
J. Cent. South Univ. (2018) 25: 1628-1641
DOI: https://doi.org/10.1007/s11771-018-3855-z

Effects of single-walled carbon nanotubes on growth and physiological characteristics of Microcystis aeruginosa
WU Yang(武阳)1, WANG Ying-jun(王应军)1, LI Yuan-wei(李远伟)1,DU Jin-ge(杜金戈)1, WANG Zhang-hong(王章鸿)2, DENG Shi-huai(邓仕槐)3
1. Department of Environmental Engineering, College of Environment, Sichuan Agricultural University, Chengdu 611130, China;
2. Department of Environmental Engineering, School of Energy and Environment, Southeast University, Nanjing 210009, China;
3. Institute of Ecological and Environmental Sciences, Sichuan Agricultural University,Chengdu 611130, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Abstract:
In order to explore a novel and potential method using carbon nanotubes (CNTs) for controlling blue-green algal blooms efficiently in future, effects of single-walled carbon nanotubes (SWCNTs) on Microcystis aeruginosa growth control were investigated under lab cultured conditions. Related physiological changes were tested involving several important enzyme of antioxidant defense system (superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), malondiadehyde (MDA), photosynthetic pigments, protein, soluble sugar and extracellular microcystin toxins (MC-LR)). Algal cell density was significantly inhibited by SWCNTs at high concentration (>5.00 mg/L), and the inhibition rate was dose-dependent. For treatment with 100 mg/L SWCNTs, the inhibitory rates even reached above 90%. 96 h IC50 was determined as 22 mg/L. Antioxidant enzyme activities were dramatically dropped with increasing lipid peroxidation at higher SWCNTs concentration, indicating intracellular generation of reactive oxygen species (ROS) and oxidative stress damage in algae. Reduction of photosynthetic pigments, soluble sugar and protein contents suggested that SWCNTs may severely ruin algal photosynthesis system, destroy the metabolism-related structure of cell, and thus lead to negative physiological status in M. aeruginosa. Besides, SWCNTs can effectively decrease the amount of extracellular microcystins in culture medium.
Key words:
single-walled carbon nanotubes; Microcystis aeruginosa; microcystin toxin; growth;
Cite this article as:
WU Yang, WANG Ying-jun, LI Yuan-wei, DU Jin-ge, WANG Zhang-hong, DENG Shi-huai. Effects of single-walled carbon nanotubes on growth and physiological characteristics of Microcystis aeruginosa [J]. Journal of Central South University, 2018, 25(7): 1628–1641.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-018-3855-z1 Introduction
In recent years, nutrient over-enrichment (eutrophication) of water systems has been a global issue. Cyanobacteria, found frequently in marine, brackish and freshwater, may remarkably cause and increase blooms due to eutrophication [1]. The cyanobacterial bloom can cause water deoxygenating, off-flavor problems, and production of toxins, such as microcystins, which can cause a series of adverse effects such as the decreasing biodiversity, and illness in animals and humans [2]. Microcystis aeruginosa, a typical species of Microcystis, is one of the utmost worldwide toxic bloom forming cyanobacteria present in eutrophic freshwaters [3] and can produce toxic microcystins. In China, the M. aeruginosa algal blooms usually outbreak at summer and autumn each year in some fresh water lakes, such as the Dianchi Lake, the Taihu Lake and Chaohu Lake. It is reported that Microcystis was the most predominant cyanobacterial genus in Hongze Lake, and the cyanobacterial abundance could be reached about 1.35×106 cell/L [4]. Simultaneously, the microcystis blooms hence create a wide range of social, ecological, and economic problems as well, involving deterioration of water quality, decrease of the esthetic value of the affected water, and damage both capture and culture fisheries [5]. Therefore, the exploration and development of the effective methods of treatment and prevention to cyanobacterial blooms, especially toxic M. aeruginosa blooms is needed urgently and crucially.
Carbon nanotubes (CNTs) have been considered a kind of promising materials in nanotechnology in recent decades [6, 7]. They are widely applied in diverse areas ranging from electronic industrial to medicine products, even energy storage area, owing to their unique physicochemical properties, such as unique mechanical, electrical, thermal and optical properties in application [7, 8]. The huge production of CNTs make them more likely to be released into the aquatic environment in potential, and in modeled prediction, surface water concentrations in the ng/L range of CNTs were evaluated [9]. This attracts a growing interest to researchers concerning about potential ecological impacts including CNTs toxicity toward aquatic algae [10, 11]. Relevant existing findings revealed that inhibition of algal growth was probably related to light absorption by the nanomaterial causing the shading effects, while agglomeration and physical interactions between algae and CNTs could lead to the cell membrane disruption and destroy cell structure with endocyte outflow, besides, the CNTs-induced production of intracellular reactive oxygen species (ROS) may elicit oxidative stress at the cellular level, with the result of membrane lipid peroxidation causing disruption of cell membranes and DNA damage, and all these above factors contribute to the aquatic toxicity mechanism rather than to a specific mode of toxic action [12–16].
However, currently, so scarce information is available on the potential impact of CNTs toward blue-green algae because a majority of related efforts are addressed mainly focusing on the species of green algae, such as C. vulgaris, Chlorella sp, Scenedesmus obliquus, as previous evidence present. Moreover, much more details in research of mechanism underlying the ecological toxicity of CNTs in aquatic are still poorly unraveled and merit further examination. In the present investigation, we assessed the effects of single-walled carbon nanotubes (SWCNTs) on physiological level by measuring antioxidant enzymes (SOD, POD and CAT) and oxidant marker (MDA), photosynthesis (photosynthetic pigment), metabolic basis substances (soluble sugar and protein) and microcystin toxin (MC-LR) release in the blue-green alga M. aeruginosa, which was regarded as a main kind species of cyanobacteria blooms forming algae, with the purpose of providing a certain reference for comprehensive understanding the ecological impact of carbon nanotubes on the aquatic environment. We also believe that the findings of this work will be beneficial for exploring the great potential application of carbon nanotubes on controlling cyanobacteria algal blooms and providing a new guide for developing promising algicide in prospect.
2 Experimental
2.1 Preparation of materials
Single-walled carbon nanotubes used in this study were purchased from Chengdu Organic Chemicals Co. Ltd., Chinese Academy of Sciences. They were synthesized using chemical vapor deposition from a CH4/H2 mixture at 700 °C, using Co as catalysts. Their characterization data were listed in Table 1. Microcystis aeruginosa (FACHB- 912) was purchased from Wuhan Freshwater Algae Culture Collection at the Institute of Hydrology, Chinese Academy of Sciences. All reagents used in experiment were in the level of chemical analysis purity (AR).
2.2 Algae culture conditions and growth inhibition test
The purchased algae were cultured at (25±0.5) ℃ under a 12 h light and 12 h dark cycle in BG-11 medium with 500 mL Erlenmeyer flasks. The BG-11 medium were composed of 1.5 g/L of NaNO3, 0.04 g/L of K2HPO4·3H2O, 0.075 g/L of MgSO4·7H2O, 0.036 g/L of CaCl2·2H2O, 0.006 g/L of citric acid, 0.006 g/L of ferric-citrate, 0.001 g/L of EDTA sodium salt, 0.02 g/L of Na2CO3 and 1 mL of trace elements mixtures, 2.86 g/L of H3BO3, 1.81 g/L of MnCl2·2H2O, 0.222 g/L of ZnSO4·7H2O, 0.39 g/L of Na2MoO4·2H2O,0.079 g/L of CuSO4·5H2O, 0.0494 g/L of Co(NO3)2·6H2O were added as well. The continuous light was provided by incandescent lamp with an automated 12 h/12 h light/dark cycle. Its light intensity during the light phase was 2000–2500 lx.
