Trans. Nonferrous Met. Soc. China 25(2015) 915-925
First-principles calculations of structural, electronic, elastic and thermal properties of phase M2SiC (M=Ti, V, Cr, Zr, Nb, Mo, Hf, Ta and W)
B. GHEBOULI1, M. A. GHEBOULI2,3, M. FATMI2, L. LOUAIL2, T. CHIHI2, A. BOUHEMADOU4
1. Laboratory of Studies of Surfaces and Interfaces of Solids Materials, University of Setif 1, Setif 19000, Algeria;
2. Research Unit on Emerging Materials, University of Setif 1, Setif 19000, Algeria;
3. Department of Physics, University of Bordj Bou-Arreridj, 34000, Algeria;
4. Laboratory for Developing New Materials and their Characterization, Department of Physics, Faculty of Science, University of Setif 1, Setif 19000, Algeria
Received 30 April 2014; accepted 4 September 2014
Abstract:
The structural, electronic and elastic properties of the M2SiC phases were studied, where M are 3d, 4d, and 5d early transition metals. The valence electron concentration (VEC) effect of Ti, V, Cr, Zr, Nb, Mo, Hf, Ta and W on these properties was examined. The C44 saturates for a VEC value in surrounding of 8.5 for each serie. Hf-s, Ta-s and W-s electrons mainly contribute to the density of states at the Fermi level, and should be involved in the conduction properties. The distortion increases with increasing VEC and decreasing kc/ka factor except for the series M=Ti, V and Cr, where it is lower at the VEC value of 8.5 (it follows a parabolic variation). The M2SiC was characterized by a profound anisotropy for the shear planes 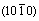 and compressibility in the direction is higher than that along the cone except for W2SiC, where it is lower.
and compressibility in the direction is higher than that along the cone except for W2SiC, where it is lower.
Key words:
ternary carbides; ab initio calculation; crystal structural; electronic structure;
1 Introduction
The MAX phases with chemical formula Mn+1AXn, where M is a transition metal, A is an A-group element, and X is C or N and n varies from 1 to 3, discovered by NOWOTNY [1], have recently attracted the interest of both material scientists and physicists due to their astonishing combination of properties. These materials combine some of the best attributes of metals and ceramics. They behave as metals in terms of their machinability, electrical and thermal conductivities. They behave as ceramics in terms of their specific stiffness and high temperature oxidation resistances [2-14]. This unique combination of characteristics makes them potential materials for many applications, such as rotating electrical contacts and bearings, heating elements, nozzles, heat exchangers, tools for die pressing [10]. Many of these applications are currently field-tested and are at various stages of development. Based on the n value, this class of materials form three groups, M2AX or 211, M3AX2 or 312 and M4AX3 or 413.
The physical properties of MAX phases vary from phase to another and depend on M, A and X elements. M2SiC (M=Ti, V, Cr, Zr, Nb, Mo, Hf, Ta and W) phases are members of this fascinating family of materials. In the present work, we report the first-principles study of the structural, electronic and elastic properties of M2SiC (M=Ti, V, Cr, Zr, Nb, Mo, Hf, Ta and W) phases as a function of valence electron concentration (VEC) (average number of valence electrons per atom), by using the state of the pseudo-potential plane-wave method (PP-PW) in the framework of the density functional theory (DFT) in conjunction with the generalized gradient approximation (GGA).
2 Computational method
The first-principles calculations were performed by employing the PP-PW approach based on the DFT [15,16] and implemented in the Cambridge Serial Total Energy Package [17]. The exchange-correlation potential was treated within the generalized gradient approximation (GGA) of Perdew, Burke and Ernzerhof [18]. In order to reduce the required number of plane waves, chemically inactive core electrons are effectively replaced with an ultra-soft pseudo-potential [19]. Two parameters that affect the accuracy of calculations are the kinetic energy cut-off which determines the number of plane waves in the expansion and the number of special k-points used for the Brillouin zone (BZ) integration. We performed convergence with respect to Brillouin zone sampling and the size of the basis set. Converged results were achieved with a 9×9×2 special k-points mesh [20]. The size of the basis set was given by cut-off energy equal to 350 eV. Careful convergence tests show that with these parameters, relative energy converged to better than 5×10-6 eV/atom. The Broyden-Fletcher- Goldfarb-Shanno (BFGS) minimization technique [21], which provides a fast way of finding the lowest energy structure, was used in the geometry optimization. The tolerances for the geometry optimization were the difference in total energy within 5×10-6 eV/atom, the maximum ionic Hellmann-Feynman force within 0.01 eV/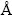 , the maximum ionic displacement within 5×10-4
, the maximum ionic displacement within 5×10-4 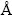 and the maximum stress within 0.02 eV/
and the maximum stress within 0.02 eV/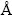 3. The elastic coefficients were determined from the first-principles calculations by applying a set of given homogeneous deformations with a finite value and calculating the resulting stress with respect to optimizing the internal degrees of freedoms, as implemented by MILMAN et al [22].
3. The elastic coefficients were determined from the first-principles calculations by applying a set of given homogeneous deformations with a finite value and calculating the resulting stress with respect to optimizing the internal degrees of freedoms, as implemented by MILMAN et al [22].
