, KOOKANA R S, SUMNER M E, HARTER R D, TILLER K G.. Cadmium sorption and transport in variablecharge soils: A review [J]. Journal of Environmental Quality, 1997, 26: 602-617.
[44] MOUTSATSOU A, GREGOU M, MATSAS D, PROTONOTARIOS V. Washing as a remediation technology applicable insoils heavily polluted by mining–metallurgical activities [J]. Chemosphere, 2006, 63(10): 1632–1640.
[45] YANG Z H, ZHANG S J, LIAO Y P, LI Q, WU B, WU R. Remediation of heavy metal contamination in calcareous soil by washing with reagents: A column washing [J]. Procedia Environmental Sciences, 2012, 16: 778-785.
[46] JOPONY M, YOUNG S D. The solid–solution equilibriaof lead and cadmium in polluted soils [J]. European Journal of Soil Science, 1994, 45: 59–70.
[47] HE H P. Studies on the interaction of clayed mineral and metallic ions [M]. Beijing: Petrolic Industrial Press, 2001. (in Chinese)
[48] JAMES R O, HEALY T W. Adsorption of hydrolysablemetal ions at the oxide–water interface III. A thermodynamic model of adsorption [J]. Journal of Colloid and Interface Science, 1972, 40: 65–81.
[49] YUAN S H, XI Z M, JIANG Y, WAN J Z, WU C, ZHENG Z H, LU X H. Desorption of copper and cadmium from soils enhanced by organic acids [J]. Chemosphere, 2007, 68: 1289–1297.
[50] LAFUENTE A L, QUINTANA J R, VAZQUEZ A, ROMERO A. Mobility of heavy metals in poorly developed carbonate soils in the Mediterranean region [J]. Geoderma, 2008, 145: 238–244.
(Edited by HE Yun-bin)
Cite this article as:
TANG Qiang, ZHOU Ting, GU Fan, WANG Yan, CHU Jia-ming. Removal of Cd(II) and Pb(II) from soil through desorption using citric acid: kinetic and equilibrium studies [J]. Journal of Central South University, 2017, 24(9): 1941–1952.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-017-3602-xFoundation item: Projects(51708377, 51678311) supported by the National Natural Science Foundation of China; Project(BK20170339) supported by the Natural Science Foundation of Jiangsu Province, China; Project(2016M591756) supported by the China Postdoctoral Science Foundation; Project(17KJB560008) supported by the Natural Science Fund for Colleges and Universities in Jiangsu Province, China; Project(1601175C) supported by the Jiangsu Planned Projects for Postdoctoral Research Funds, China; Project(2016ZD18) supported by the Jiangsu Provincial Department of Housing and Urban-Rural Development, China; Project(2016T05) supported by the Jiangsu Provincial Transport Bureau, China; Project(2017A610304) supported by the Natural Science Foundation of Ningbo City, China; Project supported by the Bureau of Housing and Urban-Rural Development of Suzhou, China
Received date: 2016-04-08; Accepted date: 2016-07-10
Corresponding author: GU Fan, PhD; E-mail: tracygufan@tamu.edu
Abstract: The desorption test was conducted to evaluate the desorption behavior of Pb(II) and Cd(II) using citric acid. The influential factors that were considered included initial Pb(II), Cd(II) contamination levels in soil, concentration of citric acid, reaction time, soil pH value and ionic strength. The test results indicated that the desorption was a rapid reaction (less than 6 h), and the removal percentages of Cd(II) and Pb(II) increased with the increasing contamination levels, concentration of citric acid and the addition of Na+, Ca2+, Cl-. However, the desorption of Pb(II) and Cd(II) decreased with the addition of SO42– because of the precipitation in the form of MSO4(s). The high pH condition indicated a negative effect on Pb(II) desorption. The removal percentage decreased from 71.39% to 10.9% as pH increased from 2 to 10.8. The desorption behavior predicted by Visual MINTEQ was in good agreement with the experimental testing result. The results of X-ray diffraction (XRD), X-ray fluorescence (XRF) and N2-BET adsorption test demonstrated that the desorption behavior of heavy metals (i.e., Pb(II) and Cd(II)) was controlled by the affinity of sorption sites for heavy metals, the competition of H+, Ca2+, Na+, Cl– and the chelating of organic ligands.