Table 1 Physicochemical properties of SWCNTs

The 96 h algal growth inhibition tests were conducted using a modified method in the guidance of OECD guideline No. 201. M. aeruginosa, obtained from its logarithmic growth phase, was grown in BG-11 medium with the carbon materials (0 denoted as control, 0.1, 0.5, 1, 5, 10, 50 and 100 mg/L) autoclaved under 121 °C at 0.1 MPa for 30 min, and bath-sonicated (150 W, 40 kHz, 25 °C) for 30 min for sake of sufficient dispersion. The initial cell densities were approximately 2×106 cell/mL in the assays for 96 h exposure. Cultivations were performed in 500 mL conical flasks (about 250 mL culture medium) in triplicate, under (25±0.5) °C and 2000–2500 lx light intensity with a 12 h light and 12 h dark cycle. Flasks were shaken by hand and their places were changed randomly three times a day. Algal cells were counted daily, using a counting chamber under a light microscope (LM-CX41, Olympus, Japan).
2.3 Enzymatic antioxidant assays, lipid peroxidation measurement, proteins and soluble sugar determination
Algal cells were harvested at 96 h cultivation by centrifugation at 10000 r/min for 10 min at 4 °C. The cell pellets were transferred into 10 mL centrifuge tubes. The pellets were washed with sterilized media and centrifuged twice. For the measurements of the antioxidant enzymes and small molecule antioxidants, the cell pellets were resuspended in 2 mL PBS solution (50 mmol/L,pH 7.0). The cells were homogenized by an ultrasonic cell pulverizer (JY92-2D, Xinzhi Co., China) at 200 W with total time of 5 min (ultrasonic time: 3 s; rest time: 10 s) under ice-bath cooling. Then the homogenate was centrifuged at 3000 r/min for 10 min at 4 °C. The supernatant, cell-free enzyme extract, was used to enzymatic and non- enzymatic substances measurements at 96 h.
The activity of SOD was measured based on its ability to inhibit the reduction of nitro blue tetrazolium (NBT) by superoxide radicals generated with xanthine oxidase according to the total superoxide dismutase assay kit. One unit of SOD activity (U) is defined as the amount of protein that inhibits the rate of NBT reduction by 50% in 1 mL of the reaction solution. POD activity was assayed using guaiacol as a hydrogen donor by measuring the change at 470 nm over 1 min as instruction to Peroxidase assay kit. CAT activity was detected through catalase assay kit by measuring the initial rate of decrease in absorbance at 240 nm as a consequence of H2O2 consumption over 1 min. The lipid of peroxidation level was determined in terms of MDA content, which was measured using the thiobarbituric acid (TBA) reaction by Malondialdehyde assay kit. Protein concentrations in the cell extracts were determined at 595 nm using the protein quantitative assay kit. Soluble sugar in the extract was quantified by anthrone-sulfuric acid assay using glucose as a standard according to sugar assay kit. All the analysis was conducted using assay kit supported by Nanjing Jiancheng Bioengineering Institute, Nanjing, China. All experiments were repeated at least three times.
2.4 Measurement of photosynthetic pigment contents
At the end of 96 h treatment, 20 mL of M. aeruginosa biomass from different experiments were put separately in 5 mL of 80% acetone with no light at 4 °C. After 24 h extraction of pigment, sample was centrifuged using the centrifuge at 2500 r/min for 5 min. The supernatant was separated and the absorbance was red at 400–700 nm using UV–visible double-beam spectrophotometer (WFJ7200, Unico Instrument Co., Ltd., USA). The contents of chlorophyll a (Chl-a), chlorophyll b (Chl-b) and carotenoids could be calculated by Eqs. (1)–(3) according to JEFFREY et al [17] with a modification methods. It was present that Chl-a showed the maximum absorbance at 663 nm, Chl-b at 645 nm, and total carotenoid at 450 nm.
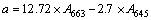 (1)
(1)
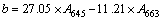 (2)
(2)
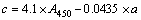 (3)
(3)
where a, b and c (mg/L) are the contents of Chl-a, Chl-b and carotenoids, respectively. A663, A645 and A450 are denoted as absorbance values at 663 nm, 645 nm and 450 nm, respectively.
2.5 Measurement of microcystin toxins production
The microcystins-LR (MC-LR) concentration at the 5th day in solutions was determined with enzyme-linked immunosorbant assay (ELISA), a highly sensitive biochemical method [18]. The measurement of MC-LR was conducted using MC-LR essay kit supported by Wuhan Institute of Hydrology, Chinese Academy of Sciences, Wuhan, China.
2.6 Data analysis
The inhibitory effect of SWCNTs on the algal growth was estimated which is defined by the following equation:
 (4)
(4)
where η is the inhibition; N0 and N are cell numbers in the control and SWCNTs-treated cultures, respectively.
When algal growth was significantly inhibited, the effective concentration causing a 50% inhibitory response at 96 h (96 h-EC50) was estimated with logistic fitting. Analysis of the data was done using Origin analysis program software 8.0. The means and standard deviations (SD) of all data were determined and graphed. Significant differences between means were identified using one-way ANOVA by SPSS software 20. Statistical significance was evaluated using significance levels at 0.05.
3 Results and discussion
3.1 Effects of SWCNTs on Microcystis aeruginosa growth
As shown in Figure 1(a), Hormesis, a dose response phenomenon characterized by low dose stimulation and high dose inhibition, was clearly observed in our results. As all M. aeruginosa grew with different initial concentrations of SWCNTs taken into considerations, the growth states can be mainly divided into three levels referred to as facilitations, independence and inhibition compare to the control. The algae density of control was exponentially increased with culture times after initial 24 h for adaption (R2=0.9921) and reached their maximum limit of 55×106 cell/mL, which indicated a pretty favorable culture conditions toward the algal. Treatments with concentrations among 0.10 and 0.50 mg/L were separately obtained their maximum algae density of 68×106 cell/mL and 59×106 cell/mL at 96 h, which were stimulated all the time higher than that of control. The effects of SWCNTs on the M. aeruginosa growth under these concentrations thus were corresponded to facilitation level. When treated at 1 mg/L, the growth trends of M. aeruginosa were very consistent with control treatment which assigned to dependence level. While higher concentrations (5.00–100.00 mg/L) which brought about bad growth status to algae can be considered as inhibition level. Specifically, M. aeruginosa subject to 100 mg/L was inhibited so severely that it almost could not grow anymore in the whole experiment. The relationship between SWCNTs concentration and corresponding algae inhibition ratio at 96 h was further investigated (Figure 1(b)). The result revealed that SWCNTs concentration and 96 h inhibition ratio showed a well positively linear relationship (R2=0.95). The highest inhibition ratio was obtained at 100 mg/L (90.45%), which was approximately three times higher than that of 10 mg/L (33.69%). These phenomena are similar with the results implemented by other researchers regarding CNTs toxicity toward green algae [12–15]. In contrast, PARK et al [19] described M. aeruginosa is more sensitive to silver nanoparticles than green algae and 1 mg/L concentration significantly inhibited the algae growth. Additionally, the 96 h IC50 value was also determined to 22 mg/L which was comparable to the earlier toxicity investigation toward algae [16, 20]. For example, CNTs were reported to have a 96 h IC50 of 24 mg/L toward C. vulgaris, and 38 mg/L toward Chlorella sp.