3 Results and discussion
3.1 Structural properties
M2SiC (M = Ti, V, Cr, Zr, Nb, Mo, Hf, Ta and W) compounds crystallize in the Cr2AlC crystal structure, with space group P63/mmc (#194). The atomic positions in the elementary cell are: C (0, 0, 0), Si (1/3, 2/3, 3/4) and M (1/3, 2/3, z). Two lattice constants a and c and the internal structural parameter z define the structure. Figure 1 shows a structural model for the crystalline structure of M2SiC phase. The optimized equilibrium lattice parameters a0, c0 and the internal structural parameter z0, for all M2SiC series as determined from geometry within GGA are given in Table 1. There is a good agreement between our calculated lattice constants and internal parameter of Nb2SiC and those previously reported by COVER et al [23] and GHEBOULI et al [24]. The compounds with M elements belonging to the same line of the periodic table are grouped together. HUG [25] defined for a parameter, ar, of the 211 MAX phases to describe the distortion as follows:
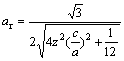 (1)
(1)
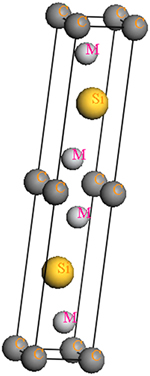
Fig. 1 Structural model for crystalline structure of M2SiC phase
where z is the internal coordinate of the C atoms. As defined, ar is the ratio of the distances between two opposite faces contained in the basal planes and two faces not in the basal planes. This factor equals unity for the cubic octahedron. The calculated ar values for the studied M2SiC phases are listed in Table 1 and plotted in Fig. 2. The distortion increases with increasing VEC except for the series M=Ti, V and Cr, where it has a lower value in V2SiC (it follows a parabolic variation). The distortion is clearly the highest in Ti2SiC, Mo2SiC and W2SiC. The variation of the lattice constants c and a as a function of the VEC for the three series is shown in Fig. 2. These parameters show a decrease with increasing VEC for all series. From Table 1, for the same VEC, c0/a0 ratio decreases when the number of valence electrons of M element is enhanced. The a0 and c0 values of the series of M2SiC phases, where M belongs the same column, increase when they go downward the column: {a0 and c0} (Ti2/V2/Cr2SiC)<{a0 and c0} (Zr2/Nb2/ Mo2SiC)<{a0 and c0} (Hf2/Ta2/W2SiC). As Si and C atoms are the same in the three compounds, this result can be easily explained by considering the atomic radii of M atoms. The larger size of M atoms forces the system to have larger lattice constants.
Table 1 Lattice constants a0 and c0, c0/a0 ratio, bulk modulus B0 and its pressure derivative B′, internal parameter z and distortion of M2SiC (M=Ti, V, Cr, Zr, Nb, Mo, Hf, Ta and W) phases at zero pressure

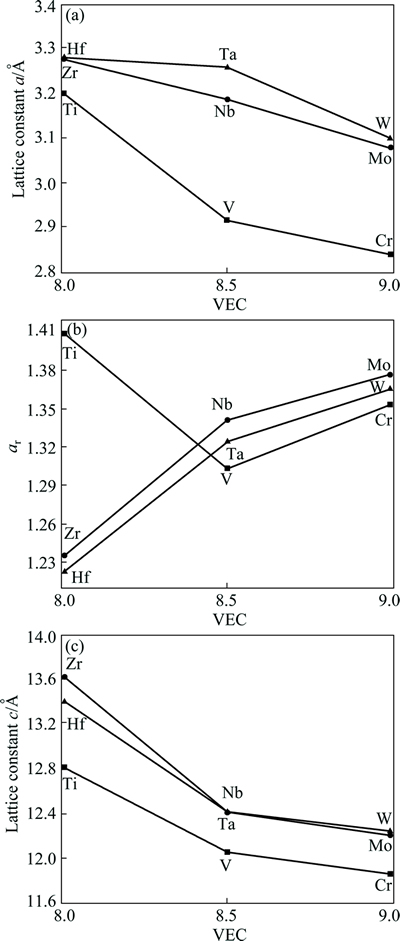
Fig. 2 Lattice constants a and c and distorsion ar in M2SiC phases as function of VEC
The computed equilibrium geometry of M2SiC unit cell at applied hydrostatic pressure in the range of 0-20 GPa with the step of 5 GPa is performed to investigate the structural parameters under pressure effect. It is assumed that no phase transformation occurred in these systems. Also, it was reported that no phase transformation was observed in the MAX phases Ti2AlN, Ti2AlC, V2AlC, Cr2AlC, Nb2AlC, Nb2AlC and Zr2InC, which were investigated under pressure of 50 GPa by using a synchrotron radiation and a diamond-anvil cell to measure the pressure dependencies of the lattice parameters [26,27]. Figure 3 plots the relative changes of the lattice parameters (a/a0 and c/c0) versus applied hydrostatic pressure (p). We clearly observe a quadratic dependence in all curves of the studied compounds in the considered range of pressure. The solid curve is the quadratic least-squares fit (a/a0, c/c0=1+αp+βp2). In contradiction to some other MAX phases [26-32], the compressibility of M2SiC along the a-axis is greater than that along c-axis, except for Nb2SiC, which are nearly identical and in W2SiC, it is greater along the c-axis. The same behaviour has been observed in other MAX phases [27,31-33]. Different reasons have been reported to explain this behaviour. With subjecting Nb2AsC to hydrostatic pressure up to 41 GPa, KUMAR et al [33] found that the pressure contraction along the a-direction was greater than that along the c-axis, and concluded that Nb-As bond must be quite resistant to compression along the c-axis. Experiments on M2AlC phases (M=Ti, V, Cr, Zr, Nb and Ta) [27] revealed that the compressibility in the c-axis was lower than that along a-axis for M = Cr and Nb. It was suggested that M—C and M—Al bonds have comparable strength in M2AlC with M=Cr, Nb. EMMERLICH et al [31] reported for M2AlC phases (M= Ti, V, Cr, Zr, Nb, Mo, Hf, Ta, W) that for the M element with a VEC of 4, the compressibility in c-axis is larger than that along the a-axis. As the VEC increases and reaches 5, it decreases and becomes comparable to that along the a-axis, whereas at VEC=6, the compressibility in the a-axis is larger than that along the c-axis. The geometric alteration of the bonding configuration in combination with the increase in M—C bond stiffness is responsible in this compressibility change. A similar observation in compressibility along both a and c axes for Ta2AlC [32] has been attributed to an increase in Ta—Al and Ta—Ta bonding strength as well as the interaction between TaC-TaC layers. Our results show that the compressibility along the a-axis is higher than that along the c-axis for all studied M2SiC compounds and has VEC of 8.0, 8.5 and 9.0, except for M=Nb and W. These results conclude that the compressibility of the lattice parameters depend on the nature of M, A and X atoms. The calculated unit cell volumes at fixed values of applied hydrostatic pressure in the range of 0-20 GPa with the step of 5 GPa were used to construct the equation of state (EOS), which was fitted to the third-order Birch-Murnaghan equation [34]. We obtained, by least-squares fitting, the bulk modulus at zero pressure B0 and its pressure derivative B′. These are listed in Table 1. Table 1 illustrates that the bulk modulus increases with increasing the number of valence electrons of the transition metal in the same row.