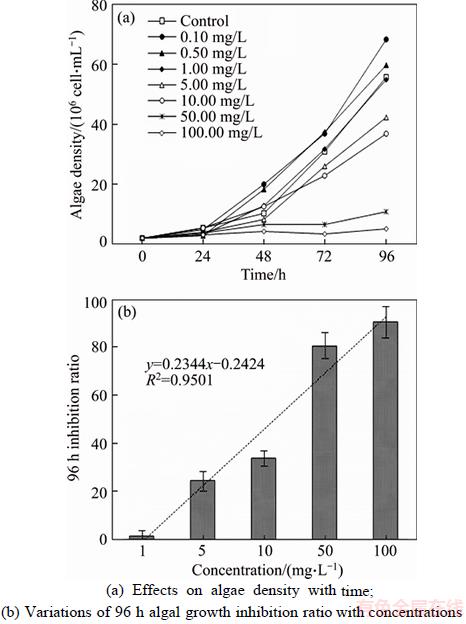
Figure 1 Effects of single-walled carbon nanotubes on M. aeruginosa growth:
Prior studies have confirmed that the effects of toxic matters were often stimulatory for phytoplankton and algal growth, and there have been similar experimental phenomena reported in some toxicological studies [13, 21]. MOU et al [15] reported that CNTs in the definite concentration range at low level (0.1–1.0 mg/L) would have a stimulative effect on the growth of Scenedesmus obliquus, while high concentration obviously inhibited the growth. RHIEM et al [14] also found an impact of CNTs at subcytotoxic concentrations of 1 mg/L CNTs, and no inhibitory effect on the growth of the algae was recorded at this CNTs level, which was a good correlation corresponding to our data. As a recognized theory, the Hormesis effect can be employed to describe the reason why the algae appeared such a phenomenon which assign to the little stimulatory growth, when involved carbon nanotubes stress at low level (0.10–1.00 mg/L).
Not only that, it has been reported that the algal toxicity of CNTs could mainly be attributed to the mechanism underlying the combined effects of shading effects, agglomeration and physical interactions, and oxidative stress [12, 16, 22]. Illumination played a key role in the algal cell propagation, and the CNTs could therefore likely form a layer adhered to the algal surface, that hence restrict light accessibility to the cells, namely, shading effects [23, 24]. CNTs can also agglomerate with algal cells through hydrophobic interactions, and/or hydrogen bonds formed between the cell surfaces and the oxygen defects of CNTs, and even pierce and disturb the cell wall or membrane, thus affecting intracellular normal physiological status. To our results, the strong inhibition which appeared at high concentration, was probably because of reducing light due to their stronger agglomeration with the algal cells, resulting in the inhibition of growth of M. aeruginosa ultimately. What’s more, the hetero- agglomeration and co-precipitation of CNTs with algal cells would perhaps enhance the direct SWCNTs-cell interaction and lead to the apparent nanotoxicity toward M. aeruginosa. Some researchers found that toxicity of CNTs toward algae was significantly correlated with CNTs agglomeration with cells [16]. There is evidence that CNTs closely attached to the cell surfaces would penetrate and disrupt the cell wall, physically interact with the biomolecules, chemically inhibit physiological cell behavior, and therefore caused the critical damage to the algae [25]. CNTs-cell interactions can be visualized by electron microscopy and related to alterations in their cell composition. ZHANG et al [13] observed severe plasmolysis and uptake and internalization of MWCNTs to Chlorella pyrenoidosa. WANG et al [26] reported that copper oxide nanoparticles were actively internalized and prompted toxicity toward M. aeruginosa. Due to smaller size and different shape of carbon nanotubes, the nanoparticles can cause increases in cell membrane permeability leading to osmotic collapse and algae growth inhibition [19]. DI et al [27] described the toxicity of single and multi walled carbon nanotubes on macrophages through a decrease in the electron density of the nucleoplasm, along with aggregation of the DNA fibrils.
According to our results, it can be speculated that during the adaption growth phase, initially the well-dispersed CNTs may become attached to algal cells, which was favorable for the growth of M. aeruginosa. Over time, agglomerates are formed and a rapid increase of the amount of SWCNTs associated with the algae was appeared. This process might be supported by the fact of sorption of extracellular polymeric substances (EPS), which were previously shown to stimulate agglomeration of nanoparticles [28], excreted by and surrounding the algae. We strongly have the reason to believe that most of the algal cells on the SWCNTs layer may lost their cell membrane integrity, and SWCNTs were capable of disrupting cell membrane by interacting with lipid components and denaturing proteins essential to cell function, which thereby contributed to the outcome of strong inhibitory effect to M. aeruginosa in the end. However, more details remain to be clarified.
3.2 Effects of SWCNTs on antioxidant enzyme activity and content of malondialdehyde of Microcystis aeruginosa
Antioxidant enzyme activity, including dismutase (SOD), peroxidase (POD) and catalase (CAT), was depicted in Figures 2(a)–(d). As recognized in general, SOD can rapidly convert O2· radicals to H2O2 when experiencing under unfavorable stress conditions. An apparent slightly increase occurred in the algae cultivation group exposed to 0.10–0.50 mg/L SWCNTs in comparison to control. The highest activity of 17.67 U/108 cells was observed at 0.5 mg/L concentration, which was 3.00 U/108 cells higher than that of the control, but not significant (p<0.05). The increase of SOD activities at low SWCNTs concentration revealed that SOD in algae was in normal stress response to SWCNTs, which was perhaps due to the activated enzyme pools, or due to the intensified expression of genes encoding SOD in plants when exposing to moderate dosages of contaminants [29]. When the concentrations varied from 1.00 to 100.00 mg/L, SOD activities were all lower than that of the control. When SWCNTs concentration increased from 5.00 to 100.00 mg/L, the SOD activity in algae was inhibited significantly (from 52.54% to 16.96% of that of control). The minimum activity was 2.53 U/108 cells at 50.00 mg/L dosage, which was almost at the similar level inhibited compared to that of 100.00 mg/L. The decrease of SOD activity at the highest concentrations suggested that SOD may be out of self-adjustment under the stress of SWCNTs, resulting in the inefficient removal of superoxide. Moreover, the results on SOD activity were comparable with previous investigations in which Chlorella vulgaris were cultured under the stress conditions of allelochemical [30].