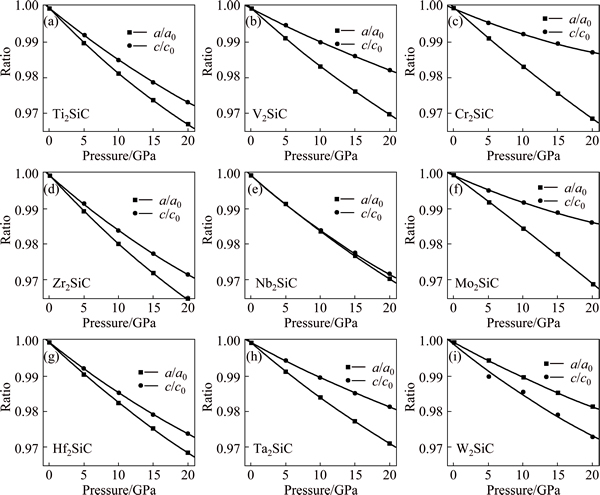
Fig. 3 Relative changes of lattice constants a/a0 and c/c0 in M2SiC (M=Ti, V, Cr, Zr, Nb, Mo, Hf, Ta and W) phases as function of pressure
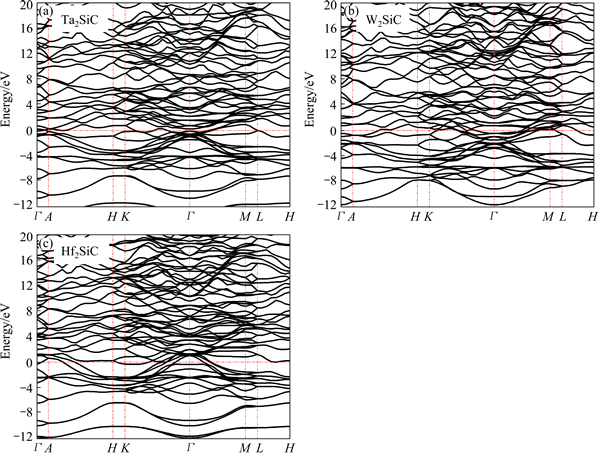
Fig. 4 Electronic band dispersion curves of Ta2SiC (a), W2SiC (b) and Hf2SiC (c) along some high symmetry directions of Brillouin zone
3.2 Electronic properties
The calculated energy band structures for Hf2SiC, Ta2SiC and W2SiC at equilibrium lattice parameters, along the high symmetry directions in the Brillouin zone, where the transition metal element belongs to the sixth period, are shown in Fig. 4. We considered them as a prototype since the band profiles of the other compounds, where the transition metal element belongs to the other periods, are quite similar. The valence and conduction bands overlap considerably and there is no band gap at the Fermi level. As a result, M2SiC will exhibit metallic properties. The noble metal carbides, such as PdC, AgC, PtC and NbC in zinc blende phase are metallic in nature [35-38]. The EF of Hf2SiC lies below the valence-band maximum near Γ point. In W2SiC, more valence electrons present in the unit cell, and the EF lies about 2.5 eV which is higher than that in Hf2SiC. This leads to some additional occupation of bonding states near the Fermi level. The initially unoccupied valence band near the Γ point shifts downward and is located below the Fermi level in W2SiC. The substitution of Hf by Ta and then by W in M2SiC introduces extra valence electrons per atom and correspondingly Fermi level moves to a higher energy.
The calculated total densities of states (TDOS) for Hf2SiC, Ta2SiC and W2SiC are presented in Fig. 5. The details of the peak structures and the relative heights of the peaks in their TDOS are rather similar, indicating similarity in chemical bonding. The computed number (N) of states at the Fermi level (EF) is 3.59, 3.01 and 2.12 eV/cell for W2SiC, Hf2SiC and Ta2SiC. Therefore, we expect that the electrical conductivity decreases in the sequence of W2SiC→Hf2SiC→Ta2SiC. The understanding of the chemical bonding in M2SiC requires the calculating their partial density of states (PDOS). The PDOS spectrum for M2SiC, where M belongs to the fourth period, is shown in Fig. 5. Carbon does not contribute to the TDOS at the Fermi level and therefore is not involved in the conduction properties. M-s electrons mainly contribute to the TDOS at the Fermi level, and should be involved in the conduction properties. Si electrons do not contribute significantly at the Fermi level. It is apparent that a covalent interaction occurs between the constituting elements. C-s and M-s as well as Si-s and M-s states are hybridized. The PDOS shows that the hybridization peak in energy of M-s and C-s is lower than that of M-s and Si-s. This suggests that the M-s=(Ti-s, V-s and Cr-s)—C-s bonds are stiffer than the (M-s)—(Si-s) bonds. The Fermi level moves from a lower to a higher energy with the substitution of the transition metals of Ti by V and Cr, which indicates that the increased extra valence electrons fill in the M-s—C-s and M-s—Si-s hybridized bonding states. The states located between -0.9 and -5.5 eV below the Fermi level in Hf2SiC are originated from the hybridization of (Hf-6s)—(C-2s) orbitals. These states shift downward and extend from -1.9 to -5.68 eV below Fermi level in Ta2SiC. This indicates that the stiffness of the M-Si and M-C bonds increases with increasing valence electron concentration.
3.3 Elastic properties
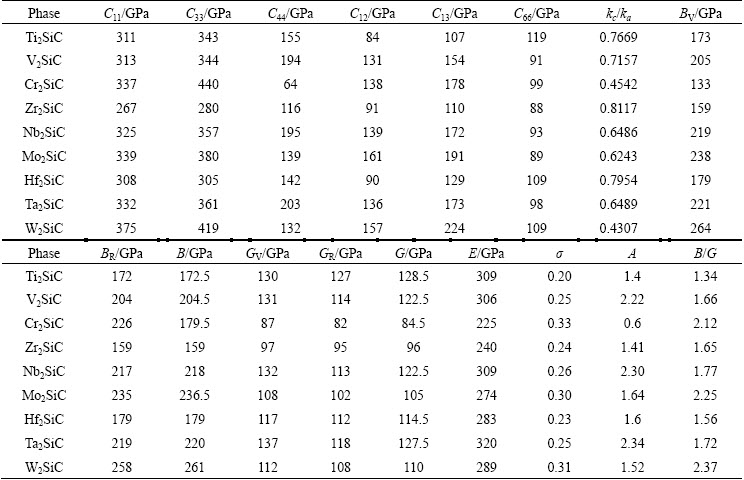
Table 2 lists our computed elastic constants of M2SiC phases (M=Ti, V, Cr, Zr, Nb, Mo, Hf, Ta, W). To date, no direct experimental elastic constants are available to be compared with our results. Future experimental measurements will test our calculated predictions. The elastic constant C11, which provides a measure of rigidity against unidirectional deformation along a-axis is slightly lower than the elastic constant C33, which provides an estimation of the elastic response of the material to a unidirectional pressure along c-direction. This is in accordance with the response of a- and c-axis under hydrostatic pressure (Fig. 3).