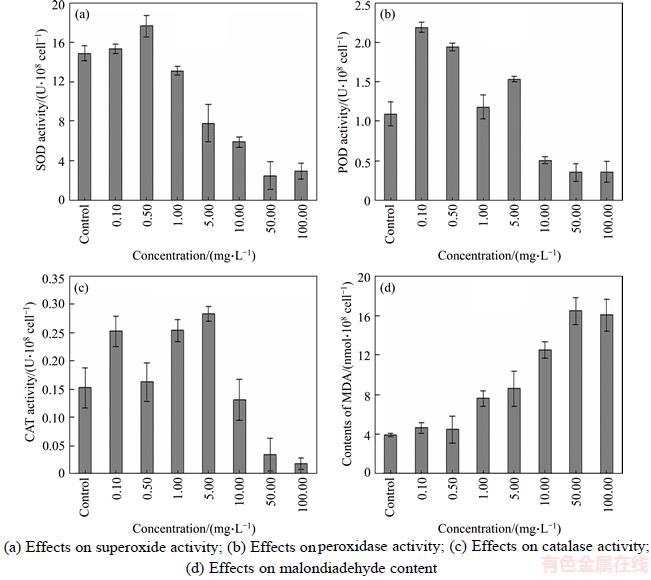
Figure 2 Effects of single-walled carbon nanotubes on M. aeruginosa:(Statistical significance was accepted at a level of p<0.05)
As a scavenger of H2O2, POD and CAT also play an essential role in oxidative stress situation, in which the produced H2O2 can be converted into H2O and O2 by the POD and CAT catalysis. As was shown in Figures 2(b) and (c), the algae exposed to 0.10–5.00 mg/L appeared an obvious enhancement in POD activity versus the control. The activity of POD in 0.10 mg/L was rised up to 2.19 U/108 cells, which was 2 times higher than that of control, with the differences being significant (p<0.05). When the algae treated with 10.00–100.00 mg/L SWCNTs, POD activity was strongly inhibited comparing to control and exhibited no apparent changes to SWCNTs. In general, the variation trends of CAT activity with concentration were similar to that of POD, in which the activities of 0.10–5.00 mg/L was almost displayed in the relative high level, whereas, decrease was observed with the minimum activities obtained at 100 mg/L. The increase of POD and CAT activity was also caused by the normal stress responses to the SWCNTs in the culture medium, possibly due to the stimulation on the expression of genes encoding POD/CAT by the overproduced H2O2, which was responsible to be removed by POD/CAT [31]. The afterwards decline was believed to cause large damage to M. aeruginosa by severe oxidation stresses occurring with the higher SWCNTs concentrations, the peroxidation of membrane lipid, and the damage to cellular membrane permeability, might also have been consequently aggravated, contributing to the result of inhibition on enzyme synthesis.
MDA is an oxidized product of membrane lipids and its accumulation reflects the damage degree caused by oxidative stress. Its content is commonly considered as a general indicator to evaluate the extent of stress level [32]. According to Figure 2(d), the MDA contents of the algal cells kept increasing with increased SWCNTs concentration, with significantly higher contents obtained at concentrations of 10 mg/L and higher in comparison to control (p<0.05). The MDA content in algae subjected to the treatments of 10 mg/L SWCNTs was 12.51 nmol/108 cells, which was 3.2-fold higher than that of the control. However, there was no notable effect on the changes of MDA content within less than 0.50 mg/L concentration. The obvious dose-dependent enhancement in the accumulation of MDA implied the fact that larger oxidative damage to the algal cells occurred at higher concentrations. These results were in accordance with previous reported literatures that sharp increase of cellular lipid peroxidation in the algal cells was clearly observed [12, 13].
The generation of oxidative stress triggered by CNTs is generally regarded as an important nanotoxic mechanism of CNTs to other hydrobiontes, as presented in previous Section 3.1 [12, 25]. Reactive oxygen species (ROS), including the superoxide anion (O2·–), hydrogen peroxide (H2O2), and the hydroxyl radical (·OH), can cause severe oxidative stress and injuries to cell when these free-radical productions exceed normal level [33]. CNTs could indeed induced plenty of intracellular ROS resulting in algal proliferation inhibition, or even cell death via an apoptotic pathway, or by necrosis [25, 34, 35], even direct oxidative DNA damage [27]. To prevent free-radical toxicity and protect cells against oxidative stress. In fact, algal cell possesses ROS scavengers, such as SOD, CAT and POD, which protect against the potential damaging effects of these ROS before they damage sensitive parts of the cellular machinery. These oxidant enzymes can scavenge excessive ROS accumulation, maintain at a dynamic balance between free radicals production and elimination, thus protect the cells functioning well. When the levels of ROS formed in cells exceed the ability of the antioxidant system to deal with, damage to cellular compounds and membrane occurs. Due to the cell membranes of alga are made of unsaturated phospholipids, which are vulnerable to ROS. MDA, as the decomposition product of this process has been utilized frequently as a biomarker for vital sign of cellular oxidative damage.
In view of our investigation, the response of SOD, POD and CAT activities to SWCNTs fundamentally seemed to have the similar changing trend, that is increase initially at relative low concentration (approximately less than 5 mg/L), whereas inhibit intensively later at high concentration (more than 10 mg/L), simultaneously, the MDA content accumulation can also be directly linked to the variation of antioxidant enzyme activities. When the moderate/low concentrations of SWCNTs (0.10–0.50 mg/L) were employed, the maximum SOD, POD values in M. aeruginosa were recorded, indicating that ROS was scavenged regularly and self-regulatory mechanism of antioxidant levels continued an important adaptive response to withstanding adverse conditions. Correspondingly, the MDA content in algae remained close to their corresponding controls, also suggesting that the peroxidation damage on the membrane lipid was not serious. In fact, maintenance of a high antioxidant capacity in cells can be associated with increasing tolerance of M. aeruginosa against unfavorable environmental stress conditions due to SWCNTs presence in the culture medium. The activities of these antioxidant enzymes were triggered by exposure to less than 1.00 mg/L SWCNTs in order to alleviate the damage partly caused by oxidation, which is similar to that found in other evidence (Figure 1) such as stimulation on growth [14, 30].
However, when higher concentrations of SWCNTs were applied (5.00–100.00 mg/L), both a dramatically dropping of antioxidant enzyme activities and intensified membrane lipid peroxidation were observed, indirectly reflecting that the dynamic balance of ROS production and elimination was disturbing. The involvement of ROS in cell can be further evidenced by consistently high level of MDA, suggesting that the antioxidant enzymes induced by SWCNTs may not completely eliminate ROS. On the other hand, exposure to more than 10 mg/L SWCNTs did not increase the activities of the antioxidants. As so much high levels of ROS accumulation could not be efficiently scavenged out of algae via antioxidant system timely, consequently, macromolecule damage in algae and damnification of the cell membrane occurred, hence contributing to the result of strong inhibitory and negative effects toward the algal growth and metabolism. These results indicated that SWCNTs could weaken or damage the antioxidant defense system and therefore exhibit the algicidal property. Correspondingly, more evidences from in vitro and in vivo experiments were put forward demonstrating that CNTs elicits oxidative stress at cellular level [36, 37].
In addition, ROS generated from SWCNTs photoreaction in water has been confirmed previously [33], meaning that extracellular ROS production in culture medium may thereby likely cause comparable oxidative damage to M. aeruginosa as well. LONG et al [12] found that MWCNTs attached to algal cells or internalized into the cells would induce oxidative stress by producing ROS, implying that the close SWCNTs- cell attachment and piercing the algal cell wall could further facilitate the accumulation of SWCNTs-induced intracellular ROS and the occurrence of oxidative damage in algae. We speculate that the greater oxidative stress along with the enhanced cell penetration and internalization of SWCNTs can be responsible for the higher cytotoxicity toward M. aeruginosa. ZHANG et al [13] reported that MWCNTs of 15–30 mg/L could apparently increase the production of intracellular ROS toward green algae Chlorella pyrenoidosa and higher ROS level was reached at higher concentration, which was very consistent with our efforts toward M. aeruginosa. SANKAR et al [38] confirmed that nanoparticles can directly interact with the M. aeruginosa membrane and generated the enhanced ROS level, thus induce the M. aeruginosa mitochondrial injury, leading to the alteration of Δψm, ultimately prominent to M. aeruginosa cell death. There are other researches indicating that residual metal catalyst impurities of CNTs were also likely to be responsible for ROS formation [39, 40]. However, more detailed investigation of the possible mechanisms of CNTs resulting in oxidative stress to algal cells was beyond the scope of this paper, and further research is required to explain the observed shifts in physiological characteristics due to SWCNTs exposure.
3.3 Effects of SWCNTs on photosynthetic pigment of Microcystis aeruginosa
The content of chlorophyll a, chlorophyll b, and carotenoids in M. aeruginosa subject to single- walled carbon nanotubes treatment are illustrated in Figure 3. Compared with the control, the dose- dependent SWCNTs treated group showed a uniform tendency of decline on chlorophyll a, chlorophyll b, and carotenoids contents at 96 h, whereas a prompt little stimulation was observed at 0.50 mg/L treatment on chlorophyll b. The logarithm regression models between exposure concentration and chlorophyll inhibition were employed and they were of good quality (R2 ranged from 79.8% to 96.2%) to describe the inhibition of chlorophyll content after 96 h exposure. This phenomenon suggests that SWCNTs exerted a sustainable negative impact on M. aeruginosa and the ability to maintain proceeding of photosynthesis was getting weakened. At the range of 50.00–100.00 mg/L concentration treatment, the significant minimum values were recorded at 7.12 and 1.16 mg/L in Chl-a and Chl-b, respectively, which was only 14.89% and 7.33% of that in control. Generally, the variation tendency of all photosynthetic pigment is consistent with the growth tendency of M. aeruginosa, which is related to the algae growth states. Huge loss on chlorophyll can be ascribed to a lot of algae being dead with no more photosynthesis in aquatic under higher toxic conditions. However, there was no significant difference (p<0.05) detected on the reduction of carotenoids between different concentrations.
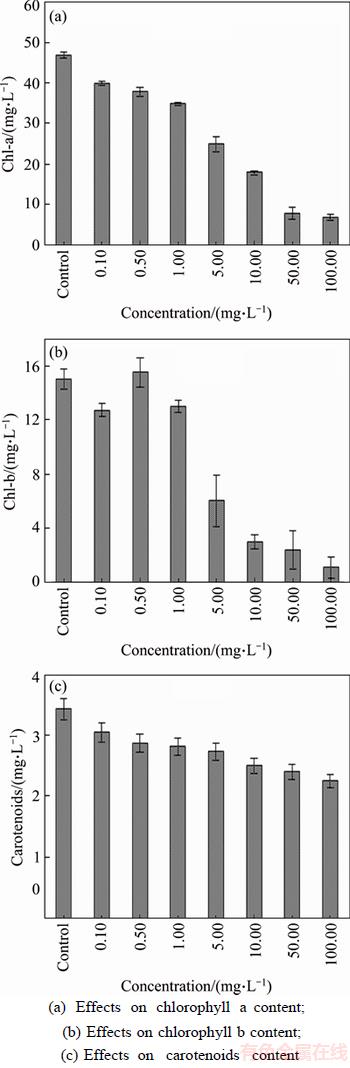
Figure 3 Effects of single-walled carbon nanotubes on photosynthetic pigment:(Statistical significance was accepted at a level of p<0.05)
Chlorophyll content has been regarded as an important factor in determining the photosynthetic rates and photosynthesis to assess activity of cell physiological metabolism to M. aeruginosa [41]. Little reduction at initial stage in chlorophyll content was observed after exposure to SWCNTs, and afterwards the prolonging decline was more obvious. This indicated that the ability of the cells to synthesize chlorophyll was decreased. Lower chlorophyll content signified a drop appeared in the antenna size of the photosynthetic reaction centre complexes. On the contrary, excess high concentrations of CNTs may induce significant toxic effect by altering the photosynthetic pigment contents. This assumption was in accordance with previous study and might be one of algal inhibiting mechanisms [38]. SAISON et al [42] specified that nanoparticles are toxic to unicellular algae by inhibiting PSII electron transport capacity.
Nanoparticles can inhibit the algae cell growth through down-regulation of lipid peroxidation and photosynthesis process, besides, CNTs may give rise to shading effects by reducing available light absorption affecting algal normal photosynthesis function as well [43]. Here, we speculate the intervention of carbon nanoparticles in the photosynthetic electron transport system might contribute to dramatical drop in the growth and pigments of M. aeruginosa. What’s more, both chloroplast and mitochondria are the main source of ROS, which can cause cell severe damage in the way of oxidative stress as discussed above. WEI et al [24] found functionalized MWCNTs (f-MWCNTs) damaged algal cells and assumed that the direct contact between f-MWCNTs and the cell surface was responsible for reduced PSII functional cross section and oxidative stress during exponential growth. SCHWAB et al [16] discussed alterations of the cell wall structure by CNTs and shading of the cells due to attached nanomaterial. The decrease of chlorophyll and carotenoid content might also be explained that M. aeruginosa may lost the capacity to sustain stability and integrity of chloroplast membrane due to constantly ROS attack or penetration in the process of CNTs internalization, which hence can be likely to destroy the photosynthesis-related structure of the algae at the same time, cause the collapse outcome of photosynthesis in algae, then further lead to malfunctions of normal physiological metabolism in algae cells ultimately.
3.4 Effects of SWCNTs on soluble sugar and protein content of Microcystis aeruginosa
When the scattering CNTs fragments at nanometer level enter into algal cell, it is quite likely that CNTs interact with the biomacromolecules physically or chemically and thus influence algal metabolism status and physiological conditions, which has been accepted as an another important interaction mechanism of CNTs to hydrobiose [16]. Soluble sugar is one of the common compounds in photosynthetic organisms acting as energy storage material and osmotic adjustment solutes. The variation on soluble sugar could reflect the energy metabolism, catabolism and anabolism to some extent. Our study showed that the sugar contents were strongly affected by SWCNTs, and there seemed to be no obvious difference with a light enhancement less than 1 mg/L SWCNTs, whereas afterwards fallen linearly with a good fitting correlated coefficient (R2=0.88). This implies that catabolism and anabolism of sugar played a key role in algal inhibition process, and the little increase at initial concentration was mainly probably caused by the normal stress in response to the SWCNTs contaminants in M. aeruginosa. MANABU et al [44] reported that the accumulation of soluble sugars in the protonema of cells is favorable for the enhancement of the cell’s tolerance to freeze. Other study considered polysaccharides and proteins as released products of photosynthesis, which may contribute to detoxification mechanisms to SWCNTs [45, 46]. These results assigned well with discussion of physiological response of M. aeruginosa in prior section that SWCNTs could damage the algal cell, and the damage degree was positive with exposure dose.