The elastic anisotropy of crystals has an important implication in engineering since it is highly correlated with the possibility to induce microcracks in the materials [39]. Essentially, all known crystals are elastically anisotropic, and a description of such anisotropic behaviour has an important implication in engineering science as well as in crystal physics. To quantify the elastic anisotropy of M2SiC, we calculated the shear anisotropic factor (A) for the shear plane 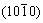 formed by the
formed by the 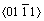 and
and 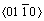 directions, which is identical to the shear anisotropy factor for the shear plane
directions, which is identical to the shear anisotropy factor for the shear plane 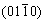 formed by the
formed by the 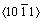 and
and 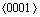 directions [40]:
directions [40]:
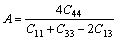 (2)
(2)
The calculated shear anisotropic factor of M2SiC is given in Table 2. For an isotropic crystal, A is equal to 1, while any value smaller or larger than 1 indicates anisotropy. The magnitude of the deviation from 1 is a measure of the degree of elastic anisotropy possessed by the crystal. According to this, M2SiC is characterized by a profound anisotropy for the shear planes described above.
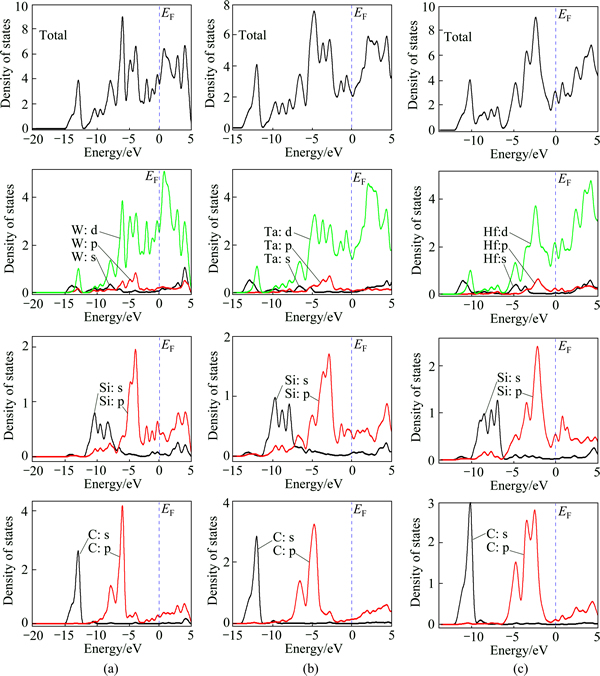
Fig. 5 Total and partial densities of states of Ta2SiC (b), W2SiC (a) and Hf2SiC (c)
We use the ratio between the linear compressibility coefficients along the c-and a-axis, i.e., kc/ka to characterize their elastic anisotropy (Table 2 and Fig. 6). For a hexagonal crystal, kc/ka can be expressed as [41,42]
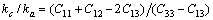 (3)
(3)
On can notice that kc/ka decreases with increasing VEC. The kc/ka values for all M2SiC phases are less than 1, which suggests that the compressibility along a-axis is higher than that along c-direction. It is observed that the distortion increases with decreasing kc/ka except for the series M=Ti, V and Cr, where it is lower for V2SiC compound.
Table 2 Calculated elastic constants Cij, ratio kc/ka, bulk modulus B and shear modulus G, elastic modulus E, Poisson ratio υ and anisotropy factor A of M2SiC (M=Ti, V, Cr, Zr, Nb, Mo, Hf, Ta and W) phases
Once the elastic constants are determined, we would like to compare our results with experiments, or predict what experiment would yield for the elastic constants. A problem arises when single-crystal samples cannot be obtained. Then, it is not possible to measure the individual elastic constants Cij. Instead, the isotropic bulk modulus B and the shear modulus G are determined [43]. These data cannot in general be calculated directly from Cij, but we can use our values to place bounds on the isotropic modulus. REUSS and ANGEW [44] found lower bounds for all lattices, while VOIGT [45] discovered upper bounds. HILL [46] showed that the Voigt and Reuss averages are limited and suggested that the actual effective modulus could be approximated by the arithmetic mean of the two bounds. The formulas for these bounds for a hexagonal lattice can be found in Refs. [47,48]. We also calculated the elastic modulus (E) and Poisson ratio (υ), which are frequently measured for polycrystalline materials when investigating their hardness. These data are related to the bulk modulus B and the shear modulus G by the following equations [49]:
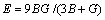 (4)
(4)
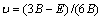 (5)
(5)
The calculated bulk modulus, shear modulus, elastic modulus and Poisson ratio of M2SiC (M=Ti, V, Cr, Zr, Nb, Mo, Hf, Ta, W) are given in Table 2. The bulk modulus values calculated from the elastic constants have nearly the same ones as those obtained from the EOS fitting. This might be an estimate of the reliability and accuracy of our calculated elastic constants. We illustrate the VEC effect on elastic and shear moduli as shown in Fig. 6. With the exception of V2SiC, which is anomalously soft, E is the maximum at VEC of 8.5. The trends in G are identical, with the G values also peaking at VEC of 8.5, again with the exception of V2SiC. The Poisson ratio falls in the range 0.2-0.33.
One can estimate the brittle and ductile behaviours of polycrystalline materials by considering B and G as the resistance to fracture and to plastic deformation. A low (high) B/G ratio is therefore associated to brittleness (ductility) of materials. The consequence of brittleness is the sensitivity for thermal shocks, as the material cannot efficiently dissipate thermal stress via plastic deformations. Thus, a brittle solid can only be subjected to limited thermal shocks before its strength drops dramatically. The ductile materials are resistant to thermal shocks; their mechanical properties decrease slowly with increasing temperature. PUGH [50] proposed a critical value which separates ductile and brittle materials. It was fixed at about 1.75, i.e., if B/G>1.75, the material behaves in a ductile manner, otherwise, the material behaves in a brittle manner. From the computed B/G ratios of Table 2 and referred to Pugh’s criterion, we can conclude that M2SiC (M=Cr, Mo and W) is brittle, whereas M2SiC (M=V, Nb and Ta) is between the two categories of materials. M2SiC, with M=Ti, Zr and Hf is ductile.