Protein plays an important role inside algal cells as well. It is closely linked to enzymes synthesis, which facilitates the biochemical reactions in cell metabolism. The soluble protein content could reflect the activity of cell metabolism. As shown in Figure 4, all the treatments except for control were significant declined and protein displayed in the approximately half level of that of control in the whole experiment. The decreased protein content in our study suggests that SWCNTs may inhibit the algal ability of protein synthesis sensitively. Protein deficiency would affect the normal physiological metabolism functions such as contributing to the disorder of antioxidant enzymes activity (evidenced from Figure 2), leading to the inhibition of photosynthesis (present by Figure 3), even resulting in the algal death, which may be the one of algal inhibiting mechanisms supposed to be further investigation and confirmation.

Figure 4 Effects of single-walled carbon nanotubes on soluble sugar and protein content (Statistical significance was accepted at a level of p<0.05)
3.5 Effects of SWCNTs on microcystin toxins production of Microcystis aeruginosa
Microcystins toxins (MCs), the secondary metabolite during the growth of algae, are greatly harmed to water quality and aquatic. Toxins can be released from algal blooms and especially those produced by microcystis species when the cell wall is destroyed [47]. Hence, the impact of carbon nanotubes on microcystins release is an important determinant of the ecological safety in potential. To assess the release of toxin from M. aeruginosa cells and the overall degradation of released toxins, the concentrations of extracellular toxins in the 5th day cultivation at different exposure concentrations were quantified as depicted in Figure 5. The control sample contained extracellular microcystins as high as 10.27 μg/L. The explanation can be that the extracellular microcystins was also released in the process of normal growth. Fortunately, the productions of microcystins in treated samples were significantly lower than that of the control group except for 0.10 mg/L treatment group, indicating that it seemed no significant influence beneath this level exposure. Specifically, when majority of M. aeruginosa died in the treatment of more than 50 mg/L, more amount of MC-LR would have liberated from fragmentized algal cells. However, the microcystins production was far lower than that in the control. This indicated that SWCNTs can not only damage M. aeruginosa cells, but can also degrade the microcystins. The present result is similar to the work by CHANG et al [48], where the proliferation of algal cells was effectively inhibited without increasing the release of cyanotoxins. The outcomes of this work shed new light on safely multipurpose use of CNTs to control algal blooms.

Figure 5 Effects of single-walled carbon nanotubes on microcystins toxins content (Statistical significance was accepted at a level of p<0.05)
4 Conclusions
This is the first report of the potential of carbon nanotubes for blue-green algae control test and may therefore provide a great potential and prospect for multipurpose use of CNTs. The present work indicates that the single-walled carbon nanotubes have potential ability to inhibit the growth of M. aeruginosa via oxidative stress and photosynthesis suppression, and the effect is normally dose-dependent. Extracellular microcystins production in treatment more than 50 mg/L of SWCNTs is far lower than that of control group. It is likely that the SWCNTs destroy the metabolism-related structure of algae cell and bring about bad physiological status. However, there are still some relations and questions between other CNTs and M. aeruginosa acting on the algal growth and physiological characteristics, for example, the multi-walled carbon nanotubes (MWCNTs) have higher purity, cheaper price and more applicability than SWCNTs, and further research on the mechanism of that needs to be investigated in future.
References
[1] MICHALAK A M, ANDERSON E J, BELETSKY D, BOLAND S, BOSCH N S, BRIDGEMAN T B, CHAFFIN J D, CHO K, CONFESOR R,  I, DEPINTO J V, EVANS M A, FAHNENSTIEL G L, HE LING-LI, HO J C, JENKINS L, JOHENGEN T H, KUO K C, LAPORTE E, LIU Xiao-jian, MCWILLIAMS M R, MOORE M R, POSSELT D J, RICHARDS R P, SCAVIA D, STEINER A L, VERHAMME E, WRIGHT D M, ZAGORSKI M A. Record-setting algal bloom in Lake Erie caused by agricultural and meteorological trends consistent with expected future conditions [J]. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110: 6448–6452.
I, DEPINTO J V, EVANS M A, FAHNENSTIEL G L, HE LING-LI, HO J C, JENKINS L, JOHENGEN T H, KUO K C, LAPORTE E, LIU Xiao-jian, MCWILLIAMS M R, MOORE M R, POSSELT D J, RICHARDS R P, SCAVIA D, STEINER A L, VERHAMME E, WRIGHT D M, ZAGORSKI M A. Record-setting algal bloom in Lake Erie caused by agricultural and meteorological trends consistent with expected future conditions [J]. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110: 6448–6452.
[2] CAREY C C, IBELINGS B W, HOFFMANN E P, HAMILTON D P, BROOKES J D. Eco-physiological adaptations that favour freshwater cyanobacteria in a changing climate [J]. Water Research, 2012, 46: 1394–1407.
[3] HUMBERT J F, BARBE V, LATIFI A, GUGGER M, CALTEAU A, COURSIN T, LAJUS A, CASTELLI V, OZTAS S, SAMSON G. A tribute to disorder in the genome of the bloom-forming freshwater cyanobacterium Microcystis aeruginosa [J]. PLoS One, 2013, 8: e70747.
[4] REN Ying, PEI Hai-yan, HU Wen-rong, TIAN Chang, HAO Da-ping, WEI Jie-lin, FENG Ya-wei. Spatiotemporal distribution pattern of cyanobacteria community and its relationship with the environmental factors in Hongze Lake, China [J]. Environmental Monitoring and Assessment, 2014, 186: 6919–6933.
[5] HU Liang-bin, ZHOU Wei, YANG Jing-dong, CHEN Jian, YIN Yu-fen, SHI Zhi-qi. Cinnamaldehyde induces PCD-like death of Microcystis aeruginosa via reactive oxygen species [J]. Water, Air, & Soil Pollution, 2011, 217: 105–113.
[6] BAUGHMAN R H, ZAKHIDOV A A, de HEER W A. Carbon nanotubes-the route toward applications [J]. Science, 2002, 297: 787–792.
[7] KHALKHALI A, KHAKSHOURNIA S, SABERI P. Optimal design of functionally graded PmPV/CNT nanocomposite cylindrical tube for purpose of torque transmission [J]. Journal of Central South University, 2016, 23(2): 362–369.
[8] KLAINE S J, ALVAREZ P J J, BATLEY G E, FERNANDES T F, HANDY R D, LYON D Y, MAHENDRA S, MCLAUGHLIN M J, LEAD J R. Nanomaterials in the environment: Behavior, fate, bioavailability, and effects [J]. Environmental Toxicology and Chemistry, 2008, 27: 1825–1851.
[9] GOTTSCHALK F, SONDERER T, SCHOLZ R W, NOWACK B. Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, fullerenes) for different regions [J]. Environmental Science & Technology, 2009, 43: 9216–9222.
[10] PETERSEN E J, ZHANG Li-wen, MATTISON N T, O’CARROLL D M, WHELTON A J, UDDIN N, NGUYEN T, HUANG Qing-guo, HENRY T B, HOLBROOK R D. Potential release pathways, environmental fate, and ecological risks of carbon nanotubes [J]. Environmental Science & Technology, 2011, 45: 9837–9856.