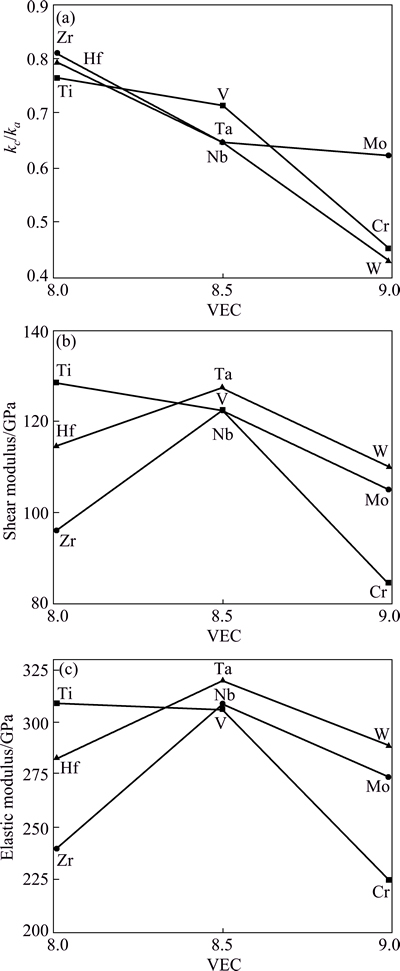
Fig. 6 Ratio between linear compressibility coefficients kc/ka along c- and a-axis (a), shear modulus (b) and elastic modulus (c) in M2SiC phases as function of VEC
The key criterion for mechanical stability of a crystal is that the strain energy must be positive [51]. For an hexagonal crystal, its five independent elastic constants should satisfy the well-known born stability criteria [52], i.e., C11-|C12|>0, (C11+C12)C33-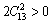 and C44>0. Our results reveal that the stability criteria are verified, implying the mechanical stability of M2SiC materials. The stability of these compounds can also be confirmed by providing the Poison ratio, whose value is usually between -1 and 0.5, corresponding to the lower and upper limit where the materials do not change their shapes.
and C44>0. Our results reveal that the stability criteria are verified, implying the mechanical stability of M2SiC materials. The stability of these compounds can also be confirmed by providing the Poison ratio, whose value is usually between -1 and 0.5, corresponding to the lower and upper limit where the materials do not change their shapes.
Elastic deformation can be reduced to volume and shape changes [53]. The bulk modulus provides an estimation of the elastic response of the material to isotropic hydrostatic pressure. The shear modules (G and C44) provide a measure of rigidity against the shape deformation. WANG and ZHOU [53] and JHI et al [54] found that in transition-metal carbonitrides TiCxN1-x, the hardness and shear module C44 reached an anomalous maximum for one valence electron number value in the unit cell, while the bulk and shear moduli did not show the maximum. C44 was demonstrated to be a better hardness predictor for this class of materials [53-55]. Because of the fact that M2SiC phase has a close relationship with the transition-metal carbides both in crystal structure and atomic bonding characteristics, one could expect direct information on predicting the hardness by examining the correlation between C44 and VEC for M2SiC. Figure 7 illustrates the calculated elastic constant C44 of M2SiC (M=Ti, V, Cr, Zr, Nb, Mo, Hf, Ta and W) as a function of the valence electron concentration (average number of valence electrons per atom). This variation is parabolic and similar in each series. In the same series, as the VEC is increased from 8.0 to 8.5, C44 increases and then decreases when the VEC reaches 9. We remark that C44 may saturate its maximum when the VEC is equal to 8.41, 8.56 and 8.48 for the compounds having M element from the fourth, the fifth and the sixth period, respectively. This trend is consistent with the literature on M2AlC (M=Ti, V, Nb, Cr) [56,57]. This implies that the maximal hardness might be achieved when the VEC is in the range of 8.40-8.42, 8.55-8.57 and 8.47-8.49 for the compounds having M element from the fourth, the fifth and the sixth period, respectively.
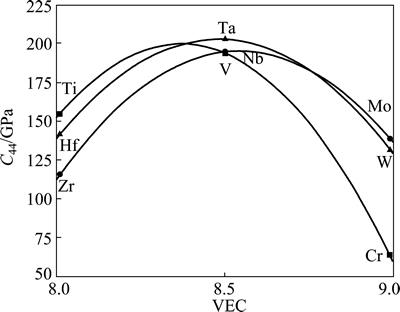
Fig. 7 Calculated elastic constant C44 of M2SiC as function of VEC (Solid lines represent the second-order polynomial fit)
3.4 Thermal properties
The thermal conductivity is the property of a material that indicates its ability to conduct heat. So, in order to know if material is a potential candidate for thermal barrier coating application, its thermal conductivity needs to be investigated. Based on the Debye model, CLARKE [58] suggested that the theoretical minimum thermal conductivity kmin can be calculated after replacing different atoms by an equivalent atom with a mean relative atomic mass Mm:
 (6)
(6)
where kB is the Boltzmann constant.
The average sound velocity vm in the polycrystalline material is given by [59]
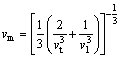 (7)
(7)
where vl and vt are the longitudinal and transverse sound velocities obtained by the shear modulus G and the bulk modulus B from the Navier’s equation [59]:
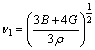 ,
,  (8)
(8)
One of the most important parameters that determine the thermal characteristics of materials is the Debye temperature (θD). The Debye temperature is closely related to many physical properties such as elastic constant, specific heat and melting temperature. A higher θD implies a higher thermal conductivity. It is used to distinguish high and low temperature regions for a solid. All modes are expected to have energy kBT if T>θD, and if T<θD one can expect high-frequency modes to be frozen [60]. At low temperature, the vibrational excitation arises solely from acoustic modes. Hence, at low temperature, the Debye temperature calculated from elastic constants is the same as that determined from specific heat measurements. The Debye temperature can be defined in terms of the mean sound velocity as follows [59]:
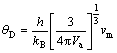 (9)
(9)
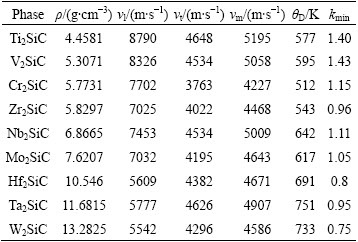
The computed thermal properties of M2SiC (M= Ti, V, Cr, Zr, Nb, Mo, Hf, Ta and W) phases, including the sound velocity, minimum thermal conductivity and Debye temperature as well as the density are summarized in Table 3. When the M element changes downward the column of periodical table, the Debye temperature and minimum thermal conductivity of M2SiC decrease, except for the series M = Ti, Zr and Hf, where it is lower for Zr2SiC. When the M element changes in the same line of the periodical table, the Debye temperature and the minimum thermal conductivity of M2SiC saturate their maximum for a VEC value about 8.5. In this formulation, the Debye temperature is directly related to the elastic constants via average elastic wave velocity, so the variations of the Debye temperature and the minimum thermal conductivity of M2SiC depending on the chemical nature of the M elements have the same trend with the average elastic wave velocities. Unfortunately, as far as we know, there are no data available related to these properties in the literature for M2SiC. Therefore, our calculated values can be considered prediction of these properties. Future experimental work will provide a comparison for our calculated results.
Table 3 Calculated density ρ, longitudinal, transverse and average sound velocities vl, vt and vm, Debye temperature θD and minimum thermal conductivity kmin for M2SiC (M=Ti, V, Cr, Zr, Nb, Mo, Hf, Ta and W) phases
4 Conclusions
1) The lattice constants decrease with increase in VEC of the M element in the same period.
2) A numerical first-principles calculation of the elastic constants was used to calculate C11, C12, C13, C33, C44 and C66. It is found that a quadratic dependence of the ratio c0/a0 as a function of valence electron concentration.
3) With the exception of V2SiC, both the elastic and shear moduli peaks are at a VEC value of 8.5.
4) The distortion and bulk modulus increase with increasing VEC.
5) The Debye temperature and the minimum thermal conductivity of M2SiC saturate their maximum at a VEC value about 8.5.
6) Like all MAX phases, the compounds studied are electrical conductors and the conductivity is assured by the s electrons of the transition metals.
7) The analysis of the partial density of states shows a strong hybridization Si-s—M-s and C-s—M-s.
References
[1] NOWOTNY V H. Strukturchemie einiger Verbindungen der 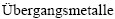 mit den elementen C, Si, Ge, Sn [J]. Progress in Solid State Chemistry, 1971, 2: 27-70.
mit den elementen C, Si, Ge, Sn [J]. Progress in Solid State Chemistry, 1971, 2: 27-70.
[2] BARSOUM M W. The Mn+1AXnphases: A new class of solids: Thermodynamically stable nanolaminates [J]. Progress in Solid State Chemistry, 2000, 28: 201-281.
[3] FINKEL P, BARSOUM M W, EL-RAGHY T. Low temperature dependencies of the elastics properties of Ti4AlN3, Ti3Al1.1C1.8, and Ti3SiC2 [J]. JournalofApplied Physics, 2000,87: 1701-1703.
[4] BARSOUM M W, EL-RAGHY T. Synthesis and characterization of a remarkable ceramic: Ti3SiC2 [J]. Journal of the AmericanCeramic Society, 1996, 79: 1953-1956.
[5] BARSOUM M W, BRODKIN D, EL-RAGHY T. Layered machinable ceramics for high temperature applications [J]. Scripta Metallurgica et Materialia, 1997, 36: 535-541.
[6] BARSOUM M W, ALI M, EL-RAGHY T. Processing and characterization of Ti2AlC, Ti2AlN and Ti2AlC0.5N0.5 [J]. Metallurgical and Materials Transactions A, 2000, 31: 1857-1865.
[7] BARSOUM M W, EL-RAGHY T. The MAX phases: Unique new carbide and nitride materials [J]. American Scientist, 2001, 89: 336-345.
[8] BARSOUM W, FARBER L, EL-RAGHY T, LEVIN I. Dislocations, kink bands, and room temperature plasticity of Ti3SiC2 [J]. Metallurgical and Materials Transactions A, 1999, 30: 1727-1738.
[9] BARSOUM M W. Physical properties of the MAX phases [M]//Encyclopedia of Materials Science and Technology. Amsterdam: Elsevier, 2006.
[10] BARSOUM M W, RADOVIC M. Encyclopedia of materials science and technology [M]. CAHN R W, et al. Amsterdam: Elsevier, 2004.
[11] HETTINGER J H, LOFLAND S E, FINKEL P, MEEHAN T, PALMA J, HARREL K, GUPTA S, GANGULY A, EL-RAGHY T, BARSOUM M W. Electrical transport, thermal transport, and elastic properties of M2AlC (M=Ti, Cr, Nb and V) [J]. Physical Review B, 2005, 72: 115-120.
[12] MANOUN B, ZHANG F X, SAXENA S K, EL-RAGHY T, BARSOUM M W. X-ray high-pressure study of Ti2AlN and Ti2AlC [J]. Journal of Physics and Chemistry of Solids, 2006, 67: 2091-2094.
[13] DRULIS M K, DRULIS H, HACKEMER A E, GANGULY A, EL-RAGHY T, BARSOUM M W. On the low temperature heat capacities of Ti2AlN and Ti2Al(N0.5C0.5) [J]. Journal of Alloys and Compounds, 2007, 433: 59-62.
[14] HU C F, ZHANG J, WANG J, LI F G, WANG J Y, ZHOU Y C. Crystal structure of V4AlC3: A new layered ternary carbide [J]. Journal of the AmericanCeramic Society, 2008, 91: 636-639.
[15] HOHENBERG P, KOHN W. Inhomogeneous electron gas [J]. Physical Review, 1964, 136: 864-871.
[16] KOHN W, SHAM L J. Self-consistent equations including exchange and correlation effects [J]. Physical Review A, 1965, 140: 1133-1165.
[17] SEGALL M D, LINDAN P L D, PROBERT M J, PICKARD C J, HASNIP P J, CLARK S J, PAYNE M C. First-principles simulation: Ideas, illustrations and the CASTEP code [J]. Journal of Physics: Condensed Matter, 2002, 14: 2717-2744.