[11] ZHAO Xing-chen, LIU Ru-tao. Recent progress and perspectives on the toxicity of carbon nanotubes at organism, organ, cell, and biomacromolecule levels [J]. Environment International, 2012, 40: 244–255.
[12] LONG Zhi-feng, JI Jing, YANG Kun, LIN Dao-hui, WU Fen-chang. Systematic and quantitative investigation of the mechanism of carbon nanotubes’ toxicity toward algae [J]. Environmental Science & Technology, 2012, 46: 8458–8466.
[13] ZHANG Lu-qing, LEI Cheng, CHEN Jia-jun, YANG Kun, ZHU Li-zhong, LIN Dao-hui. Effect of natural and synthetic surface coatings on the toxicity of multiwalled carbon nanotubes toward green algae [J]. Carbon, 2015, 83: 198–207.
[14] RHIEM S, RIDING M J, BAUMGARTNER W, MARTIN F L, SEMPLE K T, JONES K C, SCH FFER A, MAES H M. Interactions of multiwalled carbon nanotubes with algal cells: Quantification of association, visualization of uptake, and measurement of alterations in the composition of cells [J]. Environmental Pollution, 2015, 196: 431–439.
FFER A, MAES H M. Interactions of multiwalled carbon nanotubes with algal cells: Quantification of association, visualization of uptake, and measurement of alterations in the composition of cells [J]. Environmental Pollution, 2015, 196: 431–439.
[15] MOU Feng-wei, WANG Ping, LI Han-dong, ZHOU Zhi-xiang. Growth inhibitions of four types of CNTs on Scenedesmus obliquus [J]. Journal of Convergence Information Technology, 2013, 8: 176–182.
[16] SCHWAB F, BUCHELI T D, LUKHELE L P, MAGREZ A, NOWACK B, SIGG L, KNAUER K. Are carbon nanotube effects on green algae caused by shading and agglomeration? [J]. Environmental Science & Technology, 2011, 45: 6136–6144.
[17] JEFFREY S W, HUMPHREY G F. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton [J]. Biochem Physiol Pflanz, 1975, 167(2): 191–194.
[18] UENO Y, NAGATA S, TSUTSUMI T, HASEGAWA A, WATANABE M F, PARK H D, CHEN G C, CHEN G, YU S Z. Detection of microcystins, a blue-green algal hepatotoxin, in drinking water sampled in Haimen and Fusui, endemic areas of primary liver cancer in China, by highly sensitive immunoassay [J]. Carcinogenesis, 1996, 17: 1317–1321.
[19] PARK M H, KIM K H, LEE H H, KIM J S, HWANG S J. Selective inhibitory potential of silver nanoparticles on the harmful cyanobacterium Microcystis aeruginosa [J]. Biotechnology Letters, 2010, 32: 423–428.
[20] van HOECKE K, de SCHAMPHELAERE K A C, van DER MEEREN P, LCUCAS S, JANSSEN C R. Ecotoxicity of silica nanoparticles to the green alga Pseudokirchneriella subcapitata: importance of surface area [J]. Environmental Toxicology and Chemistry, 2008, 27: 1948–1957.
[21] CALABRESE E J, BALDWIN L A. Toxicology rethinks its central belief [J]. Nature, 2003, 421: 691–692.
[22] KANG S, HERZBERG M, RODRIGUES D F, ELIMELECH M. Antibacterial effects of carbon nanotubes: size does matter! [J]. Langmuir, 2008, 24: 6409–6413.
[23] NIELSEN H D, BERRY L S, STONE V, BURRIDGE T R, FERNANDES T F. Interactions between carbon black nanoparticles and the brown algae Fucus serratus: Inhibition of fertilization and zygotic development [J]. Nanotoxicology, 2008, 2: 88–97.
[24] WEI Li-ping, THAKKAR M, CHEN Yu-hong, NTIM S A, MITRA S, ZHANG Xue-yan. Cytotoxicity effects of water dispersible oxidized multiwalled carbon nanotubes on marine alga, Dunaliella tertiolecta [J]. Aquatic Toxicology, 2010, 100: 194–201.
[25] NEL A, XIA Tian, M DLER L, LI Ning. Toxic potential of materials at the nanolevel [J]. Science, 2006, 311: 622–627.
DLER L, LI Ning. Toxic potential of materials at the nanolevel [J]. Science, 2006, 311: 622–627.
[26] WANG Zhen-yu, LI Jing, ZHAO Jian, XING Bao-shan. Toxicity and internalization of CuO nanoparticles to prokaryotic alga Microcystis aeruginosa as affected by dissolved organic matter [J]. Environmental Science & Technology, 2011, 45: 6032–6040.
[27] DI GIORGIO M L, DI BUCCHIANICO S, RAGNELLI A M, AIMOLA P, SANTUCCI S, POMA A. Effects of single and multi walled carbon nanotubes on macrophages: Cyto and genotoxicity and electron microscopy [J]. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 2011, 722: 20–31.
[28] GUO Hong-xia, TIAN Yu-jing, ZHANG Ye, WANG Huai. Adsorption of extracellular polymeric substances (EPS) of activated sludge onto single-walled nanotubes [J]. Anhui Chemical Industry, 2012(4): 12. (in Chinese)
[29] ZHANG Shan-shan, ZHANG Hui-min, QIN Rong, JIANG Wu-sheng, LIU Dong-hua. Cadmium induction of lipid peroxidation and effects on root tip cells and antioxidant enzyme activities in Vicia faba L [J]. Ecotoxicology, 2009, 18: 814–823.
[30] QIAN Hai-feng, XU Xiao-yan, CHEN Wei, JIANG Hong, JIN Yuan-xiang, LIU Wei-ping, FU Zheng-wei. Allelochemical stress causes oxidative damage and inhibition of photosynthesis in Chlorella vulgaris [J]. Chemosphere, 2009, 75: 368–375.
[31] LI Yong, ZHANG Shan-shan, JIANG Wu-sheng, LIU Dong-hua. Cadmium accumulation, activities of antioxidant enzymes, and malondialdehyde (MDA) content in Pistia stratiotes L [J]. Environmental Science and Pollution Research, 2013, 20: 1117–1123.
[32] CHAOUI A, MAZHOUDI S, GHORBAL M H, EL FERJANI E. Cadmium and zinc induction of lipid peroxidation and effects on antioxidant enzyme activities in bean (Phaseolus vulgaris L.) [J]. Plant Science, 1997, 127: 139–147.
[33] CHEN Chia-ying, JAFVERT C T. Photoreactivity of carboxylated single-walled carbon nanotubes in sunlight: reactive oxygen species production in water [J]. Environmental Science & Technology, 2010, 44: 6674–6679.
[34] KAGAN V E, TYURINA Y Y, TYURIN V A, KONDURU N V, POTAPOVICH A I, OSIPOV A N, KISIN E R, SCHWEGLER-BERRY D, MERCER R, CASTRANOVA V. Direct and indirect effects of single walled carbon nanotubes on RAW 264.7 macrophages: role of iron [J]. Toxicology Letters, 2006, 165: 88–100.
[35] ZHANG Yong-bin, ALI S F, DERVISHI E, XU Yang, LI Zhong-rui, CASCIANO D, BIRIS A S. Cytotoxicity effects of graphene and single-wall carbon nanotubes in neural phaeochromocytoma-derived PC12 cells [J]. Acs Nano, 2010, 4: 3181–3186.