[18] PERDEW J P, BURKE K, ERNZERHOF M. Generalized gradient approximation mode simple [J]. Physical ReviewLetters, 1996, 77: 3858-3865.
[19] VANDERBILT D. Soft self-consistent pseudopotentials in generalized eigenvalue formalism [J]. Physical Review B, 1990, 41: 7892-7895.
[20] MONKHORST H J, PACK J D. Special points for Brillouin-zone integrations [J]. Physical Review B, 1979, 13: 5188-5192.
[21] FISCHER T H, ALMLOF J. General methods for geometry and wave function optimization [J]. Journal of Physical Chemistry, 1992, 96: 9768-9774.
[22] MILMAN V, WINKLER B, PROBERT M I J. Stiffness and thermal expansion of ZrB2: An ab initio study [J]. Journal of Physics: Condensed Matter, 2005, 17: 2233-2241.
[23] COVER M F, WARSCHKOW O, BILEK M M M, MCKENZIE D R. A comprehensive survey of M2AX phase elastic properties [J]. Journal of Physics: Condensed Matter, 2009, 21: 305403-305412.
[24] GHEBOULI M A, GHEBOULI B, BOUHEMADOU A, FATMI M. First-principles study of the structural, elastic, electronic, optical and thermodynamic properties of the cubic perovskite CsCdCl3 under high pressure [J]. Solid States Communication, 2010, 150: 1896-1901.
[25] HUG G. Electronic structures of and composition gaps among the ternary carbidesTi2M [J]. Physical Review B, 2006, 74: 184113-184118.
[26] MAMOUN B, ZHANG F X, SAXENA S K, EL-RAGHY T, BARSOUM M W. X-ray high-pressure study of Ti2AlN and Ti2AlC [J]. Journal of Physics and Chemistry of Solids, 2006, 67: 2091-2095.
[27] MAMOUN B, GULVE R P, SAXENA S K, GUPTA S, BARSOUM M W, ZHA C S. X-ray high-pressure study of Ti2AlN and Ti2AlC [J]. Physical Review B, 2006, 73: 0241100-241115.
[28] MANOUN B, LIERMANN H F, GULVE R P, SAXENA S K, GANGULY A, BARSOUM M W, ZHA C S. Compression of Ti3Si0.5Ge0.5C2 to 53 GPa [J]. Applied Physics Letters, 2004, 84: 2799-2805.
[29] MANOUN B, SAXENA S K, BARSOUM M W. High pressure study of Ti4AlN3 to 55 GPa [J]. Applied Physics Letters, 2005, 86: 101906-101912.
[30] KUKARNI S R, VENNILA R S, PHATAK N A, SAXENA S K, ZHA C S, EL-RAGHY T, BARSOUM M W, LUO W, AHUJA R. Study of Ti2SC under compression up to 47 GPa [J]. Journal of Alloys and Compounds, 2006, 448: L1-L4.
[31] EMMERLICH J, MUSIC D, HOUBEN A, DRONSKOWSKI R, SCHNEIDER J M. Systematics study on the pressure dependence of M2AlC phases (M=Ti, V, Zr, Nb, Mo, Hf, Ta, W) [J]. Physical Review B, 2007, 76: 224111-224116.
[32] MUSIC D, EMMERLICH J, SCHNEIDER J M. Phase stability and elastic properties of Tan+1AlCn(n=1-3) at high pressure and elevated temperature [J]. Journal of Physics: Condensed Matter, 2007, 19: 136207-136214.
[33] KUMAR R S, REKHI S, CORNELIUS A L, BARSOUM M W. Compressibility of Nb2AsC to 41 GPa [J]. Applied Physics Letters, 2005, 86: 111904-111909.
[34] BIRCH F. Finite strain isotherm and velocities for single-crystal and polycrystalline NaCl at high pressures and 300 K [J]. Journal of Geophysical Research, 1978, 83: 1257-1268.
[35] SONI H R, GUPTA S K, JHA P K. Ab initio total energy calculation of the dynamical stability of noble metal carbides [J]. Physica B, 2011, 406: 3556-3561.
[36] MANKAD V, RATHOD N, GUPTA S D, GUPTA S K, JHA P K. Stable structure of platinum carbides: A first principles investigation on the structure, elastic, electronic and phonon properties [J]. Materials Chemistry and Physics, 2011, 29: 816-822.
[37] RATHOD N, GUPTA S D, GUPTA S K, JHA P K. First-principles study of structural, electronic, elastic, phonon, and thermodynamically properties of niobium carbide [J]. Solid state Phenomena, 2011, 171: 67-77.
[38] RATHOD N, GUPTA S K, JHA P K. Dynamical stability and phase transition of ZrC under pressure [J]. Phase Transitions, 2012, 85: 1060-1069.
[39] MATTESINI M. Elastic properties and electrostructural correlations in ternary scandium-based cubic inverse perovskites: A first-principles study [J]. Physical Review B, 2009, 79: 125122-125129.
[40] SUN Z, LIB S, AHUJA R, SCHNEIDER J M. Calculated elastic properties of M2AlC (M=Ti, V, Cr, Nb and Ta) [J]. Solid State Commun, 2004, 129: 589-592.
[41] HE Xiao-dong, BAI Yue-lei, ZHU Chun-cheng, SUN Yue, LI Ming-wei, BARSOUM M W. General trends in the structural, electronic and elastic properties of the M3AlC2 phases (M=transition metal): A first-principles study [J]. Computational Materials Science, 2010, 49: 691-695.
[42] WANG J M, WANG J Y, ZHOU Y C, HU C F. Phase stability, electronic structure and mechanical properties of ternary-layered carbide Nb4AlC3: An ab initio study [J]. Acta Mater, 2008, 56: 1511.
[43] MEHL M J, BARRY B M, PAPACONSTANTOPOULOS D A. Principle and practice [M]. Volume I: Principles. London: John Wiley and Sons, 1995: 195-199.
[44] REUSS A, ANGEW Z. Berechnung der 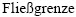 von Mischkristallen auf Grund der
von Mischkristallen auf Grund der 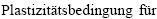 für Einkristalle [J]. Mathematics and Mechanics, 1929, 8: 55-60.
für Einkristalle [J]. Mathematics and Mechanics, 1929, 8: 55-60.
[45] VOIGT W. Lehrbush der Kristallphysik, Taubner, (Leipzig), 1928.
[46] HILL R. The elastic behaviour of a crystalline aggregate [J]. Proceedings of the Physical Society, 1952, 65: 349-355.
[47] BOUHEMADOU A. Prediction study of structural and elastic properties under pressure effect of M2SnC (M=Ti, Zr, Nb, Hf) [J]. Physica B, 2008, 403: 2707-2713.
[48] BOUHEMADOU A, KHENATA R. Prediction study of structural and elastic properties under pressure effect of M2GaC (M=Ti, V, Nb, Ta) [J]. Journal of Applied Physics: 2007, 102: 043528-043540.
[49] SCHREIBER E, ANDERSON O L, SOGA N. Elastic constants and their measurements [M]. New York: McGraw-Hill, 1973.
[50] PUGH S F. Relations between the elastic moduli and the plastic properties of polycrystalline pure metals [J]. Philosophical Magazine, 1954, 45: 823-843.
[51] NYE J F. Physical properties of crystals [M]. Oxford: Oxford University Press, 1985.
[52] BORN M, HANG K. Dynamical theory and experiments [M]. Berlin: Springer Verlag Publishers, 1982.
[53] WANG J Y, ZHOU Y C. Dependence of elastic stiffness on electronic band structure of nanolaminate M2AlC (M=Ti, V, Nb and Cr) ceramics [J]. Physical Review B, 2004, 69: 214111-214120.
[54] JHI S H, IHM J, LOUIE S G, COHEN M L. Electronic mechanism of hardness enchcement in transition-metal carbonitrides [J]. Nature, 1999, 399: 132-135.
[55] SUN Z, MUSIC D, AHUJA R, SCHNEIDER J M. Electronic origin of shearing in M2AC (M=Ti, V, Cr, A = Al, Ga) [J]. Journal of Physics: Condensed Matter, 2005, 17: 7169-7176.
[56] BOUHEMADOU A, KHENATA R, CHEGAAR M. Structural and elastic properties of Zr2AlX (X=C and N) under pressure effect [J]. European Physical Journal B, 2007, 56: 209-215.
[57] WANG J Y, ZHOU Y C. Ab-initioelastic stiffness of nano-laminate (MxM′2-x)AlC (M and M′=Ti, V and Cr) solid solution [J]. Journal of Physics: Condensed Matter, 2004, 16: 2819-2824.
[58] CLARKE D R. Materials selections guidelines for low thermal conductivity thermal barrier coatings [J]. Surface and Coatings Technology, 2003, 163: 67-74.
[59] ANDERSON O L. A simplified method for calculating the Debye temperature from elastic constants [J]. Journal of Physics and Chemistry of Solids, 1963, 24: 909-917.
[60] CHRISTMAN J R. Fundamentals of solid state physics [M]. New York: Wiley, 1988: 369.
M2SiC(MAX)相(M=Ti, V, Cr, Zr, Nb, Mo, Hf, Ta, W)结构、电子特性、弹性和热性能的第一性原理计算
B. GHEBOULI1, M. A. GHEBOULI2,3, M. FATMI2, L. LOUAIL2, T. CHIHI2, A. BOUHEMADOU4
1. Laboratory of Studies of Surfaces and Interfaces of Solids Materials, University of Setif 1, Setif 19000, Algeria;
2. Research Unit on Emerging Materials, University of Setif 1, Setif 19000, Algeria;
3. Department of Physics, University of Bordj Bou-Arreridj, 34000, Algeria;
4. Laboratory for Developing New Materials and their Characterization, Department of Physics,
Faculty of Science, University Setif 1, Setif 19000, Algeria
摘 要:研究M2SiC相的结构、电子特性、弹性和热性能(M为3 d, 4 d和5 d前过渡金属)。分析Ti、V、Cr、Zr、Nb、Mo、Hf、Ta 和W价电子浓度(VEC)对这些性能的影响。每个系列金属在VEC值约为8.5时弹性常数C44达到饱和。Hf-s、Ta-s 和 W-s电子主要在费米能级对态密度有贡献,可用于传导性能计算。M=Ti,V和 Cr系列金属在VEC值为8.5时畸变最小(遵循抛物线变化),而其他金属的变形随着VEC值的增大和kc/ka因子的减小而增大。M2SiC的主要特征是在 剪切面具有强烈的各向异性。除W2SiC外,沿该方向的可压缩性比沿锥面方向的可压缩性高。
剪切面具有强烈的各向异性。除W2SiC外,沿该方向的可压缩性比沿锥面方向的可压缩性高。
关键词:三元碳化物;从头计算法;晶体结构;电子结构
(Edited by Xiang-qun LI)
Corresponding author: M. A. GHEBOULI; E-mail: med.amineghebouli@yahoo.fr
DOI: 10.1016/S1003-6326(15)63680-9
Abstract: The structural, electronic and elastic properties of the M2SiC phases were studied, where M are 3d, 4d, and 5d early transition metals. The valence electron concentration (VEC) effect of Ti, V, Cr, Zr, Nb, Mo, Hf, Ta and W on these properties was examined. The C44 saturates for a VEC value in surrounding of 8.5 for each serie. Hf-s, Ta-s and W-s electrons mainly contribute to the density of states at the Fermi level, and should be involved in the conduction properties. The distortion increases with increasing VEC and decreasing kc/ka factor except for the series M=Ti, V and Cr, where it is lower at the VEC value of 8.5 (it follows a parabolic variation). The M2SiC was characterized by a profound anisotropy for the shear planes 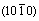 and compressibility in the direction is higher than that along the cone except for W2SiC, where it is lower.
and compressibility in the direction is higher than that along the cone except for W2SiC, where it is lower.
[15] HOHENBERG P, KOHN W. Inhomogeneous electron gas [J]. Physical Review, 1964, 136: 864-871.
[45] VOIGT W. Lehrbush der Kristallphysik, Taubner, (Leipzig), 1928.
[51] NYE J F. Physical properties of crystals [M]. Oxford: Oxford University Press, 1985.
[52] BORN M, HANG K. Dynamical theory and experiments [M]. Berlin: Springer Verlag Publishers, 1982.
[60] CHRISTMAN J R. Fundamentals of solid state physics [M]. New York: Wiley, 1988: 369.