[36] THURNHERR T, BRANDENBERGER C, FISCHER K, DIENER L, MANSER P, MAEDER-ALTHAUS X, KAISER J P, KRUG H F, ROTHEN-RUTISHAUSER, B, WICK P. A comparison of acute and long-term effects of industrial multiwalled carbon nanotubes on human lung and immune cells in vitro [J]. Toxicology Letters, 2011, 200: 176–186.
[37] PORTER A E, GASS M, BENDALL J S, MULLER K, GOODE A, SKEPPER J N, MIDGLEY P A, WELLAND M. Uptake of noncytotoxic acid-treated single-walled carbon nanotubes into the cytoplasm of human macrophage cells [J]. Acs Nano, 2009, 3: 1485–1492.
[38] SANKAR R, PRASATH B B, NANDAKUMAR R, SANTHANAM P, SHIVASHANGARI K S, RAVIKUMAR V. Growth inhibition of bloom forming cyanobacterium Microcystis aeruginosa by green route fabricated copper oxide nanoparticles [J]. Environmental Science and Pollution Research, 2014, 21: 14232–14240.
[39] KIM H S, PARK B H, KANG M S, YOON J S, JIN H J. Characterization of polycarbonate/multiwalled carbon nanotube composites [J]. Key Engineering Materials, 2006, 326–328: 1829–1832.
[40] TAN Xiao-ming, LIN Chun, FUGETSU B. Studies on toxicity of multi-walled carbon nanotubes on suspension rice cells [J]. Carbon, 2009, 47: 3479–3487.
[41] ZHANG Chao, YI Yang-lei, HAO Kai, LIU Guang-lu, WANG Gao-xue. Algicidal activity of Salvia miltiorrhiza Bung on Microcystis aeruginosa—towards identification of algicidal substance and determination of inhibition mechanism [J]. Chemosphere, 2013, 93: 997–1004.
[42] SAISON C, PERREAULT F, DAIGLE J C, FORTIN C, CLAVERIE J, MORIN M, POPOVIC R. Effect of core–shell copper oxide nanoparticles on cell culture morphology and photosynthesis (photosystem II energy distribution) in the green alga, Chlamydomonas reinhardtii [J]. Aquatic Toxicology, 2010, 96: 109–114.
[43] ARUOJA V, DUBOURGUIER H C, KASEMETS K, KAHRU A. Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata [J]. Science of The Total Environment, 2009, 407: 1461–1468.
[44] NAGAO M, MINAMI A, ARAKAWA K, FUJIKAWA S, TAKEZAWA D. Rapid degradation of starch in chloroplasts and concomitant accumulation of soluble sugars associated with ABA-induced freezing tolerance in the moss Physcomitrella patens [J]. Journal of Plant Physiology, 2005, 162: 169–180.
[45] MIAO Ai-jun, SCHWEHR K A, XU Chen, ZHANG Sai-jin, LUO Zhi-ping, QUIGG A, SANTSCHI P H. The algal toxicity of silver engineered nanoparticles and detoxification by exopolymeric substances [J]. Environmental Pollution, 2009, 157: 3034–3041.
[46] ZHANG Sai-jin, JIANG Yue-lu, CHEN Chi-shuo, CREELEY D, SCHWEHR K A, QUIGG A, CHIN Wei-chun, SANTSCHI P H. Ameliorating effects of extracellular polymeric substances excreted by Thalassiosira pseudonana on algal toxicity of CdSe quantum dots [J]. Aquatic Toxicology, 2013, 126: 214–223.
[47] LI Fang, LIU Wei-qing, ZHAO Nan-jing, DUAN Jing-bo, WANG Zhi-gang, ZHANG Yun-jun, XIAO Xue, LIU Jing, YIN Gao-fang, SHI Chao-yi. Studies on extracting microcystin-LR from Microcystis aeruginosa by water bath [J]. Journal of Environmental Protection, 2013, 4: 70.
[48] CHANG Shu-chi, LI Cheng-hao, LIN Jiang-jen, LI Yen-hsien, LEE Maw-rong. Effective removal of Microcystis aeruginosa and microcystin-LR using nanosilicate platelets [J]. Chemosphere, 2014, 99: 49–55.
(Edited by HE Yun-bin)
中文导读
单壁碳纳米管对铜绿微囊藻生长及生理特性的影响
摘要:为了探索一种潜在新型的使用碳纳米管(CNTs)高效控制水华蓝藻的处理方法,本研究调查了在实验室条件下单壁碳纳米管(SWCNTs)对铜绿微囊藻(Microcystis aeruginosa)的生长及控制的影响。相关生理变化检测包括重要的抗氧化酶,如超氧化物歧化酶(SOD)、过氧化物酶(POD)、过氧化氢酶(CAT)、丙二醛(MDA),有光合色素、蛋白、可溶性糖和胞外藻毒素(MC-LR)。藻细胞密度在高浓度SWCNTs (>5.00 mg/L)处理时受到明显抑制,抑制率具有浓度依赖性。当处理100 mg/L SWCNTs时,抑制率可达90%以上。96 h IC50 为22 mg/L。在高浓度SWCNTs处理时,抗氧化酶活性明显下降,脂质过氧化物上升,表明胞内产生了活性氧自由基(ROS)和氧化胁迫伤害。光合色素、可溶性糖和蛋白含量下降,表明SWCNTs可能摧毁了藻的光和系统,细胞的相关代谢结构,导致了Microcystis aeruginosa 糟糕的生理状态。此外,SWCNTs还可以有效地降低培养基中的微囊藻毒素。
关键词:单壁碳纳米;铜绿微囊藻;微囊藻毒素;生长
Foundation item: Project(035703011) supported by the Scientific Research Double Support Program of SICAU, China
Received date: 2017-02-21; Accepted date: 2017-04-05
Corresponding author: WANG Ying-jun, PhD, Professor; Tel: +86–28–86291390; E-mail: wwyyjj1972@163.com
Abstract: In order to explore a novel and potential method using carbon nanotubes (CNTs) for controlling blue-green algal blooms efficiently in future, effects of single-walled carbon nanotubes (SWCNTs) on Microcystis aeruginosa growth control were investigated under lab cultured conditions. Related physiological changes were tested involving several important enzyme of antioxidant defense system (superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), malondiadehyde (MDA), photosynthetic pigments, protein, soluble sugar and extracellular microcystin toxins (MC-LR)). Algal cell density was significantly inhibited by SWCNTs at high concentration (>5.00 mg/L), and the inhibition rate was dose-dependent. For treatment with 100 mg/L SWCNTs, the inhibitory rates even reached above 90%. 96 h IC50 was determined as 22 mg/L. Antioxidant enzyme activities were dramatically dropped with increasing lipid peroxidation at higher SWCNTs concentration, indicating intracellular generation of reactive oxygen species (ROS) and oxidative stress damage in algae. Reduction of photosynthetic pigments, soluble sugar and protein contents suggested that SWCNTs may severely ruin algal photosynthesis system, destroy the metabolism-related structure of cell, and thus lead to negative physiological status in M. aeruginosa. Besides, SWCNTs can effectively decrease the amount of extracellular microcystins in culture medium.


